Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista de la Sociedad Entomológica Argentina
versión impresa ISSN 0373-5680versión On-line ISSN 1851-7471
Rev. Soc. Entomol. Argent. v.66 n.3-4 Mendoza ago./dic. 2007
Composition and structure of aquatic insect assemblages of Yungas mountain cloud forest streams in NW Argentina
Composición y estructura de asociaciones de insectos acuáticos de arroyos de selva nublada de Yungas del NO Argentino
Von Ellenrieder, Natalia
Instituto de Bio y Geociencias, Museo de Ciencias Naturales de Salta, Universidad Nacional de Salta, Mendoza 2, 4400 Salta, Argentina; e-mail: natalia.ellenrieder@gmail.com
RESUMEN. Treinta y tres ambientes lóticos en las selvas nubladas de montaña de las Yungas del NO Argentino, fueron muestreados tanto en ambientes no modificados como alterados por actividades humanas. Insectos acuáticos de 143 taxones en 55 familias fueron colectados. El análisis de agrupamientos sugirió que la altura es una de las variables principales en la estructuración de las comunidades de insectos en estos arroyos, y la importancia de esta variable fue confirmada mediante un ordenamiento no-métrico multi-dimensional (NMS); los parámetros ambientales que mejor se correlacionaron con la ordenación fueron: altura, temperatura del agua, latitud y variables del canal (ancho, porcentaje de detritos leñosos grandes y pequeños, de bancos excavados, de piedras y grava gruesa). Procedimientos de permutación de respuestas múltiples (MRPP), mostraron que los arroyos en áreas bien conservadas difieren significativamente en su composición de los arroyos en áreas modificadas. La proporción de individuos de Elmidae y Plecoptera, y el número de taxones de Trichoptera, fueron los métricos biológicos mejor correlacionados con el gradiente de alteración ambiental local, sugiriendo que un índice 'ElPT' podría ser un componente útil para la evaluación del estado ecológico de estos ambientes. Los análisis de indicadores de especies, identificaron algunos indicadores potenciales de la condición de los arroyos y de los factores de alteración que los afectan.
PALABRAS CLAVE. Diversidad; Estructura de la comunidad; Especies indicadoras; Índices biológicos; Elmidae.
ABSTRACT. Thirty three lotic environments in the Yungas mountain cloud forest of NW Argentina were sampled both in undisturbed forest areas and sites altered by human activities. Aquatic insects of 143 taxa in 55 families were collected. Cluster analysis suggested altitude as one of the main structuring variables of aquatic insect communities in these streams, and its importance was confirmed by non-metric multidimensional scaling (NMS); the environmental parameters measured that were best correlated with the ordination were altitude, water temperature, latitude and channel variables (width, percentage of large and small woody debris, of undercut banks, cobble and coarse gravel). Multi response permutation procedures (MRPP) showed streams in well preserved areas to significantly differ in their composition from streams in disturbed areas. Proportion of Elmidae and of Plecoptera individuals and number of Trichoptera taxa were the biological metrics best correlated with the local disturbance gradient, suggesting that an 'ElPT' index could be a useful component in the evaluation of the ecological status of these environments. Indicator species analyses identified some potential indicators of stream condition and disturbance factors affecting these streams.
KEY WORDS. Diversity; Community structure; Indicator species; Biological metrics; Elmidae.
INTRODUCTION
The Yungas are cloud forests extending from the south of Venezuela into NW Argentina along the Andean mountain range. In Argentina they are distributed discontinuously within the provinces of Salta, Jujuy, Tucumán and Catamarca, along part of the Subandean chains, and they represent one of the most species-rich biogeographic provinces (Brown, 1995). Climate is warm and humid to sub-humid, and altitude ascends from 300 to 2400 m.a.s.l. Temperature and humidity vary in relation to altitude, latitude and aspect of slopes. Average yearly precipitation ranges from 900- 1000 mm, reaching 1300 mm in some areas. Rains are concentrated within 5 to 6 months in summer, and during colder months, fog partly compensates for lack of rainfall (Burkart et al., 1994). With increasing altitude, different altitudinal floors or vegetation levels are recognizable: warm and humid foothill jungle; temperate-warm and humid mountain jungle; and temperate and humid mountain forest with frequent winter frost.
The Yungas have become intensively fragmented during the last few decades due to ongoing agricultural practices (Reboratti, 1989). Human activities such as conversion of forest to agricultural or residential areas, logging, mining, road construction and recreation, modify reach and channel variables which are well known factors structuring aquatic insect assemblages (Allan, 2004). Sponseller et al. (2001) found that macroinvertebrate indices were more closely related to land-cover patterns evaluated at a local 200 m corridor scale, suggesting that streamside development effectively alters assemblage structure. In Patagonia, loss of riparian cover as a consequence of overgrazing and other land use practices was shown to have negative effects on aquatic environments (Miserendino, 2004). Several studies on benthic macroinvertebrates of lotic environments have been conducted in the Yungas of NW Argentina during the last years (Fernández & Fernández, 1995; Domínguez & Fernández, 1998; Romero & Fernández, 2001; Fernández et al., 2001; 2002; 2006; Fernández & Molineri, 2006), reflecting the rising concern for loss of biodiversity and alteration of landscape in the region. In order to evaluate the ecological status (Prat et al., 1999) of Yungas streams, studies integrating information from their riparian areas into the analysis are needed (Fernández et al., 2006).
