Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista de la Sociedad Entomológica Argentina
versión impresa ISSN 0373-5680versión On-line ISSN 1851-7471
Rev. Soc. Entomol. Argent. v.66 n.3-4 Mendoza ago./dic. 2007
New contributions to the study of Corixoidea: cytogenetic characterization of three species of Sigara from Argentina and the plausible mechanisms of karyotype evolution within Nepomorpha
Nuevas contribuciones al estudio de Corixoidea: caracterización citogenética de tres especies de Sigara de Argentina y los posibles mecanismos de evolución del cariotipo en Nepomorpha
Bressa, María José and Alba Graciela Papeschi
Laboratorio de Citogenética y Evolución, Departamento de Ecología, Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Ciudad Universitaria. Pabellón 2, C1428EGA, Ciudad Autónoma de Buenos Aires, Argentina; e-mail: alpape@ege.fcen.uba.ar
RESUMEN. Los estudios citogenéticos en Heteroptera contribuyen al análisis de las tendencias evolutivas en el taxón. Los Heteroptera se caracterizan por poseer cromosomas holocinéticos, diferentes sistemas de cromosomas sexuales y un par de cromosomas m en algunas especies. En este trabajo describimos el cariotipo y la meiosis masculina de Sigara denseconscripta (Breddin), S. chrostowskii Jaczewski y S. rubyae (Hungerford). Las tres especies tienen un número diploide de 24, con un par de cromosomas m y un sistema de cromosomas sexuales XY/XX. Con estos resultados son 30 las especies de Corixoidea estudiadas citogenéticamente y el cariotipo modal de la superfamilia es 2n= 20+2m+XY en machos. La información citogenética disponible hasta el presente en Heteroptera nos permite sugerir que la presencia de cromosomas m y cromosomas sexuales XY/XX, serían caracteres plesiomórficos para Nepomorpha. La ausencia de cromosomas m en especies de Nepoidea y Ochteroidea, y los sistemas de cromosomas sexuales X0 y Xn0 (en machos) en especies de Corixoidea, Naucoroidea y Nepoidea, serían caracteres derivados que habrían surgido evolutivamente más tarde.
PALABRAS CLAVE. Citogenética; Heteroptera; Cromosomas holocinéticos; Meiosis; Cromosomas m.
ABSTRACT. Cytogenetic studies in Heteroptera contribute to the analysis of evolutionary trends within the group. Heteroptera are characterized by the possession of holokinetic chromosomes, different sex chromosome mechanisms and a pair of m chromosomes in some species. In the present work, the male karyotype and meiosis in Sigara denseconscripta (Breddin), S. chrostowskii Jaczewski, and S. rubyae (Hungerford) are described. The three species share a diploid chromosome number of 2n= 24 with a pair of m chromosomes and an XY/XX sex chromosome system. With this study the chromosome number of 30 species of Corixoidea are known and the modal karyotype is 2n= 20+2m+XY in males. The available cytogenetic information in Heteroptera led us to suggest that the presence of a pair of m chromosomes and an XY/XX sex chromosome system could be considered as plesiomorphic for Nepomorpha. The absence of m chromosomes in species of Ochteroidea and Nepoidea, and the sex chromosome systems X0 and Xn0 (male) in species of Corixoidea, Naucoroidea, and Nepoidea should be considered as derived characters, which arose later in evolution.
KEY WORDS. Cytogenetics; Heteroptera; Holokinetic chromosomes; Meiosis; m chromosomes.
INTRODUCTION
Corixoidea is the largest nepomorphan group and their members are commonly called water boatmen. They occur worldwide in various types of stable and temporary, continental and insular, fresh and saline waters (Bachmann, 1981; Schaefer & Panizzi, 2000). Corixids have a high dispersal potential, which allows them to utilize various available habitats (Jannson, 1986). Most of them fly very well, and they are the typical insect invaders of water bodies, including newly developed ones (Bachmann, 1981; Schaefer & Panizzi, 2000). Corixids represent one of the most important predators; they can feed on algal cells, filamentous blue-greens, diatoms, microscopic protozoans and rotifers, small invertebrates, fish eggs and detritus (Bachmann, 1981; Schaefer & Panizzi, 2000). Various corixid species are incidental or obligatory predators on mosquito larvae in various regions of the world (Reynolds, 1975; Reynolds & Scudder, 1987a, b) and may be important control agents of mosquitoes. They can even be of significant importance for the development of some young stages of fish, as food in small water bodies (Rask, 1983). However, Corixids can be pests of fish culture; some species are facultative predators of fish eggs and larvae (Schaefer & Panizzi, 2000).
