Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista de la Sociedad Entomológica Argentina
versión impresa ISSN 0373-5680versión On-line ISSN 1851-7471
Rev. Soc. Entomol. Argent. v.66 n.3-4 Mendoza ago./dic. 2007
Megamelus bellicus (Hemiptera: Delphacidae): immature stages and biology
Megamelus bellicus (Hemiptera: Delphacidae): estados inmaduros y biología
Mariani, Roxana*, Alejandro Sosa** and Ana María Marino de Remes Lenicov*
*Universidad Nacional de La Plata, División Entomología, Facultad de Ciencias Naturales y Museo, Paseo del Bosque s/n, La Plata (1900), Buenos Aires, Argentina; e-mail: rmariani@fcnym.unlp.edu.ar
** USDA-ARS-South American Biological Control Laboratory, Bolivar 1559 (B1686) Hurlingham, Buenos Aires, Argentina
RESUMEN. Se describen e ilustran los estados inmaduros de Megamelus bellicus Remes Lenicov & Sosa (Hemiptera: Delphacidae) y se presenta una clave para identificarlos. La descripción de cada estadio se realizó sobre la base de ninfas extraidas 24 horas posteriores a la eclosión, de colonias de laboratorio y criadas sobre trozos de hojas de camalote Eichhornia crassipes (Martius) Solms Laubach. Los principales caracteres para distinguir los distintos estadios son: tamaño del cuerpo, color, número de tarsómeros, espinulación de la metatibia y número de dientes del calcar y presencia de sensorios en el pedicelo antenal. Datos biológicos basados en observaciones en el laboratorio y en el campo, muestran que M. bellicus realiza su ciclo biológico exitosamente sobre Pontederiaceae. Los huevos, dispuestos de 1 a 9 por postura, son colocados profundamente en el aerénquima del pecíolo; son más frecuentes de 3-4, menos frecuentes 5, 2 y 6, y raramente 1, 7 y 9. Se registra el porcentaje de parasitoidismo de un Hymenoptera oófilo de la familia Eulophidae, Aprostocetus (Ootetrastichus) sp. Debido a que M. bellicus ocupa el mismo hábitat ecológico que M. scutellaris Berg, se resaltan las principales diferencias morfológicas y de comportamiento entre las mismas.
PALABRAS CLAVE. Megamelus bellicus; Pontederiaceae; Estados inmaduros; Morfología; Biología; Clave.
ABSTRACT. The immature stages of Megamelus bellicus Remes Lenicov & Sosa (Hemiptera: Delphacidae) are described, keyed and illustrated. The description of each stage was based on 24-h hatched nymphs from the laboratory colony. The main characters that distinguish the various stages are: body size, color, number of tarsomeres, espinulation of the metatibia and number of teeth on the spur; presence of sensoria on antennal pedicel. This insect was reared on pieces of water hyacinth, Eichhornia crassipes (Martius) Solms Laubach. Biological data based on lab and field observations show that M. bellicus carries out its biological cycle successfully on Pontederiaceae. One to nine eggs per scar are laid deeply into the aerenquima of the petiole, being most frequent 3-4, less frequent 5, 2 and 6 and rarely 1, 7 and 9. Eulophid wasps Aprostocetus (Ootetrastichus) sp, known as eggs parasitoid, was registered and quantified. As M. bellicus occupies the same ecological habitat that M. scutellaris Berg, we highlighted some morphological and biological aspects that allow their differentiation.
KEY WORDS. Megamelus bellicus; Pontederiaceae; Immature stages; Morphology; Biology; Key.
INTRODUCTION
The genus Megamelus Fieber 1866 (Hemiptera: Delphacidae) includes 25 species in the Americas, of which six are found in the Neotropical region: M. bifurcatus Crawford, M. iphigeniae Muir, M. electrae Muir, M. timehri Muir, M. scutellaris Berg and M. bellicus Remes Lenicov & Sosa (Sosa et al., in press). Although all of them are known as associated with aquatic plants, only the life cycle of the North American M. davisi Van Duzee on water lily Nuphar advena (Aiton) and the South American M. scutellaris Berg on water hyacinth, Eichhornia crassipes (Martius) Solms-Laubach (Wilson and McPherson, 1981a, b; Sosa et al., 2005) have been studied.
