Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Anales de la Asociación Química Argentina
Print version ISSN 0365-0375
An. Asoc. Quím. Argent. vol.94 no.4-6 Buenos Aires Aug./Dec. 2006
REGULAR PAPERS
Scale-up of pilot plant for adsortion of heavy metals
Moreno-Piraján, J.C.a; Rangel, D.b; Amaya, B.b ; Vargas, E.M.b; Giraldo, L.c
a Departamento de Química, Facultad de Ciencias, Universidad de los Andes
Grupo de Investigación en Sólidos Porosos y Calorimetría, Carrera 1 No. 18A-10/70, Bogotá, Colombia
FAX: +57 1 3324366, E-mail: jumoreno@uniandes.edu.co
b Departamento de Ingeniería Química, Facultad de Ingeniería, Universidad de Los Andes, Carrera 1 No. 18A-10/70, Bogotá, Colombia
c Departamento de Química, Universidad Nacional de Colombia, Carrera 30 calle 45, Bogotá, Colombia
Received May 8th, 2006. In final form December 30th, 2006
Abstract
The present work shows a simple way to design a process scale-up, for an adsorber, from laboratory to industrial level using breakthrough curves in fixed beds developed through the continuous detection of the Pb(II) ion concentration in the effluent of the bed. This procedure was developed in order to examine its efficiency and to be compared with an experimental method developed in the laboratory, which produced similar results. The feasibility of this design lies on the fact that neither tedious calculations nor mass transfer coefficients are required in order to plot the mentioned curves. The procedure was developed by applying concepts such as mass transfer zone (MTZ) and length of unused bed (LUB), which are the dynamic basis of the adsorption process in fixed beds.
As a complement to the experimentation, within a pilot plan scale, a filter was developed to handle the most important adsorption parameters and to control the variables that change the operating conditions during the process.
Resumen
Este trabajo describe una manera simple de diseñar un proceso de escalado, para un adsorbedor, desde nivel de laboratorio a escala industrial usando curvas de paso en lechos fijos, desarrollado a través de la detección continua de la concentración de Pb(II) en el efluente del lecho. El procedimiento fue medido con el objeto de examinar su eficiencia y para ser comparado con un método experimental desarrollado en el laboratorio que dio lugar a resultados similares. La factibilidad del diseño se basa en la ausencia de cálculos tediosos y coeficientes de transferencia en el graficado de las curvas mencionadas. El procedimiento fue desarrollado aplicando conceptos tales como los de zona de transferencia de masa (MTZ) y longitud del lecho no usado (LUB), que constituyen la base dinámica del proceso de adsorción en lechos fijos.
Como complemento a la parte experimental en el proceso de escalado, se desarrolló un filtro para controlar los parámetros de adsorción más importantes y para observar las variables que modifican las condiciones de operación durante el proceso.
Introduction
Due to the increasing level of toxic metals found in residual streams originated in industrial discharges, new methods and techniques are being developed for environmental control. Metal adsorption by porous solids has been one of the most useful tools for handling these wastes.
Even though several publications have been reported concerning water treatment in batch systems, nowadays, continuous columns carry out the treatments with fixed beds due to the fast process that takes place. If an optimisation process is desired, it is necessary to undertake scale-up studies that can be used to adequate industrial equipments, owing to the dynamic behaviour that can be described in terms of time-concentration profiles commonly called breakthrough curves.
A breakthrough curve is constructed based on the concentration pursuit that may be emptied into the effluent (C) while a contaminated influent with a known concentration (Co) is continuously poured into the fixed bed absorber. The characteristic shape of this curve will depend on the equilibrium between the solid and liquid phase, based on the kinetic adsorption process, which is divided into four phenomena: i) diffusion in the bulk fluid, ii) external mass transfer, iii) intraparticular mass transfer and iv) micropore adsorption [1-3].
During the first period, the contaminant concentration at the final part of the bed is practically zero, however, with time, the contaminated influent is continuously fed, a limit concentration is reached, which is known as breakthrough point (Cb) at the time (tb). In high-level processes this point will determine the moment in which the feed has to be interrupted for a determined bed and deviated to a new or fresh one, while the adsorbent material is renewed [2]. If the feed is not interrupted into the adsorber, the concentrations that appear in the effluent after the breakthrough point start to increase quickly until a ratio of concentrations C/Co equal to 1 is achieved.
An excellent summary of mathematical procedures to predict the breakthrough curves appears in the recent literature [4-7]. However, due to its intricate complexity for the determination of some of the parameters and calculations, it is preferable to make an analysis of the operation in fixed bed based on experimental data.
To formulate a generalized procedure corresponding to the pore diffusion mechanism, the following assumptions are made [8-12]:
1. The system operates under isothermal conditions.
2. The adsorption equilibrium is described by the Langmuir isotherm.
3. Intraparticle mass transport is due to Fickian diffusion, characterized by pore diffusion coefficient, Dp.
4. Mass transfer across the boundary layer surrounding the solid particles is characterized by external-film mass transfer coefficient, kf.
5. The macroporous adsorbent particles are spherical and homogeneous in size and density.
Based on the preceding assumptions, for the control volume, Adz, and for the limiting situation z → 0, the net rate of accumulation or depletion is given by Eq. 1:

The following initial conditions (Eqns. 2 and 3) are considered:
Cb = Cbo z = 0, t = 0 (2)
Cb = 0 0 < z ≤ L, t = 0 (3)
The contour conditions at both ends of the column are given by Eqns. 4 and 5:

The superficial velocity, V in fixed-bed adsorption is not constant because of adsorption.
The following equation is used to estimate (dV/dz), the variation of velocity of bulk fluid along the axial direction of the bed. For liquid adsorption, assuming the liquid density to be constant, the total mass balance gives Eq. 6.

Velocity boundary conditions are given by Eqns. 7 and 8:

The inter-phase mass transfer rate may be expressed as given by Eq. 9

The intra-pellet mass transfer is due to the adsorbate molecules diffusion through the pore. The macroscopic conservation equation is given by Eq. 10

Rearranging Eq. 3, we obtain Eq.11

The considered initial condition is given by Eq. 12:
c = 0, q = 0 a < r < ap, t = 0 (12)
The symmetry condition at the center of the particles and continuity condition on the external surface of the adsorbent bed are expressed as (equations 13 and 14):

The adsorption isotherm was nonlinear and described by that of Langmuir (Eq. 15):

By means of the application of the mass transfer zone (MTZ) and the length of unused bed (LUB) concepts, the scale-up of the process to a major length filter was possible and a new curve of the scaled procedure was obtained. A theoretical development of the problem was made and the experimental procedures confirmed that the mathematical model describes properly the phenomenon in a fixed bed of an adsorbent.
Experimental
The activated carbon used throughout the experiment was obtained from sawdust, carbonised at 300 ºC for two hours under an inert gas flux (N2) at 110 ml/min, activated by means of a 110 ml/min current of CO2 during two hours at 600 ºC. The concentration of ion Pb+2 was determined by Atomic Absorption on a Perkin-Elmer Analyst 300 equipment.
Table 1. Specifications of the designed procedure.
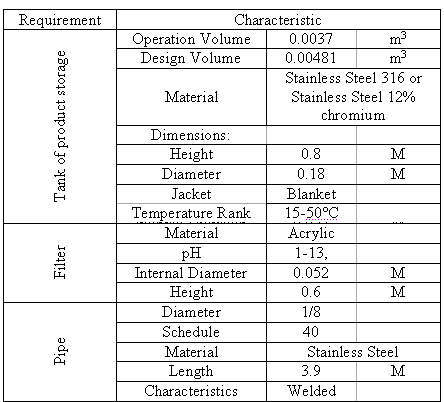

Figure 1 shows the detection of the effluent concentration with time to obtain breakthrough curves of the treated systems. An influent of a well-known concentration that at the exit of the process reach a relative concentration of 0.05 was poured into a bed of 13 cm length and 0.71 cm of diameter. This first curve, after algebraic manipulation, was used in the scale-up process to a column of 37 cm length.