The superfamily Corixoidea comprises about 400 species, which are included in Corixidae and Micronectidae. The former contains the majority of known species of the superfamily and 26 genera, with the large and widespread genus Sigara Fabricius being divided into many subgenera (Bachmann, 1981; Schuh & Slater, 1995). The cosmopolitan genus Sigara comprises approximately 70 species in America, distributed from Canada to southern Argentina in Santa Cruz province and the Malvinas Islands (Bachmann, 1981; Morrone et al., 2004). Micronectidae includes two genera: Micronecta Kirkaldy distributed in the Old World and Australia, and Tenagobia Bergroth distributed from Mexico to central Argentina in Buenos Aires province (Bachmann, 1981; Morrone et al., 2004).
True bugs, the Heteroptera, have many cytogenetic characteristics that make them unique among most insect groups: the possession of chromosomes without a primary constriction, the centromere, namely holokinetic chromosomes; a pair of «m chromosomes» in 16 families, belonging to four infraorders; a different meiotic behaviour for autosomes and sex chromosomes; and a mean chiasma frequency of only one chiasma per bivalent (Ueshima, 1979; Nokkala, 1986; Papeschi & Bressa, 2006). As a rule, autosomal bivalents are chiasmatic while the sex chromosomes and m chromosomes are achiasmatic. The m chromosomes are generally of small size and show allocycly with respect to both the autosomes and the sex chromosomes during male meiosis; they are usually unpaired and thus achiasmatic during early meiotic prophase (Ueshima, 1979; Papeschi & Bressa, 2006).
The pattern of meiosis in the Heteroptera varies between species, particularly for the behaviour of the sex chromosomes and the m chromosomes. During the early prophase, the sex chromosomes X and Y are positively heteropycnotic and remain in this condition until diakinesis. By late diakinesis, the X and Y chromosomes are separated from each other, become isopycnotic and each is composed of two sister chromatids (Ueshima, 1979; Papeschi & Bressa, 2006).
The autosomal bivalents segregate reductionally during the first meiotic division and equationally during the second division. The achiasmatic m chromosomes associate at late diakinesis end-to-end, through the socalled «touch-and-go pairing»; they form a pseudobivalent, which segregates reductionally at anaphase I, and divides equationally at the second meiotic division. On the other hand, sex chromosomes behave as univalents in male meiosis I; they divide equationally at anaphase I, associate at meiosis II through the touch-and-go pairing and segregate reductionally at anaphase II (Ueshima, 1979; Manna, 1984; Papeschi & Bressa, 2007).
In all heteropteran species, the autosomes tend to form a ring on the periphery of the spindle at both meiotic metaphases, with the sex chromosomes lying side by side in the centre of the ring. Usually, when m chromosomes are present, the X and Y form part of the ring of the autosomal bivalents at first metaphase, and the m pseudobivalent lies in its centre. At second metaphase, both the X-Y pseudobivalent and the m chromosome lie in the centre of a ring formed by the autosomes (Ueshima, 1979). This arrangement at the metaphase plates has been recorded in the Corixidae (Peters & Kleba, 1971), the Lygaeidae (Ueshima & Ashlock, 1980), and the Stenocephalidae (Lewis & Scudder, 1958).
