Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de la Sociedad Entomológica Argentina
Print version ISSN 0373-5680On-line version ISSN 1851-7471
Rev. Soc. Entomol. Argent. vol.63 no.1-2 Mendoza Jan./July 2004
Calliphoridae (Diptera) from Southeastern Argentinean Patagonia: Species Composition and Abundance
Calliphoridae (Diptera) del Sudeste de la Patagonia Argentina: Composición Específica y Abundancia
Schnack, Juan A.* and Juan C. Mariluis**
* División Entomología, Museo de La Plata, Paseo del Bosque. 1900 La Plata, Argentina; e-mail: js@netverk.com.ar
*Servicio de Vectores, ANLIS, Instituto Nacional de Microbiología Dr. Carlos Malbrán, Avda. Vélez Sarsfield 563, 1281, Buenos Aires, Argentina; e-mail: jcmariluis@yahoo.com.ar
ABSTRACT. Species composition and spatial and temporal numerical trends of blow flies (Diptera, Calliphoridae) species from three southeastern Patagonia localities: Río Grande (53° 48' S, 67° 36' W) (province of Tierra del Fuego), Río Gallegos (51° 34' S, 69° 14' W) and Puerto Santa Cruz (50° 04' S, 68° 27' W) (province de Santa Cruz) were studied during November, December (1997), and January and February (1998). Results showed remarkable differences of overall fly abundance and species relative importance at every sampling site; nevertheless, they shared a poor species representation (S £ 6). The cosmopolitan Calliphora vicina Robineau-Desvoidy, the nearly worldwide Lucilia sericata (Meigen), and the native Compsomyiops fulvicrura (Robineau-Desvoidy) prevailed over the remaining species. Records of Protophormia terraenovae (Robineau-Desvoidy), a species indigenous to the Northern Hemisphere are noteworthy.
KEY WORDS. Calliphoridae. Patagonia. Species Composition. Spatial and Temporal Numerical Trends.
RESUMEN. Se estudian la composición y las variaciones numéricas espacio-temporales de especies de Calliphoridae (Diptera) de tres localidades del sudeste patagónico: Río Grande (53° 48' S, 67° 36' W) (provincia de Tierra del Fuego), Río Gallegos (51° 34' S, 69° 14' W) y Puerto Santa Cruz (50° 04' S, 68° 27' W) (provincia de Santa Cruz), a partir de muestreos realizados en noviembre, diciembre (1997), enero y febrero (1998). Comparando los sitios de muestreo, los resultados obtenidos muestran diferencias marcadas en la abundancia total de la taxocenosis como de cada una de sus especies, destacándose la escasa riqueza específica de cada uno de ellos (S £ 6). Calliphora vicina Robineau-Desvoidy (cosmopolita), Lucilia sericata (Meigen) (de amplia distribución mundial) y Compsomyiops fulvicrura (Robineau-Desvoidy) (nativa), son las mejor representadas numéricamente. Se subraya el hallazgo, en el área de estudio, de Protophormia terraenovae (Robineau-Desvoidy), especie indígena del hemisferio norte.
PALABRAS CLAVE. Calliphoridae. Patagonia. Composición Específica. Tendencias Numéricas Espacio-temporales.
INTRODUCTION
Studies concerning species composition and numerical trends of Calliphoridae from Patagonia have been recently started in the following Argentinean western localities and neighboring areas: San Carlos de Bariloche (41° 08' S, 72° 00' W), province of Río Negro (Mariluis & Schnack, 1996), Esquel (42° 55'S, 71° 52' W), province of Chubut (Schnack et al., 1998), and El Calafate (50° 25' S, 71° 50' W), province of Santa Cruz (Mariluis et al., 1999). Furthermore, blow flies were also surveyed at Ushuaia (54° 47' S, 68° 18' W), province of Tierra del Fuego (Mariluis et al., 1999).
This paper provides further information on blow fly species composition and abundance regarding three locations still unexplored from the Atlantic Patagonian coast: Río Grande (53° 48' S, 67° 36' W) (province of Tierra del Fuego), Río Gallegos (51° 34' S, 69° 14' W) and Puerto Santa Cruz (50° 04' S, 68° 27' W) (province of Santa Cruz). It is expected that the data here provided would contribute to further studies aimed to identify the potential impacts of local and non indigenous blow fly species on domestic animals and public health in the Andean-Patagonian Dominion.