Sites of present study are within the Bermejo basin in Salta and Jujuy provinces, where lotic environments (streams and small rivers) included in the three altitudinal floors of the Yungas were sampled, both in protected and non-protected areas. My goals were to document composition and structure of insect assemblages of Yungas streams in relation to natural gradients, evaluate the response of stream condition to a disturbance gradient at the local scale, and explore the potential for indicators of stream condition and disturbance factors. I dedicate this paper with affection to Dr. A.O. Bachmann, our father of aquatic entomology, who has always been available to provide advice on biological, systematic or nomenclatorial questions when answers from books were not forthcoming.
MATERIAL AND METHODS
Study area and sampling protocol
Thirty three lotic environments located in both protected and non-protected areas (Fig. 1) within four of the Yungas forest fragments in Salta and Jujuy provinces were sampled between August 2005 and May 2006. In each locality three samples each lasting 15 minutes were taken and later pooled for standardization, encompassing all microhabitats of different depths, substrate and current along three transects separated by 10 m across the stream. Aquatic insects were collected with aquatic nets of 20 cm diameter and mesh size of 300 μm, and manually by visually inspecting rocks, substrate and aquatic vegetation. Specimens are preserved in the collections of the Museo de Ciencias Naturales de Salta and Instituto y Fundación Miguel Lillo, Tucumán (Argentina). Biotic and abiotic data were recorded at each station (Appendix 1) including qualitative (visual estimates) and quantitative in-stream, channel and riparian characteristics, as well as evidence of habitat disturbance (presence of roads, garbage, cattle, crops, logging, channelization) at the local reach scale, extending 100 m in width on each bank and 300 m in length upstream. Stations were classified (Table I) according to altitudinal level as follows: 1. foothill rainforest (ca. 300-900 m), 2. mountain rainforest (ca. 900-1500 m) and 3. mountain forest (ca. 1500-2400 m), as well as according to the disturbance level observed at the local reach scale (riparian area and channel) as follows: 1. well preserved: vegetative cover of tall trees (about 25 m high), thick riparian vegetation with no evidence of habitat alteration, and disturbed, in three categories: 2. preserved: trees 15-20 m high, bushes comprising large part of the cover, as indirect evidence of habitat alteration; 3. modified: vegetative cover with bushes making up for most of it, direct evidence of habitat alteration; 4. highly modified: vegetative cover scarce and riparian forest deteriorated, direct evidence of habitat alteration.

Fig. 1. Study area. Hatched: Yungas forest; Grey polygons: Protected areas; 1: National Park Baritú; 2: Private Reserve El Pantanoso; 3: National Park Calilegua; 4: National Park El Rey; Contour: Biosphere Yungas Protected area. Coded squares: Localities: Yu1: stream 1 affluent to Baritú River; Yu2: Baritú River; Yu3: stream 2 affluent to Baritú River; Yu4: stream 1 crossing road Baritú-Lipeo; Yu5: stream 2 crossing road Baritú-Lipeo; Yu6: stream affluent to Cayotal River; Yu7: Cayotal River at Las Juntas; Yu8: stream affluent to Lipeo River; Yu9: stream 1 crossing road Lipeo-Los Toldos; Yu10: stream 2 crossing road Lipeo-Los Toldos; Yu11: stream 3 crossing road Lipeo-Los Toldos; Yu12: effluent river from Dique Itiyuro; Yu13: Pantanoso River; Yu14: Zora River; Yu15: San Francisco River; Yu16: stream at Route 6 to Palma Sola; Yu17: Tres Cruces Stream; Yu18: stream in Lesser; Yu19: La Sala Stream 1; Yu20: La Sala Stream 2; Yu21: stream on trail to Pozo Verde; Yu22: stream in La Angostura; Yu23: stream in Quebrada de San Lorenzo; Yu24: Vaqueros River; Yu25: stream in Las Costas; Yu26: Las Nieves Stream; Yu27: Yuto River; Yu28: stream in Quebrada de Tilián; Yu29: Zanjón Seco Stream; Yu30: stream 20 km SE to Isla de Cañas; Yu31: stream 15 km SE to Isla de Cañas; Yu32: stream 5 km SE to Isla de Cañas; Yu33: Anta Muerta Stream.

Table I. Locality classification and summary statistics. BMWP (Domínguez & Fernández, 1998: >40= non-polluted (water quality class 1); 30-40= slightly polluted (water quality class 2); 20-30: polluted (water quality class 3). See text for explanation of other codes.