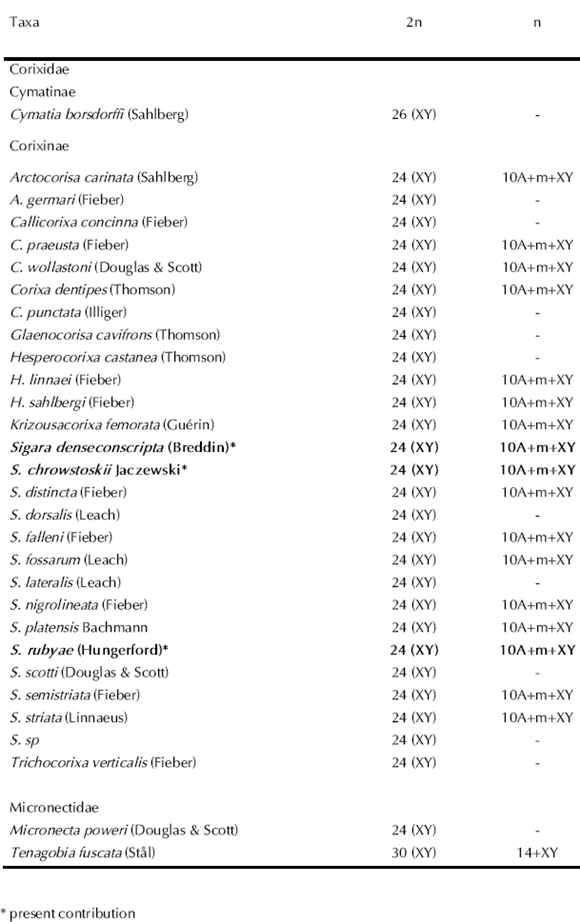
Previous cytogenetic studies on 27 species of Corixoidea have shown a noticeable karyotypic uniformity with a diploid chromosome number of 24, a pair of m chromosomes, and an XY/XX sex chromosome system (male/female) (Table I) (Ueshima, 1979; Ituarte & Papeschi, 2003, 2004). Up to these days only two species of Micronectidae, Micronecta poweri (Douglas & Scott) and Tenagobia fuscata (Stål), have been cytogenetically analyzed (Table I) (Southwood & Leston, 1959; Ituarte & Papeschi, 2004). While M. poweri has the modal chromosome number of Corixidae (2n= 24), T. fuscata presents many interesting cytogenetic features, such as achiasmatic male meiosis. Besides, both species are characterized by the absence of an m chromosome pair. Within Corixidae, nine genera and 25 species belonging to the subfamilies Cymatinae (Cymatia borsdorfii (Sahlberg)) and Corixinae (24 species) have been cytogenetically analyzed (Table I) (Ituarte & Papeschi, 2004). All species of Corixinae show a diploid chromosome number of 24, with a pair of m chromosomes and an XY/XX sex chromosome system (male/ female), while C. borsdorfii has 2n= 26= 24+XY (Ueshima, 1979). Sigara platensis Bachmann was the only cytogenetically described species inhabiting Argentina. It possesses the diploid chromosome number of 24 (2n= 20+2m+XY/XX, male/female) and all the specimens analyzed showed from one to three supernumerary chromosomes (Ituarte & Papeschi, 2003).
Table I. Diploid chromosome number in species of Corixoidea (previous data cited in Ituarte & Papeschi, 2003, 2004 are included for comparisons)
In the present study, male karyotype and meiotic behaviour of Sigara denseconscripta (Breddin), S. chrostowskii Jaczewski, and S. rubyae (Hungerford) from the National Park Pre-Delta (Entre Ríos province, Argentina) are described. The cytogenetic results are discussed at the superfamily level, and evolutionary trends within Nepomorpha are proposed.
MATERIAL AND METHODS
All adult males of Sigara denseconscripta, S. chrostowskii and S. rubyae were collected in the National Park Pre-Delta (Entre Ríos province, Argentina). Immediately after their capture, all specimens were fixed in ethanol: chloroform: glacial acetic acid (6:3:1) and the gonads were dissected under a binocular stereoscopic microscope and kept in 70% ethanol at 4°C. Slides were made by the squash technique in ferric acetic haematoxylin following conventional procedures.
RESULTS
Sigara denseconscripta, S. chrostowskii, and S. rubyae possess a male diploid chromosome number of 24 (2n= 20+2m+XY) and a haploid chromosome number of 13 (n= 10+m+XY) (Fig. 1). All of them present three large, six medium-sized and one small pair of autosomes. The m chromosomes are the smallest of the complement and negatively heteropycnotic. The sex chromosome system is XY/XX (male/female), being the X chromosome as big as the medium-sized pair of autosomes. The Y chromosome is similar in size to the smallest autosomal pair in Sigara rubyae and S. chrostowskii, whereas it is smaller than the smallest bivalent in S. denseconscripta.

Fig. 1. Male meiosis of Sigara denseconscripta (a-f), S. chrostowskii (g) and S. rubyae (h, i). a) Pachytene; b) Diakinesis; c) Late Diakinesis; d) Metaphase I; e) Telophase I; f) Metaphase II; g) Metaphase I; h) Diakinesis with a pair of univalents (I); i) Metaphase I. Arrows point the sex chromosomes and arrowheads point the smallest autosomal bivalent. N= nucleolus; m= m chromosomes. Scale bar = 10 μm.