Megamelus bellicus is widely distributed in Argentina on different species of Pontederiaceae, occupying the same ecological habitat that M. scutellaris, in the subregions Delta of Paraná River and La Plata River. They also appear in the south of Brazil and north of Peru (Sosa et al., 2004, in press). Its host plants are several Pontederiaceae species: Pontederia cordata L., P. rotundifolia L., E. crassipes -commonly called water hyacinth-, and E. azurea (Swartz) Kunth, and plants from other families: Echinodorus grandiflorum (Chamisso et Schlechtedahl) Micelli (Alismathaceae) and Limnobium laevigatum (Heine) (Hydrocharitaceae). An unidentified eulophid wasp-Aprostocetus (Ootetrastichus) sp- is known as eggs parasitoid (Sosa et al., in press). From studies in field and laboratory, several differences between M. bellicus and M. scutellaris may be highlighted particularly in reproductive and feeding behaviour (Sosa et al., 2005, in press).
In this article the egg and nymphal instars of M. bellicus are described and illustrated as well as some biological information from the laboratory regarding oviposition and parasitoidism.
MATERIAL AND METHODS
Laboratory rearing and biological aspects
In a 3-yr period (2003-2006), adults and nymphs of M. bellicus were periodically collected by aspirating on water hyacinth in different localities in Buenos Aires, Argentina and used to establish a laboratory colony. Several generations were reared in two cages (61 by 61 by 61 cm), in outdoor conditions, on E. crassipes and on two other Pontederiaceae species: Pontederia subovata (Seubert) Low and Heteranthera limosa (Swartz) Willdenow. Plants were added every week or when required.
Petioles (6) of E. crassipes with 458 scars of M. bellicus were dissected to study the ovipositional pattern of the species. Eggs and nymphs from field and lab were examined daily to detected parasitoids under microscope.
Morphological studies
The description of each stage was based on 24-h hatched nymphs from the laboratory colony. Specimens were anaesthetized with 95% ethyl ether to register coloration, and then cleared in cold 10% KOH solution and fixed in Faure liquid for microscopic examination and illustration. The eggs were studied from leaves with oviposition scars taken from the laboratory colony and removed from plant tissue.
The first instar is described in detail; only major changes that differ from the previous instars are highlighted in the later stages. Specimens used for the descriptions were the brachypterous, as it is the only morphotype obtained in lab. The reported measurements derive from 10 eggs and specimens of each instar nymph and are given in millimetres. The dimensions are expressed as length of egg (LE), width of egg (WE); total body length (L) from the tip of the vertex to the distal apex of the abdomen; width (W), measured across the widest part of the metathorax; and thoracic length (TL), from the anterior margin of the pronotum to the posterior margin of the metanotum. The nomenclature for arrangement of sensorial pits follows Vilbaste (1968). Drawings were made with the aid of a camera lucida on a Leica stereomicroscope. Average is expressed as mean ± SE.
Series of six specimens per instar were deposited in the collection of the División Entomología, Facultad de Ciencias Naturales y Museo de La Plata.
RESULTS
Morphological studies
Eggs (Fig. 1). LE: 0.97 ± 0.06, WE: 0.20 ± 0.01. Ellipsoidal with cephalic apex sharp and opposite end rounded; ventral surface slightly concave, dorsal convex. Color milky white when laid, turning yellowish white before hatching. Chorion translucent, smooth.

Figs. 1-6. Megamelus bellicus. 1, eggs. 2-6, habitus: 2, first instar; 3, second instar; 4, third instar; 5, fourth instar; 6, fifth instar. Scales = 0.5 mm.
First-instar nymph (Figs. 2, 7, 12). Measurements L: 1.10 ± 0.04; W: 0.32 ± 0.02; TL: 0.35 ± 0.01. Ground color pale yellowish; vertex with carinae and basal compartment, frons, clipeous- except the area around the frontoclypeal suture-, rostrum, antennae, tergites of thorax and abdomen - except the distinguishable longitudinal stripe on midline, and legs, except apex of procoxa and mesocoxa, brown. Eyes red.