STUDY SITES
From the biogeographical viewpoint, the study sites belong to the Provincia Patagónica within the Andean-Patagonian Dominium (Cabrera & Willink, 1980). There, the weather is dry, moderately cold, with snow during winter, and intense west winds most of the year. Within this biogeographical province, the annual mean temperature's amplitude fluctuates between 13.4° C in Chos Malal, (37° 23' S, 70° 17' W) and 5.0° C in Río Grande, and the annual rainfall can reach 500 mm at its eastern border. Two main districts may be distinguished in the study area (Soriano, 1956; Cabrera, 1976): the Sub-Andean district and the Fuegian district. The first stretches approximately from Puerto Santa Cruz to Cabo Vírgenes, the eastern mouth of the Strait of Magellan, and is made up of a grassy steppe dominated by Festuca pallescens (Soriano, 1956). The Fuegian district extends from the north of Tierra del Fuego to a few kilometers south of Río Grande, where forests of Nothofagus spp. commence. In this area, the steppes are dominated by Festuca gracillima (Morrison et al., 1989).
MATERIAL AND METHODS
Samplings were undertaken during the warmer months of the year when the flies are active: November, December (1997), January and February (1998). At each sampling locality and date, three sampling sites with different degrees of human intervention were chosen, as follows: dense urban settlements, isolated dwellings and uninhabited areas; they were called, following Nuorteva (1963) as eusynanthropy, hemisynanthropy and asynanthropy, respectively. Adult flies were captured with an entomological net hourly, from 10:15 to 16:15 h while placed on a bait composed by 200 g of rotten cow meat exposed to fly colonization during 15 minutes each time. The sampling dates at each surveyed location and contour are detailed elsewhere (Tables I, II and III). Air temperature was recorded hourly at each sampling site of the three studied localities.
Table I. Monthly variations of relative abundance of Calliphoridae at Río Grande (November 1997-February 1998).

Table II. Monthly variations of relative abundance of Calliphoridae at Río Gallegos (November 1997-February 1998).

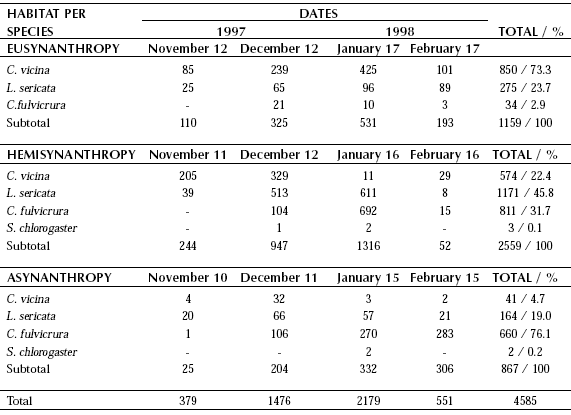
Table III. Monthly variations of relative abundance of Calliphoridae at Puerto Santa Cruz (November 1997- February 1998).
The sampled flies are currently deposited at the Servicio de Vectores, ANLIS, Instituto Nacional de Microbiología Dr. Carlos Malbrán. They will be deposited at the División Entomología, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata once morphological studies carried out by the second author are finished.
RESULTS
The following species were recorded: Calliphora vicina Robineau-Desvoidy, Chlorobrachycoma versicolor (Bigot), Compsomyiops fulvicrura (Robineau-Desvoidy), Lucilia sericata (Meigen), Protophormia terraenovae (Robineau-Desvoidy), and Sarconesia chlorogaster (Wiedemann). Summing up the whole set of captures carried out during this study, 8167 adult flies were counted. Suggestive differences were observed either in overall abundance or in species relative importance at every sampling site. They are below described separately.
Sex ratio was female biased in all the recorded species, except S. chlorogaster whose low occurrence (N = 6) did not allow to observe a definite trend.
The lowest and highest recorded temperature were, including all localities and contours, 10.9 and 27.6 °C, respectively. Excluding C. versicolor and S. chlorogaster, of almost null occurrence, the temperature ranks could be recorded for C. vicina (11.9 - 25.5 °C), L. sericata (13.4 – 27.6 °C), P. terraenovae (15.1 – 27.6 °C), and C. fulvicrura (16.8 – 25.5°C).