Data analysis
Characterization of the community
Taxonomic identifications were made to genus and species level when possible. In cases of doubtful identification, taxa were either combined (i.e. Massartellopsis/ Meridialaris in Leptophlebiidae and Banyallarga/Phylloicus in Calamoceratidae) or identified to family (i.e. Chironomidae in Diptera). Information was arranged in a matrix of 143 taxa by 33 sampling stations (Appendix 2) based on which biological metrics of number of individuals and of taxa (at taxonomic level from Appendix 2) per order, and proportions of both to the total were calculated for each station.
Three diversity indices were estimated based on Whittaker (1972): alpha diversity, calculated as the average specific richness per locality; beta diversity, a measurement of the heterogeneity of the data, calculated as the ratio between total number of species and average number of species; and gamma diversity, or diversity at landscape level, calculated as total number of species across all localities. Specific richness, evenness and diversity (Shannon and Simpson indices) were also calculated for each site.
Natural factors affecting the community
In order to avoid the masking effect that pollution could have on the analyses of natural factors affecting community composition, potentially polluted sites were excluded from these analyses. Polluted sites were identified based on the BMWP biotic index adapted for the region by Domínguez & Fernández (1998). Since this index is based on presence/ absence of families of benthic macroinvertebrates, non-benthic insects were excluded from the calculation, which included only benthic insects and Crustacea (no Oligochaeta, Mollusca or Hydracarina were collected). Although BMWP values obtained might be underestimated (for exclusion of some non insect taxa) and not comparable to values of other studies, they do allow the identification of non- polluted sites.
Composition of aquatic insect communities was analyzed using multivariate cluster and ordination techniques with the program PC-ORD (McCune & Grace, 2002). Both multivariate analyses were based on abundance data (values transformed as log (x+1) to homogenize the variance, reduce the effect of total quantity emphasizing relative quantities, and equalize relative importance of common and rare species) from the 30 non- polluted sites (water quality class 1, Table I).
For the cluster analysis, Sorensen (Bray- Curtis) distance coefficient was used, with flexible beta as linkage method at a value of β = -0.25. The resulting dendrogram was based on Wishart's objective function converted to a percentage of remaining information. A value of 25% of remaining information was selected to prune the dendrogram.
Because of its ability to extract information from nonlinear relationships, non-metric multidimensional scaling (NMS; Mather, 1976) was chosen as ordination method. Its use and interpretation differ from other ordination methods in that: 1. for a given number of dimensions the solution for a particular axis is unique; 2. the appropriate number of dimensions (axes) is determined graphing or tabulating final stress versus number of dimensions, choosing a number of axes after which reduction in stress is small; and 3. axis numbers are arbitrary, so that the percentage of variance on a given axis does not necessarily form a descending series with increasing axis number. Sorensen was chosen as distance coefficient. Forty runs were carried out with real data and 50 with random data (Monte Carlo test) starting from a random configuration, and with a possible maximum of 6 axes and 400 iterations. Final instability was calculated as standard deviation in stress over the preceding 15 iterations (value of < 10-4 indicates a stable solution; McCune & Grace, 2002). Proportion of variance represented by ordination axes was calculated by the correlation (determination coefficient r2) between Euclidean distances in the ordination space and distances in the original space. Distances in the original space were calculated with the same distance measure used in the NMS analysis. Since pH data were not available for all localities, the NMS analysis was run twice, once including all localities and the second time excluding localities lacking pH data.
Quantitative environmental variables (transformed as log (x+1) to make the units of attributes measured in different scales comparable) listed in Appendix 1 were correlated with NMS ordination axes and overlaid onto the ordination diagrams as joint plots (diagrams of radiating lines whose angle and length indicate direction and strength of relationships between variables and ordination axis) where only variables from the environmental matrix with an r2 > 0.20 were represented. Groups obtained with the cluster analysis were also overlaid onto the NMS ordination to aid in the interpretation of their relationships.
An indicator species analysis based on the method described by Dufrêne & Legendre (1997) was conducted to identify potential indicators for the three altitudinal floors of the Yungas. This method combines information about species abundance and frequency of occurrence in each group. A perfect indicator for a particular group (indicator value of 100) must be faithful (always present) and exclusive to the group (never occurring in other groups). Statistic significance of the indicator values was established with a Monte Carlo test (with 1000 permutations).
Analysis of disturbance
Spearman rank correlations between disturbance level gradient (from well preserved, class 1, to highly modified, class 4) and natural environmental variables were performed in order to determine if they covary (values transformed as log (x+1)).
Sites in well preserved areas according to the local disturbance level (11 localities, disturbance level 1 in Table I) were compared to sites in disturbed areas (22 localities, disturbance levels 2-4 in Table I), using the following methods:
- A MRPP (multiple response permutation procedure) test. This method provides a multivariate non-parametric test of differences among two or more groups based on the analysis of a distance matrix. Sorensen was used as distance coefficient. Delta (average weighted distance within a group; lower delta value indicates better cohesion within a group) was calculated according to the procedure detailed by Mielke & Berry (2001). The statistic of this method, T= observed - expected delta/ √ variance of delta, describes separation between groups (the more negative the value of T, the larger the separation between groups), A = describes homogeneity within each group compared to one due to chance, and p= the probability of obtaining a delta as high as or higher than observed given the distribution of possible deltas (probability that observed difference is due to chance).