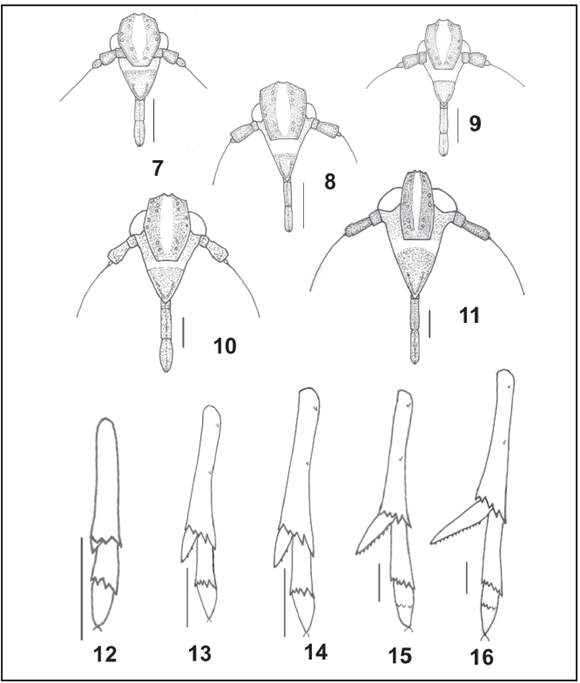
Figs. 7-16. Megamelus bellicus. 7-11, front view of head: 7, first instar; 8, second instar; 9, third instar; 10, fourth instar; 11, fifth instar. 12-16, ventral view of metatibial spur and metatarsi: 12, first instar; 13, second instar; 14, third instar; 15, fourth instar; 16, fifth instar. Scales = 0.2 mm.
Body elongated subcylindrical, widest across mesothorax. Vertex as long as broad, posterior margin straight, lateral margins carinate; projecting beyond eyes about half its length (Fig. 2). Frons broader than clypeus, oval, convex in profile; slightly longer than wide; lateral margin convex and carinate; median carinae converging, not reaching apical margin, distance between them wider than distance between lateral carinae and eyes. Clypeus convex, as broad as long, narrowing distally. Rostrum three-segmented (Fig. 7) reaching the mesocoxae, segment I almost completely hidden by anteclypeus, segment II and III subequal. Antennae threesegmented, relatively long; segment I short, slightly wider than long; II subcylindrical, 1.3 times longer than wide, without sensory pits; III bulbous, basally bearing a plaque-organ, ending in an elongated bristle as long as the length pronotum + mesonotum (Fig. 7).
Thoracic nota with three pairs of plates placed on both sides of longitudinal middorsal line. Pronotal plates subtrapezoidal, lateral carinae divergent, slightly convex toward posterior margin. Mesonotum and metanotum plates subrectangular, posterior margin concave, metanotum slightly longer laterally. Legs subcylindrical; metatrochanter bearing 11-12 cuticular folds medially; metatibiae unarmed laterally, bearing apical row of three black-tipped spines and a short, moveable subconical spike-like spur; slightly longer than the longest apical spine, without marginal teeth. Tarsi two-segmented; protarsi and mesotarsi with divisions between tarsomeres obscure; metatarsomeres equal in length, metatarsomere I bearing apical row of four black-tipped spines, with external longest; tarsomeres II of all legs subconical, slightly curved, with pair of black claws and pulvilli apically (Fig. 12).
Abdomen nine-segmented, subcylindrical, widest in segments III to V; IX elongate, surrounding anus. The first two tergites rather small and appearing as short trapezoidal sclerites in dorsal view. Hind tergites trapezoidal cross-plates bearing sensorial pits from fifth segment onward.
Arrangement of pits (Figs. 2 and 7). Head: with 11 sensorial pits on both sides; one pair on vertex behind lateral carinae; three pairs of sensorial pits -upper, median and lower, respectively- on the laterofrons; one dorsal pit at the lateral carinae; one pair pits near the anterior corner of eyes. Thorax: 12 on pronotum, two between midline and carinae, four between carinae and lateral margin bordering posterior margin on each side; eight on mesonotum, two on each side of midline and two -on antero-lateral and postero-lateral angles- on each side; two on metanotum on both sides of midline. Abdomen: tergum of segments V: 1+0; VI to VIII: 1+ 1 and IX: 3+3.