Río Grande. Blow fly overall abundance (N = 933) was lower than in the remaining locations. The number of captures showed a decrease from the urban to the uninhabited areas if all samples are summed. However, this trend was not verified at every sampling date and was notably influenced by the high number of specimens taken in February, when the three surveyed habitats exhibited the highest abundance. Overall abundance trends showed a progressive increase at every habitat except asynanthropy, as follows: November < December < January < February. Regarding December and January, the highest number of captures took place at hemisynanthropy. Hourly records did not show a definite numerical trend, thus exhibiting an even distribution. Temporal and spatial numerical trends exhibited by the recorded species clearly show the dominant role played by C. vicina at all studied contours and sampled dates, being the remaining recorded species scarcely represented. It is important to remark, however, the presence of P. terraenovae, a species from the Northern Hemisphere, which exhibited similar abundance to that of L. sericata, the former prevailing at asynanthropy, the latter at hemisynanthropy and eusynanthropy. Nevertheless, both species taken together represent less than 20% of the total number of C. vicina (Table I).
Río Gallegos. Blow fly overall abundance (N = 2649) showed a progressive numerical increase from the first to the last sampling date. Even though the highest number of flies was sampled in February, both at hemisynanthropy and at asynanthropy, the same trend was not assessed at eusynanthropy where the number of flies decreased markedly from January to February. If all sampling dates are regarded, a numerical increase can be observed from the urban to the uninhabited areas, notably expressed in the latter (Table II). Regarding the whole set of samples taken at all dates and sites, L. sericata showed the highest relative abundance, followed in decreasing order by C. vicina, C. fulvicrura and P. terraenovae. Nevertheless, the relative importance of these species differed between habitats. The overall abundance of the dominant species at hemisynanthropy and asynanthropy was notably influenced by the captures taken in February. At that time of the year L. sericata prevailed over the remaining species at all study sites (Table II). Observed variations in number of captured flies at hourly intervals, totaling the four sampling dates, were negligible.
Puerto Santa Cruz. Blow fly overall abundance was highest at this locality (N = 4585). Comparison between sampling dates either at each sampling section or gathering them up together, showed the same tendency. This bias was expressed by a progressive increase from November to January with lower figures in the first and last sampling dates. Cumulative number of sampled flies did not show remarkable variations comparing the different hourly records. Regarding the studied habitats, the highest overall abundance record was observed at hemisynanthropy while the lowest at asynanthropy. Species relative abundance showed noticeable differences between the three studied habitats. While C. vicina was the dominant species at eusynanthropy, L. sericata did so at hemisynanthropy and C. fulvicrura dominated over the remaining species at asynanthropy. At Puerto Santa Cruz, overall abundance of blow flies at all sampling sections was notably governed by the captures taken in January. Compsomyiops fulvicrura and S. chlorogaster, were the only recorded species restricted to the Neotropical Region. Both species have not been reported at Río Grande. However, the former species exhibited a different pattern of abundance at the asynanthropy of Puerto Santa Cruz, where it was the dominant species (Table III). Only six specimens of S. chlorogaster were recorded totaling the samples taken from Río Gallegos and Puerto Santa Cruz.
DISCUSSION
The low number of recorded species agrees with former studies undertaken in other Patagonian locations (Mariluis & Schnack, 1996; Schnack et al., 1998; Mariluis et al., 1999). Six species were recorded in the three sampled localities. While five species were reported at Río Gallegos, four species were identified at each of the remaining localities. If it were not for just one specimen of S. chlorogaster being captured at Río Gallegos, every surveyed locality would have had the same species richness.
The female biased sex ratio observed for most species is likely due to the fact that the bait was an oviposition substrate.
As it has been suggested by Mariluis & Schnack (1996) alluding to Argentina, a trend towards a species density decrease as the latitude increases would occur at asynanthropy. Within the study area, a species richness contraction was remarkable at Río Grande where only three species were recorded. In a recent survey undertaken at Parque Nacional Tierra del Fuego, in the southern extreme of the homonymous island, only C. vicina was reported (Mariluis et al., 1999).