- Comparison of richness, diversity indexes and biological metrics through Student t tests, illustrated with side by side boxplots.
- Spearman Rank correlations between the disturbance level gradient and biological indexes and metrics (values transformed as log (x+1)).
- Indicator species analyses based on Dufrêne & Legendre's (1997) method (described in previous section) to identify potential indicators for sites in well preserved and disturbed areas, and for groups of sites defined by presence or absence of different factors of habitat disturbance (cattle, crops, logging, etc., listed in Appendix 1).
RESULTS
Characterization of the community
A total of 143 aquatic insect taxa was found (Appendix 2). Alpha diversity was 20.2, beta diversity 7.07, and gamma diversity 143. Species richness varied from 7 to 41 across localities, and localities with the highest richness values corresponded to well preserved environments within protected areas (Yu19, Yu18 and Yu20 in National Park El Rey, and Yu13 in El Pantanoso Reserve). Localities with the highest diversity values were Yu19 and Yu21 (National Park El Rey), Yu 13 (El Pantanoso Reserve), Yu3 and Yu8 (National Park Baritú), and Yu31 (Arroyo 15 Km E to Isla de Cañas, not protected) (Table I).
Natural factors affecting the community
Thirty localities were classified as 'nonpolluted' according to the BMWP index approximation (water quality class 1, Table I) and were included in the analyses evaluating influence of natural factors.
Hierarchical cluster analysis identified five groups with 25 % of remaining information among members for each group. The cluster including groups 1, 2 and 3 joined all localities encompassed within the mountain forest altitudinal floor, while the cluster including groups 4-5 encompassed those within the foothill rainforest altitudinal floor (Fig. 2), suggesting that altitudinal gradient is an important factor in shaping aquatic insect assemblages in the Yungas.

Fig. 2. Cluster analysis dendrogram based on similarity of aquatic insect composition among sampling stations. Numbers indicate groups formed when pruning the dendrogram at a level of 25 % of remaining information (vertical line). Altitudinal floor is superimposed to show correlation with stations.
A three dimensional NMS final solution was reached after 122 iterations, with a final stress of 15.07, final instability of 10-5, and a proportion of randomized runs with stress lower than or equal to observed stress of 0.0196. Variance represented by the three ordination axes was of 30.7, 18.3 and 30.5 (cumulative variance of 79.6) respectively.
Correlation of environmental variables with ordination axes (Table II) shows that the most important environmental variables explaining community composition include channel variables of percentage of coarse gravel in the substrate, and large woody debris and undercut banks in the channel along ordination axis 1 (Figs. 3.1, 3.2), while altitude and wet channel width, percentage of cobble in the substrate and of small woody debris in the channel significantly contributed to axis 2 (Figs. 3.1, 3.3), and altitude, latitude, and water temperature to axis 3 (Figs. 3.2- 3.3). Biological groups obtained from cluster analysis overlaid onto ordination diagrams can be characterized according to the same variables: groups 4 and 5 including the totality of foothill rainforest localities, of lower elevation, higher water temperature and wider channel (Figs. 3.1-3.2), are separated by percentages of undercut banks, large woody debris in the channel and coarse gravel in the substrate (higher for group 4; Fig. 3.2). Groups 1 to 3, including all mountain forest localities of higher elevation with lower water temperature and narrower channels (Figs. 3.1-3.2), are ordered on the basis of latitude and percentage of cobble and large woody debris (Figs. 3.1-3.3). Disturbance level was not significantly correlated with ordination axes, and neither was pH in the second NMS analysis (run excluding 8 localities lacking pH data and taxa present only in them).
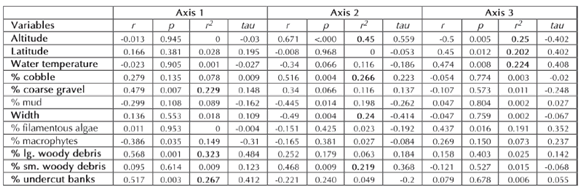
Table II. Correlations of environmental variables with NMS ordination Axes. Variables with an r2 larger than 0.20 shown in joint plots of Fig. 3 are highlighted in bold. r: Pearson Product-Moment coefficient; tau: Kendall's tau rank coefficient.

Fig. 3. Joint plots showing relationships between environmental variables and axes of NMS ordination of localities. 3 D solution reached after 122 iterations, with a final stress of 15.07 and final instability of 10-5. 3.1: Axes 1 and 2; 3.2: Axes 1 and 3; 3.3: Axes 2 and 3.