Second-instar nymph (Figs. 3, 8, 13). Measurements. L: 1.38 ± 0.02; W: 0.48 ± 0.02; TL: 0.50 ± 0.01. Coloration pattern heavily marked in brown; vertex pale on posterolateral angles, pronotum and mesonotum pale between the lateral carinae as well as on each side; metanotum with pale irregular longitudinal marks on each side of midline, and laterally. Abdomen adding two lateral pale spots on each side on tergites II, III, IV, V and VIII; VI without marks; VII with one lateral spot and IX uniform brown.
Vertex slightly longer than wide (Fig. 3). Frons longer than wide (1.2:1), broadest just anterior margin of eyes. Rostrum segment II slightly longer than III (Fig. 8). Antennal segment II 1.3 times longer than wide, bearing two sensory pits on dorsal surface near apex (Fig. 8). Mesonotum with straight lateral carinae divergent posteriorly (Fig. 3). Metatibiae (Fig. 13) bearing two small blacktipped spines on lateral margin, one near base, another nearly mid-length, bearing apical row of four black-tipped spines; spur twice the length of longest apical spine, bearing one apical tooth. Tarsi with visible divisions between tarsomeres; metatarsomere I and II subequal in length (Fig. 13).
Arrangement of pits (Figs. 3 and 8): Similar pattern to former instar with two dorsal additional pits on each lateral carinae of frons and one on abdominal segment VIII completing: 1 + 2.
Third-instar nymph (Figs. 4, 9, 14). Measurements. L: 2.00 ± 0.02; W: 0.55 ± 0.01; TL: 0.70 ± 0.05. Coloration pattern similar to former instar but darker. Mesonotum adding one lateral pale spot on each side; metanotum adding one lateralanterior pale spot on each side; apex of metafemur and tibiae at base and apex, pale.
Frons longer than wide (1.4:1). Antennal segment II 1.5 times longer than wide, bearing four sensory pits (Fig. 9). Mesonotal wingpads slightly lobate, as long as one-third of the metanotum length on mid-line. Metanotum slightly longer than mesonotum at midline, wingpads slightly extending laterally. Metatibiae bearing apical row of five blacktipped ventral spines; spur slightly flattened, less than two-thirds of metatarsomere I length, bearing one apical and two marginal teeth. Metatarsomere I bearing apical row of five black-tipped ventral spines (Fig. 14).
Arrangement of pits (Figs. 4 and 9): similar pattern to former instar, with one additional pit on abdominal segments VI and VII completing: 1 + 2.
Fourth-instar nymph (Figs. 5, 10, 15). Measurements. L: 3.00 ± 0.09; W: 1.00 ± 0.06; TL: 1.07 ± 0.05. Coloration pattern similar to former instar but darker, mesonotum and metanotum irregularly pale marked and metatibial spur at apex, lighter.
Vertex lengths one and a half its width. Frons longer than wide (1.6: 1). Antennal segment II bearing eight sensory pits (Fig. 10). Mesonotal wingpads distinctly lobate, half as long as the metanotum length on mid-line; metanotal wingpads reaching the first abdominal segment laterally (Fig. 5). Metatibial spur lengths a quarter shorter than metatarsomere I, bearing one apical tooth and a row of 6-8 marginal teeth; metatarsi twosegmented, with tarsomere I bearing apical row of six black-tipped ventral spines; tarsomere II with row of three weakly developed black-tipped ventral spines near middle of partially subdivided tarsomere (Fig. 15).
Arrangement of pits (Figs. 5 and 10): similar pattern to former instar, with two additional pits on both sides of lateral carinae of mesonotum.
Fifth-instar nymph (Figs. 6, 11, 16). Measurements. L: 3.50 ± 0.04; W: 1.00 ± 0.06; TL: 1.20 ± 0.05. Coloration pattern similar to former instar but darker.
Frons considerably longer than previous instars, twice as long as wide, parallel submedian carinae approaching each other. Antennal segment II with 12 sensory pits (Figs. 11).