Similarly to other Patagonian locations recently examined between 41-54° S and 62-72° W (Mariluis & Schnack, 1996; Schnack et al., 1998; Mariluis et al., 1999), three species prevailed over the remaining ones in the geographic range here examined; they were the synanthropic and non indigenous species C. vicina and L. sericata, and the asynanthropic and native C. fulvicrura. Among these species, L. sericata, a nearly worldwide and common species in temperate regions of the Holarctics, would be the most medically important blowfly. This endophilic and highly synanthropic species is a primary myiasis producer in part of Europe, and to a lesser extent in Sudan, South Africa, and Australia (Baumgartner & Greenberg, 1985). Furthermore, cases of human myiasis from L. sericata have been recorded in area hospitals of Chicago, Illinois, USA (Greenberg, 1984) and Buenos Aires, Argentina (Mariluis & Guarnera, 1983). In the sense of Nuorteva (1963), C. vicina, a cosmopolitan species, has been distinctly regarded either as asynanthropic (e.g. Mihályi, 1967; Schnack et al., 1995) or as eusynanthropic (Mariluis & Schnack, 1996). Like L. sericata, C. vicina was highly synanthropic in Río Grande and Puerto Santa Cruz. Its endophily and communicative habits in urban settlements, as well as its capacity to produce myiasis on man and animals, would make this species potentially important from the sanitary viewpoint in the study area. Currently, the sanitary importance of C. fulvicrura is poorly known. Its preference for the wilderness would allow us to regard this species as of lower sanitary importance.
Sixty seven and 140 specimens of P. terraenovae were captured at Río Grande and Río Gallegos, respectively. These findings are more than occasional and would suggest that this species, indigenous to the Northern Hemisphere (Evenhuis, 1989), is being established in the southernmost eastern continental sections of Argentina. Historically, its earliest detection out of its original distributional scope – in this case together with C. vicina – was from the Falkland Islands almost twenty years ago (Robinson, 1984). In late summer 1994 one single specimen was found at Husvik, South Georgia, around 1500 km east of the Falkland Islands (Hänel et al., 1998). Furthermore, adult specimens of P. terraenovae were collected in Chile, at Punta Arenas in January 1995 and at this locality and Puerto Natale in January 1996 (Mariluis, 1999). In the Arctics, P. terraenovae produces myiasis to wild animals like reindeers as well as to live stock, mainly cattle and sheep (Hall, 1948; Nuorteva, 1971). It has also been recovered from sores and wounds in people in the USA (Beesley, 1998). Its establishment in Argentine Patagonian localities adds a new sanitary threat, especially for sheep which are an important economic resource in Patagonia. Despite its documented occurrence at eusynanthropic habitats in the Palearctics (Gregor & Povolny, 1959) this species has also been found in urban and suburban sections in southeastern Patagonia.
One evidence that comes forth through analyzing the observed species' relative abundance is the prevalence of two non indigenous species, such as C. vicina and L. sericata over the native ones. The latter, except C. fulvicrura, are quite occasional. This is particularly true for urban and suburban sections expressing the changes caused by urbanization which allows the settlement of non indigenous and mostly synanthropic blowfly species.
ACKNOWLEDGMENTS
This work was supported by a grant from the Technical and Scientific National Research Council (CONICET), Argentina, (PIA Nro 7271). The authors appreciate valuable suggestions on this manuscript by Dr. Gustavo R. Spinelli. We are grateful to two anonymous reviewers for comments and criticism on the manuscript.