Indicator analysis for altitudinal floors yielded ten taxa with a significant indicator value (Table III), of which the best are Americabaetis (Ephemeroptera, Baetidae) for foothill rainforest streams, and Meridiallaris/ Massartellopsis (Ephemeroptera, Leptophlebiidae) for mountain forest streams. In accordance with the results shown by the ordination analyses, no indicator taxa were found for the intermediate altitudinal level (mountain rainforest).

Table III. Indicator taxa for altitudinal floors obtained by combining abundance and frequency data according to Dufrêne & Legendre's (1997) method. Only taxa with a statistically significant indicator value (larger than expected by chance, p < 0.05) are shown. Indicator values range from 0 to 100 (perfect indicator).
Analysis of disturbance
Disturbance gradient was not significantly correlated with any of the natural environmental variables measured.
Comparison of sites in well preserved areas (11 localities; mean inner distance of 0.799) with sites in disturbed areas (22 localities; mean inner distance of 0.852) by means of the MRPP test showed the difference in their aquatic insect assemblage composition to be significant (observed delta: 0.835; expected delta: 0.843; variance of delta: 0.17-4; T: -2.088; p: 0.035; A: 0.011).
Richness, diversity and several biological metrics (i.e. Plecoptera individuals, proportion of Elmidae individuals, Trichoptera taxa, proportion of Ephemeroptera individuals and ElPT taxa, Fig. 4) were found to be significantly different among sites in well preserved and in disturbed areas, not so EPT taxa (Fig. 4).

Fig. 4. Side by side box plots of Shannon diversity and some biotic metrics comparing median, quartiles (25th and 75th percentiles) and 95% confidence intervals of sites in well preserved (WP, N=11) and disturbed (Di, N=22) areas. p values obtained with Student t tests.
Both Shannon and Simpson diversity indexes were significantly correlated with the disturbance gradient. Ephemeroptera metrics were either directly correlated with it (number of individuals and proportions of individuals and of taxa), increasing with higher disturbance levels, or not correlated (number of taxa), and the traditional EPT taxa index was accordingly not sensitive to this gradient (Table IV). Correlations of metrics calculated for Plecoptera, Elmidae, Trichoptera, and combination of these three taxa (ElPT) were highly significant. Among these, the best correlated with the disturbance gradient were proportion and number of Plecoptera individuals (r -0.68 and -0.63), and ElPT taxa (r -0.635), all of them beyond the 0.001 significance level (Table IV).

Table IV. Spearman rank correlations of biotic metrics with classes of disturbance level. Only metrics with a statistically significant value (p < 0.05) are shown, and the highest Spearman coefficients are highlighted in bold.
Twelve taxa with a significant indicator value for disturbance level were identified (Table V), with Anacroneuria (Plecoptera, Perlidae) as the best indicator for sites in well preserved areas. Both positive and negative indicators significantly correlated with individual disturbance factors were found (Table VI).

Table V. Indicator taxa for disturbance level (WP: well preserved, N=11; Di: disturbed, N=22) obtained by combining abundance and frequency data according to Dufrêne & Legendre's (1997) method. Only taxa with a statistically significant indicator value (larger than expected by chance, p < 0.05) are shown. Indicator values range from 0 to 100 (perfect indicator).

Table VI. Indicator taxa for factors of habitat disturbance (A: absence of, P: presence of) obtained by combining abundance and frequency data according to Dufrêne & Legendre's (1997) method. Only taxa with a statistically significant value (larger than expected by chance, p < 0.05) are shown. Indicator values range from 0 to 100 (perfect indicator).
DISCUSSION
Of the 55 families of aquatic insects found, 51 correspond to benthic insects, and the number of families per site is similar to that found in other studies of benthic macroinvertebrates in Andean mountain streams (Salinas et al., 1999 in Bolivia, Fernández, et al., 2002 in Tucumán, and Miserendino, 1995 in Patagonia). Diversity values are also comparable to those obtained by Fernández et al., 2001 in a river in the Yungas of NW Argentina. The highest richness and diversity values observed in protected areas emphasize the importance of habitat conservation, and show that these areas are fulfilling their role in preserving the aquatic insect biodiversity of the Yungas.
Altitude was the strongest variable shaping studied communities of aquatic insects, but in accordance to the results of Maldonado & Goitía (2003) for benthic macroinvertebrates in Yungas streams of Bolivia, communities were not perfectly correlated with the three altitudinal floors; those from the intermediate altitude level (mountain rainforest) were not separated as a distinct group but were interspersed between low and high altitude level assemblages. The significant correlation of channel width and substrate size with community composition observed here was found also in studies of benthic macroinvertebrates of lotic Yungas environments (Fernández et al., 2001; Moya et al., 2003; Oller & Goitía, 2005), and of Patagonian rivers (Miserendino & Pizzolón, 2000; 2003).
An understanding of relationships between anthropogenic land use and the ecological status of streams can be complicated by covariation between anthropogenic and natural gradients (Allan, 2004). In this study no covariation between both gradients was found, and disturbance level was not significantly correlated with any ordination axis, thus indicating that natural variables are the primary factors influencing composition of aquatic insect communities of Yungas streams.