Figure 1. Breakthrough curve
Using the later column, a new curve was obtained by simulating an identical system to the 13 cm column; the activated carbon had the same nature as the first used, the treated solute had the same Co, and the most important parameter for the scale-up process, the velocity of fixed bed (Uo), was maintained fixed in order to ensure constant MTZ and LUB in both procedures. The design of the adsorption equipment for a pilot plant scale-up was done taking into account the most important parameters for an adsorption process, with the purpose of handling all variables by changing only the operation conditions. The procedure has the following specifications.
The storage tank for the fluid problem was constructed in stainless steel with Cr covering, since the samples could corrode the vessel interior because the pH levels range between 1 and 13. Additionally, the material had to bear temperatures between 15 and 50 ºC to study the effect of temperature on the adsorption process; therefore the system should have an electrical heating blanket. As a safety and stability measure, the height of the tank and the diameter are the same.
It is necessary to use a centrifugal pump, instead of peristaltic, for filling operations because a pulsating flow would change the residence time of the liquid within the fixed bed, and therefore it would be impossible to moderate the delta pressure that would lead to a remarkable fluctuation in the flux. The procedure also includes two pressure gauges installed before and after the filter to record the pressure drop of the fluid flux through the bed.

Figure 2. Adsorption equipment at pilot plant for heavy metals
Results and discussion
As the carbon is saturated, the adsorption zone moves towards the bottom of the bed; this zone is known as the mass transfer zone (MTZ) and corresponds to the region where the initial concentration of the influent decays to zero [4-7]; as shown in Figure 3.

Figure 3. Movement of the frontal adsorption zone through the bed.
The movement of the frontal adsorption zone through the bed is obtained by means of a mass balance, where:

The breakthrough curve has certain characteristic parameters as: i) the saturation time (ts), ii) the capacity in the breakthrough point (qb), iii) the capacity at the saturation time (qs) and iv) t*, the time at which C/Co reaches the value of 0.5 [2], as long as the curve is symmetrical as shown in Figure 4, (A1 = A2).

Figure 4. The breakthrough curve and its characteristic parameters.
If the curve is not symmetrical, it is hard to set up the stoichiometric time and a new mass balance for both areas should be compared. It entails an iterative process to determine the exact location of t* established by the following relation: [1].

The breakthrough (tb) and saturation times (ts), are calculated by means of interpolation on the graph, but the breakthrough and saturation capacities are determined by integration:

Using the previously displayed curve (Figure 4), it is possible to obtain the length of unused bed (LUB), which represents the distance that is not saturated at the breakthrough time. The foundation of the scale-up process lies on the fact that the amount of the unused bed does not vary with the overall length of the bed, since the slope of the curve does not vary [2]. The later is calculated as:

Once the constant LUB for both procedures (experimental and scale-up) is obtained, and knowing the desired new length of the bed, the breakthrough time (tb2) for the column to be scaled-up is obtained using the following relation:

If we observe the units of the preceding equation, we can realize that they correspond to the velocity units of the frontal fixed bed (Uo), that is, if maintained constant during the process, it should lead to an invariable MTZ and LUB for both cases, which implies that LUB1 and LUB2 are the same, the relation parameter for both procedures.
A way to keep constant the velocity of the frontal bed (Uo) is by using the Ergun equation (17).

By working with the same material and the same influent, it is guaranteed that the right part of the equation will be constant, with the exception of Uo. However, Uo could be kept constant if the relation Dp/L for both procedures is maintained; this means that when the distance of the bed is changed, the pressure drop also varies within the same relation. For our particular case, the previously described equation will be developed to verify that the mathematical procedure is in fact comparable to the experimental procedure.
For the 13 cm length column, the calculations are the following ones according to Figure 5:

Figure 5. Breakthrough curve using a scaled procedure.