At early meiotic prophase, the sex chromosomes X and Y are positively heteropycnotic and lie close to each other (Fig. 1a). From pachytene to diplotene, a conspicuous nucleolus is observed associated with both sex chromosomes (Fig. 1a). At diplotene-diakinesis, the X and Y univalents remain close to each other, while the m chromosomes are always separated and negatively heteropycnotic. Autosomal bivalents generally present one chiasma terminally located, but the three largest bivalents can show one chiasma at interstitial position (Fig. 1b, d, h, i). From diakinesis onwards, the m chromosomes begin to get closer and associate end-to-end to form a pseudobivalent (pII) (Fig. 1b, c, h). At metaphase I, the m pseudobivalent lies in the centre of the ring formed by the autosomal bivalents, and the X and Y univalents orientated side-by-side (Fig. 1d, f, i). At anaphase I the autosomal bivalents, as well as the m pseudobivalent, divide reductionally while the sex chromosomes segregate equationally (Fig. 1e).
Second meiotic metaphase follows immediately after telophase I, without an interkinesis stage. At metaphase II, the X and Y chromosomes are associated forming a pseudobivalent (Fig. 1f). The autosomes and the sex pseudobivalent dispose at the equatorial plane forming a ring, and the m chromosome lies in its centre. At anaphase II, the X and Y segregate to opposite poles.
DISCUSSION
Heteroptera comprises eight infraorders, and cytogenetic reports are unequally distributed not only among these major groups, but also within them. At present, approximately 1600 heteropteran species belonging to 46 families have been cytogenetically analyzed. The male diploid chromosome number of Heteroptera ranges from 2n= 4 (Lethocerus sp., Belostomatidae) to 80 (four species of Lopidea Uhler, Miridae). Even though the most represented diploid number is 14 (460 species), 70% of the species have diploid numbers between 12 to 34 chromosomes (Papeschi & Bressa, 2006). The study of karyotype evolution and the mechanisms of chromosome change can contribute to the understanding of the evolution and taxonomic relationships because the karyotypes are species-specific characters. Discussions on karyotype evolution in Heteroptera use the concept of modal number, i.e., the commonest diploid number present in a group, such as family, tribe or genera. The modal number is frequently considered as the ancestral one for the group under analysis (Ueshima, 1979; Papeschi & Bressa, 2006).
Within Nepomorpha, 96 species have been cytogenetically characterized, and the families Belostomatidae and Corixidae have been the most extensively analyzed (27 and 25 species, respectively). So far, from the 27 cytogenetically studied species of Corixoidea, two belong to Micronectidae and the remaining 25 are included in Corixidae. Within the former, Micronecta poweri has the modal chromosome number of Corixidae (2n= 24), while Tengobia fuscata presents a higher diploid number (2n= 30) and an achiasmatic male meiosis. Besides, both species are characterized by the absence of an m chromosome pair (Ituarte & Papeschi, 2004; Papeschi & Bressa, 2007).
On the other hand, all the species of Corixidae show a diploid chromosome number of 24, with a pair of m chromosomes and an XY/XX sex chromosome system (male/ female), except Cymatia borsdorfii (2n= 26= 24+XY) (Ueshima, 1979; Ituarte & Papeschi, 2003, 2004; Papeschi & Bressa, 2006). Considering that fusions and fragmentations are the main mechanisms of karyotype evolution in Heteroptera, the chromosome number of C. borsdorfii could probably originate by the fragmentation of a pair of autosomes. Furthermore, the m chromosome pair characteristic of Corixoidea could lose its particular meiotic behaviour and become a regular autosomal pair, contributing thus to the increase in the diploid chromosome number of the species.
Up to these days, 11 species of Sigara have been cytogenetically analyzed and only one of them inhabits Argentina, namely Sigara platensis Bachmann. Sigara platensis possesses the diploid chromosome number of 24 (2n= 20+2m+XY/XX, male/female), and all the specimens analyzed had from one to three supernumerary chromosomes (Ituarte & Papeschi, 2003).
Sigara denseconscripta, S. rubyae, and S. chrostowskii show similarities in karyotype (2n= 24= 20+2m+XY) and meiotic behaviour to the species previously analyzed. From all these results, we suggest that Corixidae is a cytogenetically homogenous family, and an ancestral karyotype of Corixoidea should be 2n= 24= 20+2m+XY.
Concerning the chromosome arrangements at the meiotic metaphase plates in the three species of Sigara analyzed in this paper, there is a deviation from the typical disposition described in Corixidae. During metaphase II, the sex pseudobivalent disposes at the equatorial plane forming part of the ring of autosomes, and only the m chromosome lies in its centre. The significance of this different pattern of chromosome arrangement at metaphase plates remains obscure.