In a study of four sites at the Lules River in the Yungas of Tucumán province with values of physical and chemical parameters and of biotic indexes (BMWP, ASPT, EPT) within the ranges of non-polluted water, Fernández et al. (2002) found that neither BMWP nor EPT indexes reflected the riparian disturbance observed in two of these sites. Approximations to BMWP and EPT in the present study did not correlate with riparian disturbance either, but metrics comprising Elmidae, Trichoptera and Plecoptera presented highly significant correlations with it (Table IV). Although Coleoptera and Elmidae are not always included in biological indexes (Lenat, 1993; Prat et al., 1999; Klemm et al., 2003), their potential as bioindicators and as part of biological metrics has been studied in Europe (Richoux & Forestier, 1989; García-Criado & Fernández-Alaez, 1995; 2001) and the USA (Ode et al., 2005). Fossati et al. (2001) found that Elmidae were among the most affected insects by sediment releases due to road construction in eight sites along an Andean river in Bolivia, and this study suggests that they would be a useful component in an index sensitive to the disturbance level at the local scale for Yungas streams.
EPT metrics have been found to be negatively correlated with disturbance in several studies (i.e. Sponseller et al., 2001; Klemm et al., 1990; 2003; Miserendino, 2004; Morais et al., 2004). The positive correlation of Ephemeroptera metrics with the disturbance gradient found here accounts for the lack of correlation of EPT with that gradient. A number of environmental stressors can have positive influence at low to moderate concentrations; riparian thinning and low intensity agriculture increase light, nutrients, and water temperatures, leading to an increase of periphyton biomass (Allan, 2004), which can benefit some species in spite of their negative effects (i.e. decrease of bank stability, inputs of litter and wood, and retention of nutrients, and increase of bank and channel erosion and input of pesticides; Gregory et al., 1991). Some Ephemeroptera taxa benefited from disturbance, and this explains the positive correlation of Ephemeroptera metrics with the disturbance gradient; Baetodes (Ephemeroptera, Baetidae) was found with significantly higher abundance and frequency in disturbed sites (Table V), and was significantly associated to the presence of particular disturbance factors, as was Thraulodes (Ephemeroptera, Leptophlebiidae) (Table VI).
The high values found for Progomphus (Odonata, Gomphidae) and Thraulodes (Ephemeroptera, Leptophlebiidae) as indicators of channelization could be accounted for by changes in the representation of their preferred habitats resulting from both direct and indirect effects of channelization. Many channel or stream bank stabilization structures provide increased instream habitat for certain aquatic species; Sandheinrich & Atchison (1986) reported increases in densities of epibenthic insects within revetments and stone dike areas and more suitable substrate for bottomdwelling insects in revetment areas, which could explain the high value obtained for Thraulodes. One of the secondary instream habitat alteration effects of channelization is the trapping of large quantities of sediment (EPA, 2006), which could favor an increase in populations of Progomphus larvae (burrowers in sediments). In spite of the high indicator and significance values that the analysis shielded for these two taxa, further study is required to verify their validity because they were based on only two sites showing evidence of channelization.
Results suggest that composite measures, such as a multimetric index including richness, diversity and biological metrics such as an ElPT (Elmidae, Plecoptera, Trichoptera) index, could be useful in detecting overall disturbance at a local scale in Yungas streams. Further studies are needed to develop and test such an index. In order to carry out sound management and restoration actions effectively, it is necessary to diagnose causes as well as to assess disturbance; because of the aggregated nature of multimetric indexes, they might be less easily interpreted than the behavior of individual variables (Watzin & McIntosh, 1999). Although indicator values for individual disturbance factors were in general low in this study, these taxa are worth investigating in future studies analyzing their tolerance values to specific stressors resulting from disturbance factors (Black et al., 2004; Yuan & Norton, 2004), thus providing the diagnostic tools necessary to identify and monitor disturbance factors acting upon Yungas streams.
Appendix 1. Environmental variables per locality. Depth: 1= >30 cm; 2= 30-60 cm; 3= <60 cm; width: 1= >2 m; 2= 2-5 m; 3= 5-10 m; 4= <10 m. Disturbance factors: 0= absent; 1=present
Appendix 2. Matrix of taxa collected per locality. For locality codes see Fig. 1.
ACKNOWLEDGEMENTS
I thank the authorities of APN, of the Secretary of the Environment and Sustainable Development of Salta and Jujuy provinces, and of the private Reserve El Pantanoso for permission to conduct this study within areas under their jurisdiction; the personnel of the National Parks Baritú, Calilegua and El Rey for their assistance when visiting the parks; C. Molineri and V. Manzo for their help with the identifications of Ephemeroptera and Coleoptera Elmidae respectively; A.C. Rehn for helpful suggestions on an earlier version of the manuscript; R.W. Garrison, J. Muzón and C. Molineri for critically reading the manuscript; L. Miserendino and two anonymous reviewers for their valuable suggestions; and G. von Ellenrieder for his indefatigable company and support in the field. This study was financed by CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina).