Therefore, the capacities at the breakthrough point and at the saturation point are:

It is logical that the capacity at the saturation point be smaller than that at the breakthrough point because at this point the activated carbon is already saturated. In order to facilitate the calculations we assume that the curve is symmetrical enough and therefore the value of the stoichiometric time will be t* = 3166.6s
Rearranging algebraically equation (20), the value of LUB = 7.49 cm is obtained. In order to scale-up our procedure to a new column of 37 cm length, the values in equation (21) are replaced, and the breakthrough time of the new curve is obtained:

Once this time is obtained, the new curve of the fixed bed may be determined by adding the time corresponding to the first column and plotting them according to the same relative concentrations.
As shown in figure 6, the experimental procedure of 37 cm is very similar to that obtained by means of the scale-up; this exactitude in both procedures was obtained by reproducing the experimental procedures that were performed, trying to keep working with the same material and maintaining a constant relation Dp/L to obtain the same velocity flow. Additionally, if we compare the LUB values, by using the equation (21) for both beds of 37 cm, the value of the experimental curve does not give a significant error (4.6 %), since it has a LUB value of 7.15 cm, and within the experimental error, the lengths of unused bed are congruent.

Figure 6. Breakthrough curve from the experimental procedure.
Conclusions
In this work, a simple and viable procedure of scale-up has been proposed. It provides an excellent approach to industrial procedures through experimental data obtained from fixed bed adsorption columns.
The proposed method does not require intricate calculations, use of correlations, nor the determination of any type of coefficients, which makes it the nearest procedure to reality as long as the reproducibility of the system under treatment could be assured. Therefore, the adsorption phenomenon must guarantee constant design parameters, as i) activated carbon used for the fixed bed, ii) the nature of the influent and iii) the superficial velocity of the system that keeps the retention time steady.
It is important to mention that the same pressure drop relation is fixed for both, the experimental and industrial procedure, assuring a constant MTZ, otherwise the curves slopes would change the characteristic times of the plots and therefore all the calculations.
The shown results from procedureling fixed beds may be used as a practical tool for designing industrial adsorbers due to their simplicity and accuracy.
1 Editor's note: Authors use the symbol *, at line-heigth, to indicate multiplication.
Acknowledgements
The project was supported by CIFI (Engineering Faculty Investigation Centre) and the Sciences Faculty Research Group, Universidad de Los Andes (Colombia).
Our special gratitude to the people of the Chemistry Department for their support. The authors thank the Departments of Chemistry of the Colombia and Andes Universities for their cooperation agreement.
References
[1] Basso, M.C.; Cerrella, E.G.; Cukierman, A.L., Programa de Investigación y Desarrollo de Fuentes Alternativas de Materias Primas y Energía - PINMATE. Departamento de Industrias. Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires, 1996. [ Links ]
[2] Blanco, J.; Castro, P.R.; Bonelli, E.G., Programa de Investigación de Fuentes Alternativas de Materias Primas y Energía (PINMATE), Departamento de Industrias, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, 1996. [ Links ]
[3] Hayashi, J.; Atsuo, K., Carbon, 2000, 38, 1873. [ Links ]
[4] Kobya, M.; Bioresource Technology, 2004, 40, 317. [ Links ]
[5] Marsh, H.; Heintz A.; Rodriguez-Reinoso F., Introduction to Carbon Technologies. Chapter 2, Universidad de Alicante, Secretariado de Publicaciones, 1998. [ Links ]
[6] Shaobin, Y., Carbon, 2002, 40, 277. [ Links ]
[7] Tolmachev, A.M., Carbon, 2002, 40, 1401. [ Links ]Babu, B.V.; Gupta, S., “Proceeding and Simulation for Dynamics of Packed Bed Adsorption”, Proceedings of the International Symposium & 57th Annual Session of IIChE in association with AIChE (CHEMCON-2004), Mumbai, December 27-30, 2004. [ Links ]
[8] Babu, B.V., Chaurasia, A.S., Energy Conversion and Management, 2003, 44 (13) 2135. [ Links ]
[9] Babu, B.V.; Chaurasia, A.S., Chemical Engineering Science, 2004, 59(3), 611. [ Links ]
[10] Bautista, L.F.; Martinez, M.; Aracil, J., AIChE Journal, 2003, 49(10), 2631. [ Links ]
[11] Liao, H.T.; Shiau, C.Y., AIChE Journal, 2000, 46 (6), 1168. [ Links ]
[12] Sotelo, J.L.; Uguina, M.A.; Delgado, J.A.; Celemin, L.I., Separation and Purification Technology, 2004, 37 (2), 149. [ Links ]