Nymphs of the brachypterous morph: wingpads lobate, as long as the metanotum length on mid-line; metanotal wingpads reaching second abdominal segment laterally (Fig. 6). Spur foliaceous, as long as basal metatarsomere, four times longer than broad at base, with 11-14 marginal black-tipped teeth of varying size. Metatarsi threesegmented, tarsomere II bearing apical row of four black-tipped ventral spines, tarsomere II and III subequal. (Fig. 16).
Arrangement of pits: similar pattern to former instar (Figs. 6 and 11).
Key to instars of Megamelus bellicus
1- Metatibia with two lateral spines on shaft, one near base, the other beyond midlenght (Figs. 13-16). Metatibial spur more than 2 times the length of the longest apical spine (Figs. 13-16). Antennal pedicel with several sensorial pits (Figs. 8-11) ................................................... 2
1´- Metatibia without lateral spines on shaft (Fig. 12). Metatibial spur less than 2 times the length of the longest apical spines. Antennal pedicel without sensorial pits ………………………. First-instar nymph
2- Metatarsi two-segmented (Figs 13-14) ……….……….........….………………... 3
2´- Metatarsi three-segmented (Fig. 16); if two-segmented, then with row of three weakly developed black-tipped ventral spines in the middle of tarsomere II (Fig. 15) ……..........................................…. 4
3- Metatibial spur without marginal teeth (Fig. 13). Metatarsomere I with apical row of four black-tipped spines (Fig. 13) ……………………. Second-instar nymph
3´- Metatibial spur with two marginal teeth (Fig. 14). Metatarsomere I with apical row of five black-tipped spines (Fig. 14) ……………………..… Third-instar nymph
4- Metatibial spur with 6-8 marginal teeth (Fig. 15). Mesonotal wingpads half as long as the metanotum length on mid-line (Fig. 5 ).……………...…... Fourth-instar nymph
4´- Metatibial spur with 11-14 marginal teeth (Fig. 16). Mesonotal wingpads as long as metanotum length on mid-line (Fig. 6) ………………………. Fifth- instar nymph
Biological studies
Although the adults were maintained in the laboratory on P. cordata, P. rotundifolia, E. crassipes, and E. azurea (Pontederiaceae), as well as plants from other families: E. grandiflorum (Alismathaceae) and Limnobium laevigatum P. subovata and Heteranthera limosa (Hydrocharitaceae), nymphs and oviposition scars were only observed on Pontederiaceae. It was also observed that mating occurs close to water level and the females insert their eggs deeply into the aerenquima of the petiole, more frequently on the two basal third and extending toward the leaf apex when they reach high densities. One to four holes in the inner septa and one to four eggs per hole were most frequently observed in the scars. The scars are irregularly distributed in the petioles and they are notorious due to the necrosis produced (Sosa et al., in press).
According to the lab observations on the oviposition pattern test, one to nine eggs per ovipostion were registered; most frequently recorded were three-four (34.1 %, 29.7 %), less frequently five, two and six (16.1%, 12.7 % and 5.7%) and rarely one, seven and nine (0.4%, 0.9 % and 0.4%) eggs/ scars.
Natural enemies
A member of Eulophidae: Aprostocetus (Ootetrastichus) sp. is known as eggs parasitoid and one undetermined species of Dryinidae as nymphs parasitoid (Sosa et al., in press). Populations heavily parsitoidized with the Eulophid wasp were analyzed from 15 petioles. We registered that 23.6% oviposition scars (49 out of 208) were parasitized, consuming ca. 99 of 395 eggs, which means that the real percentage of parasitism was 25.1%.
Remarks
As it was mentioned, M. bellicus occupies the same ecological habitat that M. scutellaris; when they share a water hyacinth leave, the former shows an aggressive behaviour, pushing it (Sosa et al., in press).
As M. bellicus and M. scutellaris share hosts and coexist in sympatry, we consider to highlight the main differences between them in morphology and coloration pattern as well as those in oviposition behaviour.
According to coloration, M. bellicus adults and nymphs are uniformly light brown with only one lighter stripe on frontoclypeal suture and some longitudinal pale marks on meso and metanotum, spur more indented, reaching 15-20 marginal regular-sized teeth in adults and in general aspects they are longer. M. scutellaris shows distinguishable transversal dark broad stripes on frons and below the eyes and a whitish one on frontoclypeal margin, a V-like brown spot on mesonotum and metanotum and conspicuous annular dark brown stripes on legs, spur reaching 13-14 marginal irregular-sized teeth in adults.