LITERATURE CITED
1. ALLAN, J. D. 2004. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evol. Syst. 35: 257-284.
2. BLACK, R. W., M. D. MUNN & R. W. PLOTNIKOFF. 2004. Using macroinvertebrates to identify biota-land cover optima at multiple scales in the Pacific Northwest, USA. J. N. Am. Benthol. Soc. 23: 340-360.
3. BROWN, A. D. 1995. Las selvas de montaña del noroeste de Argentina: problemas ambientales de importancia en su conservación. In: Brown, A.D. & H.R. Grau (eds.), Investigación, Conservación y Desarrollo en Selvas Subtropicales de Montaña, Proyecto de Desarrollo Agroforestal/ L.I.E.Y, pp. 9-18.
4. BURKART, R., L. DEL VALLE RUIZ, C. DANIELE, C. NATENZON, F. ARDURA & A. BALABUSIC. 1994. El Sistema Nacional de Áreas Protegidas. Diagnostico de su patrimonio natural y su desarrollo institucional. APN, Buenos Aires, 132 pp.
5. DOMÍNGUEZ, E. & H. R. FERNÁNDEZ. 1998. Calidad de los ríos de la cuenca del Salí (Tucumán, Argentina) medida por un índice biótico. Serie Conservación de la Naturaleza 12. Fundación Miguel Lillo, Tucumán, 40 pp.
6. DUFRÊNE, M. & P. LEGENDRE. 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Mon. 67: 345-366.
7. EPA, 2006. U.S. Environmental Protection Agency: National Management Measures to Control Nonpoint Source Pollution Hydromodification. Draft 841-D-06-001, 324 pp.
8. FERNÁNDEZ, H. R. & L. A. FERNÁNDEZ. 1995. La biodiversidad del zoobentos en ríos de montaña de Tucumán, la trucha como amenaza. In: Brown, A.D. & H. R. Grau (Eds.), Investigación, Conservación y Desarrollo en Selvas Subtropicales de Montaña. Proyecto de Desarrollo Agroforestal/ L.I.E.Y, pp 149-156.
9. FERNÁNDEZ, H. R., E. DOMÍNGUEZ, F. ROMERO & M. G. CUEZZO. 2006. La calidad del agua y la bioindicación en los ríos de montaña del Noroeste Argentino. Serie Conservación de la Naturaleza 16. Fundación Miguel Lillo, 36 pp.
10. FERNÁNDEZ, H. R. & C. MOLINERI. 2006. Toward a Sustainable Experience in an Intermountain Valley in Northwestern Argentina. Ambio 35(6): 262-266.
11. FERNÁNDEZ, H. R., F. ROMERO, M. PERALTA & L. GROSSO. 2001. La diversidad del zoobentos en ríos de montaña del noroeste de Argentina: comparación entre seis ríos. Ecol. Austral. 11: 9-16.
12. FERNÁNDEZ, H. R., F. ROMERO, M. B. VECE, V. MANZO, C. NIETO & M. ORCE. 2002. Evaluación de tres índices bióticos en un río subtropical de montaña (Tucumán - Argentina). Limnetica 21: 1-13.
13. FOSSATI, O., J. G. WASSON, C. HERY, G. SALINAS & R. MARIN. 2001. Impact of sediment releases on water chemistry and macroinvertebrate communities in clear water Andean streams (Bolivia). Arch. Hydrobiol. 151(1): 33-50.
14. GARCÍA-CRIADO, F. & M. FERNÁNDEZ-ALAEZ. 1995. Aquatic Coleoptera (Hydraenidae and Elmidae) as indicators of the chemical characteristics of water in the Orbigo River basin (N-W Spain). Ann. Limnol. 31(3): 185-199.
15. GARCÍA-CRIADO, F. & M. FERNÁNDEZ-ALAEZ. 2001. Hydraenidae and Elmidae assemblages (Coleoptera) from a Spanish river basin: good indicators of coal mining pollution? Arch. Hydrobiol. 150(4): 641-660.
16. GREGORY, S. V., F. J. SWANSON, W. A. McKEE & K. W. KUMMINS. 1991. An ecosystem perspective of riparian zones: focus on links between land and water. BioScience 41: 540- 551.
17. KLEMM, D. J., P. A. LEWIS, F. A. FULK & J. M. LAZORACK. 1990. Macroinvertebrate field and laboratory methods for evaluating the biological integrity of surface waters. U.S. Environmental Protection Agency, Cincinnati. EPA. 600/4-90/ 030.
18. KLEMM, D. J., K. A. BLOCKSOM, F. A. FULK, A. T. HERLIHY, R. M. HUGHES, P. R. KAUFMANN, D. V. PECK, J. L. STODDARD, W. T. THOENY, M. B. GRIFFITH & W. S. DAVIS. 2003. Development and evaluation of a macroinvertebrate biotic index (MBII) for regionally assessing Mid-Atlantic highlands streams. Env. Manag. 31(5): 656-669.