LITERATURE CITED
1. BAUMGARTNER, D. L. & B. GREENBERG. 1985. Distribution and medical ecology of the blowflies (Diptera: Calliphoridae) of Perú. Ann. Entomol. Soc. Am. 78: 565-587. [ Links ]
2. BEESLEY, W. N. 1998. The myiases. In: Palmer, S.R., Lord Soulsby & D.I.H. Simpson, D.I.H. (eds.). Zoonoses: biology, clinical practice, and public health control. Oxford University Press. Oxford, pp. 881 - 891. [ Links ]
3. CABRERA, A. 1976. Regiones Fitogeográficas Argentinas. ACME, Buenos Aires. [ Links ]
4. CABRERA, A. L. & A. WILLINK. 1980. Biogeografía de América Latina. Organización de los Estados Americanos, serie Biología. Monografía N° 13, Washington, D.C. [ Links ]
5. EVENHUIS, N. L. 1989. Catalogue of Oceanic and Australasian Diptera. Bishop Museum Press, Honolulu, and E.J. Brill, Leiden. [ Links ]
6. GREENBERG, B. 1984. Two cases of human myiasis caused by Phaenicia sericata (Diptera: Calliphoridae) in Chicago area hospitals. J. Med. Entomol. 21(5): 615. [ Links ]
7. GREGOR, F. & D. POVOLNY. 1959. Kritischer Beitrag zur Kenntniss der Tribus Phormiini (Diptera, Calliphoridae). Acta Soc. Entomol. Csl. 56: 26–50. [ Links ]
8. HALL, D. G. 1948. The blow flies of North America. Entomol. Soc. Amer. 4: 1-477. [ Links ]
9. HÄNEL, C., S. L. CHOWN & L. DAVIES. 1998. Records of alien insect species from sub-Antarctic Marion and South Georgia Islands. African Entomol. 6 (2): 366-369. [ Links ]
10. MARILUIS, J. C. 1999. Presencia de Protophormia terraenovae (Robineau-Desvoidy, 1830) en Chile, Sud América (Calliphoridae, Chrysomyinae, Phormiini). Bol. R. Soc. Esp. Hist. Nat. (Sec. Biol.) 95 (3-4): 75-77. [ Links ]
11. MARILUIS, J. C. & E. F. GUARNERA. 1983. Miasis producida por Phaenicia sericata (Meigen, 1826) (Calliphoridae, Luciliini). Rev. Soc. Entomol. Argent. 42 (1-4): 143-147. [ Links ]
12. MARILUIS, J. C. & J. A. SCHNACK. 1996. Elenco específico y aspectos ecológicos de Calliphoridae (Insecta, Diptera) de San Carlos de Bariloche, Argentina. Bol. R. Soc. Esp. Hist. Nat. (Sec. Biol.), 92(1-4): 203-213. [ Links ]
13. MARILUIS, J. C., J. A. SCHNACK, G. R. SPINELLI & J. MUZÓN. 1999. Calliphoridae (Diptera) de la Subregión Andino-Patagónica. Composición específica y abundancia relativa. Bol. R. Soc. Esp. Hist. Nat. (Sec. Biol.), 95 (3-4): 79-87. [ Links ]
14. MIHÁLYI, F. 1967. The danger index of the synanthropic flies. Acta Zool. Hung. 13: 373- 377. [ Links ]
15. MORRISON, R. I. G., P. CANEVARI & R. K. ROSS. 1989. Argentina. In: R.I.G. Morrison and R. K. Ross (Principal Authors). Atlas of Nearctic Shorebirds on the Coast of South America. Vol. 2, Chapter 11, Canadian Wildlife Service Special Publication, Ottawa, pp. 219-245. [ Links ]
16. NUORTEVA, P. 1963. Synanthropy of blow flies (Dipt. Calliphoridae) in Finland. Ann. Zool. Fenn. 8: 547-553. [ Links ]
17. NUORTEVA, P. 1971. Annoying mass occurrence of Phormia terraenovae R. D. (Diptera, Calliphoridae) in the surroundings of a rendering plant in southwestern Finland. Ann. Zool. Fenn. 8: 336–339. [ Links ]
18. ROBINSON, G. S. 1984. Insects of the Falkland Islands: A Checklist and Bibliography. British Museum (Natural History), London. [ Links ]
19. SCHNACK, J. A., J. C. MARILUIS, N. CENTENO & J. MUZÓN. 1995. Composición específica, ecología y sinantropía de Calliphoridae (Insecta: Diptera) en el Gran Buenos Aires. Rev. Soc. Entomol. Argent. 54 (1-4): 161-171. [ Links ]
20. SCHNACK, J. A., J. C. MARILUIS, G. SPINELLI & J. MUZÓN. 1998. Ecological Aspects of urban blowflies in mid-west Argentinean Patagonia (Diptera: Calliphoridae). Rev. Soc. Entomol. Argent. 57 (1-4): 127-130. [ Links ]
21. SORIANO, A. 1956. Los distritos florísticos de la Provincia Patagónica. Rev. Invest. Agric. Buenos Aires 10: 323-348. [ Links ]
Recibido: 3-XI-2003
Aceptado: 23-IV-2004