Nymphs and oviposition scars of M. bellicus were only observed on Pontederiaceae specie: Pontederia cordata, P. rotundifolia, Eichhornia crassipes, and E. azurea. Nymphs and oviposition scars of M. scutellaris were only observed on E. crassipes, carrying out its biological cycle successfully only on this host plant (Sosa et al., 2005).
M. bellicus female inserts its eggs deeply into the aerenquima of the petiole, more frequently on the two basal third and extending toward the leaf apex when they reach high densities. The ovipositional scars are irregularly distributed and the incisions in the petiole tissue are single; in each one, inside the aerenquima, the female ovipositor makes 1-4 holes in the inner septa, where one to four eggs per hole are laid (Sosa et al., in press). M. scutellaris female inserts its eggs in the aerenchyma in apical portion of petiole and pseudolamina of water hyacinth. The ovipositional scars are three recognizable parallel marks. One to four eggs are aligned in the shortest central mark, being two eggs per scars the most frequently recorded (Sosa et al., 2005)
M. bellicus dif-fer from the American species M. davisi not only in the coloration pattern and morphological features but also in biological aspects. According to the coloration, we noticed that the first instar M. davisi nymphs have the frons yellow with brownish gray dorsal markings changing to two dark brown longitudinal lines be-tween each inner and outer carinae in the next instars, and legs yellow infused with brown, a larger body size (male 3.2, female 3.8), spur with 5-10 and 12-19 marginal teeth in the fourth and fifth instars respectively. As for biological data, Wilson and McPherson (1981b) were not able to record eggs from field-collected or laboratory-reared females of M. davisi, only registered one egg per scar, and, in late fall, found the fifth instars feeding on several plants, including common anemone (Anemone virginiana L.), white snakeroot (Eupatorium rugosum Houttuyyn), round-leafed cat-brier (Smilax ritundifolia L.) and blackberry (Rubus sp) after the water lilies died. The eggs were inserted into leaves, midveins and stem without apparent external ovipositions scars.
ACKNOWLEDGEMENTS
We thank the reviewers for the valuable suggestions and comments that improved the manuscript. This work was supported by USDA-South American Biological Control Laboratory (SABCL), Universidad Nacional de La Plata (UNLP) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) in Argentina.
LITERATURE CITED
1. FIEBER, F. X. 1866. Grudzüge zur generischen Theilung der Delphacini. Ver. Zool. Bot. Ges. Weia. 16: 517-534.
2. SOSA, A. J., A. M. MARINO de REMES LENICOV, R. MARIANI & H. A. CORDO. 2004. Redescription of Megamelus scutellaris Berg (Hemiptera: Delphacidae), a candidate for biological control of water hyacinth. Ann. Entomol. Soc. Am. 97: 271-275.
3. SOSA, A. J., A. M. MARINO de REMES LENICOV & R. MARIANI. 2005. Life history of Megamelus scutellaris Berg with description of the immature stages (Hemiptera: Delphacidae). Ann. Entomol. Soc. Am. 98 (1): 66-72.
4. SOSA, A. J., A. M. MARINO de REMES LENICOV & R. MARIANI. In press. Species of Megamelus Fieber (Hemiptera: Delphacidae) associated with Pontederiaceae in South America. Ann. Entomol. Soc. Am.
5. VILBASTE, J. 1968. Preliminary key for the identification of the nymphs of North European Homoptera Cicadina. I. Delphacidae. Ann. Entomol. Fenn. 34 (2): 65-74.
6. WILSON, S. W. & J. E. McPHERSON. 1981a. Ontogeny of the tibial spur in Megamelus davisi (Homoptera: Delphacidae) and its bearing on delphacid classification. The Great Lakes Entomol. 14: 49-50.
7. WILSON, S. W. & J. E. McPHERSON. 1981b. Life history of Megamelus davisi with descriptions of immature stages. Ann. Entomol. Soc. Am. 74: 345-350. [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
Recibido: 7-06-2007;
aceptado: 24-09-2007