The m chromosomes in Heteroptera are defined by their special meiotic behaviour: achiasmatic, and associated through a touchand- go pairing at first meiotic division. However, nothing is known about the genetic information that the m chromosomes carry and their function in the genetic system of the species possessing them. The m chromosomes have been reported in 14 heteropteran families, but are absent in Gerromorpha and Cimicomorpha (Papeschi & Bressa, 2006, 2007).
From a cytogenetic point of view Nepomorpha may be regarded as containing five groups: Nepoidea, Ochteroidea, Naucoroidea, Corixoidea, and Notonectoidea. All of them with the only exception Lethocerus sp. (Belostomatidae) share a high diploid chromosome number (Fig. 2). A total of 38 species analyzed from Nepoidea lack m chromosomes and show both simple and multiple sex chromosome systems (XY, XnY); the diploid number ranges between 4 and 46. In the second group, the Ochteroidea, only one species has been studied; Gelastocoris oculatus has 2n= 30+X1X2X3X4Y, and no m chromosomes have been recognized. In Naucoroidea, ten species of Naucoridae are characterized by the possession of a pair of m chromosomes and an X0 sex chromosome system. The diploid number varies from 20 to 51 in males. Within Notonectoidea, the 17 species analyzed present m chromosomes, simple and multiple sex chromosome systems (XY, X0, Xn0), and a diploid number 2n= 23-26 (Ueshima, 1979; Manna, 1984; Papeschi & Bressa, 2006) (Fig. 2).

Fig. 2. Distribution of male chromosome numbers, sex chromosome systems and presence/absence of m chromosomes in the families and superfamilies of Nepomorpha based on cytogenetic evidence.
Taking into account the phylogenetic relationships of families and superfamilies of Nepomorpha proposed by Rieger (1976) and Mahner (1993), the «primitive» superfamilies Nepoidea and Ochteroidea show both simple and multiple sex chromosome systems (XY and XnY), and lack m chromosomes. The Corixoidea and two other superfamilies, Naucoroidea and Notonectoidea, present a pair of m chromosomes and show variable sex chromosome systems (XY, X0 and Xn0).
Based on the presence of a Y chromosome in very primitive heteropteran species, Nokkala & Nokkala (1983, 1984) and Grozeva & Nokkala (1996) suggested that the X0 system is a derived condition from the ancestral XY that is present in the majority of the species cytogenetically analyzed. Furthermore, from the finding of a pair of m chromosomes in three species of Dipsocoridae and two species of Schizopteridae (Dispsocoromorpha), Grozeva & Nokkala (1996) suggested that this pair of chromosomes might be included in the ancestral karyotype of the Heteroptera. Under these hypotheses, and according to the available cytogenetic data of Nepomorpha, we suggest that the presence of XY sex chromosome system and a pair of m chromosomes could be considered plesiomorphic characters for this infraorder (Fig. 2). On the one hand, the sex chromosome systems X0 and Xn0 of the Corixoidea, Naucoroidea and Nepoidea most probably originated during the evolution through the loss of the Y chromosome (X0) and in some species fragmentation of the original X chromosome (Xn0). On the other hand, the m chromosomes had become lost at the origin of the superfamilies Nepoidea and Ochteroidea. Summarizing, both the X0 and Xn0 systems in Corixoidea, Naucoroidea and Nepoidea, and the absence of a pair of m chromosomes in Nepoidea and Ochteroidea should be considered as derived characters within Nepomorpha.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Axel O. Bachmann for taxonomical identification of the specimens and his continuous encouragement for the study of aquatic heteropterans. The authors also thank Administración de Parques Nacionales (Dirección de Conservación y Manejo). The present study was supported by grants from the Buenos Aires University (UBA) (X317) and CONICET (PIP 5261). M. J. Bressa and A. Papeschi are members of the National Council of Scientific and Technological Research (CONICET).
LITERATURE CITED
1. BACHMANN, A. O. 1981. Insecta Hemiptera Corixidae. In: Ringuelet, R. A. (ed.), Fauna de agua dulce de la República Argentina, FECIC, La Plata, pp. 270+plates I-XXXIV.
2. GROZEVA, S. & S. NOKKALA. 1996. Chromosomes and their meiotic behavior in two families of the primitive infraorder Dipsocoromorpha (Heteroptera). Hereditas 125: 31-36.
3. ITUARTE, S. & A. G. PAPESCHI. 2003. Complemento cromosómico y comportamiento meiótico de Sigara platensis Bachmann, 1962 (Corixidae, Heteroptera). In: Huerta Grande, Córdoba, pp. 101.