19. LENAT, D. R. 1993. A biotic index for the southeastern United States: derivation and list of tolerance values, with criteria for assigning water-quality ratings. J. N. Am. Benthol. Soc. 12: 279-290.
20. MALDONADO, M. & E. GOITÍA. 2003. Las hidroecoregiones del departamento Cochabamba. Rev. Bol. Ecol. 13: 116-141.
21. MATHER, P. M. 1976. Computacional methods of multivariate analysis in physical geography. J. Wiley & Sons, London, 530 pp.
22. MCCUNE, B. & J. B. GRACE. 2002. Analysis of ecological communities. MJM Software Design, Gleneden Beach, Oregon, 300 pp.
23. MIELKE, P. W., Jr. & K. J. BERRY. 2001. Permutation methods: a distance function approach. Springer Series in Statistics. Springer Verlag, Dordrecht, vii + 352 pp.
24. MISERENDINO, M. L. 2004. Effects of landscape and desertification on the macroinvertebrate assemblages of rivers in Andean Patagonia. Arch. Hydrobiol. 159 (2): 185-209.
25. MISERENDINO, M. L. & L. A. PIZZOLÓN. 2000. Macroinvertebrates of a fluvial system in Patagonia: altitudinal zonation and functional structure. Arch. Hydrobiol. 150: 55-83.
26. MISERENDINO, M. L. & L. A. PIZZOLÓN. 2003. Distribution of macroinvertebrate assemblages in the Azul-Quemquemtreu river basin, Patagonia, Argentina. N. Z. J. Mar. Fresh. 37: 525-539.
27. MORAIS, M., P. PINTO, P. GUILHERME, J. ROSADO & I. ANTUNES. 2004. Assessment of temporary streams: the robustness of metric and multimetric indices under different hydrological conditions. Hydrobiol. 516: 229-249.
28. MOYA, N., E. GOITÍA & M. SILES. 2003. Tipología de ríos de la región del piedemonte Andino en Cochabamba. Rev. Bol. Ecol. 13: 95-115.
29. ODE, P. R., A. C. REHN & J. T. MAY. 2005. A Quantitative Tool for Assessing the Integrity of Southern Coastal California Streams. Env. Manag. 35(4): 493-504.
30. OLLER, C. & E. GOITÍA. 2005. Macroinvertebrados bentónicos y metales pesados en el río Pilcomayo, Tarija, Bolivia. Rev. Bol. Ecol. 18: 17-31.
31. PRAT, N., A. MUNNÉ, C. SOLA, N. BONADA & M. RIERADEVALL. 1999. Perspectivas en la utilización de los insectos acuáticos como bioindicadores del estado ecológico de los ríos. Aplicación a ríos mediterráneos. Rev. Soc. Entomol. Argent. 58: 181-192.
32. REBORATTI, C. 1989. La Frontera Agropecuaria en el Umbral al Chaco. Desarrollo, balance y perspectivas. Instituto de Geografía, Facultad de Filosofía y Letras, Universidad de Buenos Aires.
33. RICHOUX, P. & M. C. FORESTIER. 1989. Esolus parallelepipedus Mueller (Coleoptera, Elmidae): Indicator and functional describer of interstitial habitat linked to the river system. Elytron 3: 149- 155.
34. ROMERO, F. & H. R. FERNÁNDEZ. 2001. Abundance and diversity of a mayfly taxocene in a South American subtropical mountain stream. In: Domínguez, E. (ed.), Trends in Research in Ephemeroptera and Plecoptera, Kluwer Academic/Plenum Publishers, New York, 489 pp.
35. SALINAS, G., R. MARÍN, C. HENRY, O. FORSATI & J. G. WASSON. 1999. Efecto de la materia en suspensión sobre los invertebrados bénticos de los ríos de aguas claras en las Yungas de Bolivia. Rev. Bol. Ecol. 6:183-186.
36. SANDHEINRICH, M. B. & G. J. ATCHISON. 1986. Environmental effects of dikes and revetments on large riverine systems. National Technical Information Service, Springfield VA. 22161, Technical Report E-86-5, AD-A171000, 51 pp.
37. SPONSELLER, R. A., E. F. BENFIELD & H. M. VALLET. 2001. Relationships between land use, spatial scale and stream macroinvertebrate communities. Freshw. Biol. 46: 1409-1424.
38. WATZIN, M. C. & A. W. McINTOSH. 1999. Aquatic ecosystems in agricultural landscapes: a review of ecological indicators and achievable ecological outcomes. J. Soil Water Cons. 54: 636-644.
39. WHITTAKER, R. H. 1972. Evolution and measurement of species diversity. Taxon 21: 213- 251.
40. YUAN, L. L. & S. B. NORTON. 2004. Assessing the relative severity of stressors at a watershed scale. Env. Monit. Assess. 98: 323-349. [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
Recibido: 30-04-2007;
aceptado: 29-06-2007