4. ITUARTE, S. & A. G. PAPESCHI. 2004. Achiasmatic male meiosis in Tenagobia (Fuscagobia) fuscata (Heteroptera, Corixoidea, Micronectidae). Genetica 122: 199-206.
5. JANNSON, A. 1986. The Corixidae (Heteroptera) of Europe and some adjacents regions. Acta Entomol. Fenn. 47: 1-94.
6. LEWIS, K. R. & G. G. E. SCUDDER. 1958. The chromosomes of Dicranocephalus agilis (Hemiptera-Heteroptera). Cytologia 23: 92-104.
7. MAHNER, M. 1993. Systema cryptoceratorum phylogeneticum (Insecta, Heteroptera). Zoologica 143: IX+302.
8. MANNA, G. K. 1984. Chromosomes in evolution in Heteroptera. In: Sharma, A. K. & A. Sharma (eds.), Chromosomes in evolution of eukaryotic groups, CRC Press, Boca Raton Florida USA, pp. 189-225.
9. MORRONE, J. J., S. A. MAZZUCONI & A. O. BACHMANN. 2004. Distributional patterns of Chacoan water bugs (Heteroptera: Belostomatidae, Corixidae, Micronectidae and Gerridae). Hydrobiologia 523: 159-173.
10. NOKKALA, S. 1986. The mechanisms behind the regular segregation of the m-chromosomes in Coreus marginatus L. (Coreidae, Hemiptera). Hereditas 105: 73-85.
11. NOKKALA, S. & C. NOKKALA. 1983. Achiasmatic male meiosis in two species of Saldula (Saldidae, Hemiptera). Hereditas 99: 131-134.
12. NOKKALA, S. & C. NOKKALA. 1984. The occurrence of the X0 sex chromosome system in Dictyonota tricornis (Schr.) (Tingidae, Hemiptera) and its significance for concepts of sex chromosome system evolution in Heteroptera. Hereditas 100: 299-301.
13. PAPESCHI, A. G. & M. J. BRESSA. 2006. Evolutionary cytogenetics in Heteroptera. J. Biol. Res. 5: 3-21.
14. PAPESCHI, A. G. & M. J. BRESSA. 2007. Classical and molecular cytogenetics in Heteroptera. In: Mohan, R. M. (ed.), Research Advances in Entomology, Kerala, pp. 1-9.
15. PETERS, W. & M. KLEBA. 1971. Meiotic chromosomes of Krizousacorixa femorata (Heteroptera: Corixidae). Ann. Entomol. Soc. Am. 64: 965-968.
16. RASK, M. 1983. Differences in growth of perch (Perca fluviatilis) in two small forest lakes. Hydrobiologia 101: 139-144.
17. REYNOLDS, J. D. 1975. Feeding in corixids (Heteroptera) of small alkaline lakes in central B.C. Verh. Int. Verein. Limnol. 19: 3073-3078.
18. REYNOLDS, J. D. & G. G. E. SCUDDER. 1987a. Experimental evidence of the fundamental feeding niche in Cenocorixa (Hemiptera: Corixidae). Can. J. Zool. 65: 967-973.
19. REYNOLDS, J. D. & G. G. E. SCUDDER. 1987b. Serological evidence of realised feeding niche in Cenocorixa species (Hemiptera: Corixidae) in sympatry and allopatry. Can. J. Zool. 65: 974- 980.
20. RIEGER, C. 1976. Skelett und Muskulatur des Kopfes und Prothorax von Ochterus marginatus Latreille. Zoomorphologie 83: 109-191.
21. SCHAEFER, C. W. & A. R. PANIZZI. 2000. Heteroptera of economic importance. CRC Press, Florida.
22. SCHUH, R. T. & J. SLATER. 1995. True bugs of the world (Hemiptera: Heteroptera): classification and natural history. Cornell University Press, New York.
23. SOUTHWOOD, T. R. E. & D. LESTON. 1959. Land and water bugs of the British Isles. Frederick Warne and Co., London.
24. UESHIMA, N. 1979. Hemiptera II: Heteroptera. In: John, B. (ed.), Animal Cytogenetics, Gebrüder Borntraeger, Berlin-Stuttgart, pp. V+117.
25. UESHIMA, N. & P. D. ASHLOCK. 1980. Cytotaxonomy of the Lygaeidae (Hemiptera - Heteroptera). Univ. Kansas Sci. Bull. 51: 717- 801. [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
Recibido: 22-05-2007;
aceptado: 10-07-2007














