Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista de la Sociedad Entomológica Argentina
versión impresa ISSN 0373-5680versión On-line ISSN 1851-7471
Rev. Soc. Entomol. Argent. v.66 n.3-4 Mendoza ago./dic. 2007
Locomotor activity of Cycloneda sanguinea (Coleoptera: Coccinellidae) exposed to volatile semiochemicals and to direct contact with the odour source
Actividad locomotriz de Cycloneda sanguinea (Coleoptera: Coccinellidae) expuesta a semioquímicos volátiles y al contacto directo con la fuente de olor
Heit, Guillermo E.*, Pedro Sardoy*, Graciela R. Cohen** and Graciela Mareggiani*
*Cátedra Zoología Agrícola, Facultad de Agronomía, Universidad de Buenos Aires. Avda. San Martín 4453. (1417) Buenos Aires, Argentina; e-mail: gheit@agro.uba.ar, mareggia@agro.uba.ar
**Departamento de Biodiversidad y Biología Experimental, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires. Pabellon 2, Ciudad Universitaria. (C1428EHA) Buenos Aires, Argentina; e-mail: cohen@bg.fcen.uba.ar
RESUMEN. Cycloneda sanguinea (Linnaeus) (Coleoptera: Coccinellidae) es un predador polífago oportunista y cuando su menú está dominado por áfidos, es esperable un comportamiento complejo de forrajeo. Un movimiento activo, caracterizado por alta actividad locomotriz, juega un rol importante en la búsqueda de esta presa. En este trabajo se analizó el comportamiento de adultos de C. sanguinea expuestos a los semioquímicos volátiles, emitidos por sustratos de Capsicum annuum Linnaeus (Solanaceae) sanos o infestados con Myzus persicae (Sulzer) (Hemiptera: Aphididae), o simplemente puestos en contacto directo con esos sustratos. Los tratamientos evaluados fueron: A) hojas de pimiento infestadas con alta densidad de áfidos, B) hojas de pimiento infestadas con baja densidad de áfidos, C) hojas de pimiento sanas y D) control. La actividad locomotriz no difirió significativamente entre los distintos tratamientos cuando C. sanguinea se expuso solamente a los volátiles. En cambio, cuando los coccinélidos se pusieron en contacto directo con los sustratos evaluados, se encontraron diferencias significativas entre los tratamientos. Estos resultados preliminares indicarían que la sola presencia de un estímulo de olor, no sería suficiente para modular un patrón locomotor diferente en C. sanguinea.
PALABRAS CLAVE. Comportamiento de forrajeo; Capsicum annuum; Cycloneda sanguinea; Myzus persicae; Actómetro.
ABSTRACT. Cycloneda sanguinea (Linnaeus) (Coleoptera: Coccinellidae) is an opportunistic polyphagous predator and when aphids dominate its menu, a complex foraging behaviour can be expected. An active movement characterized by a high locomotor activity plays an important role in the search of this prey. The behaviour of C. sanguinea adults exposed to volatile semiochemicals emitted by Capsicum annuum Linnaeus (Solanaceae) substrates, healthy or infested with Myzus persicae (Sulzer) (Hemiptera: Aphididae), or in direct contact with these substrates was here analyzed. The treatments evaluated were: A) high aphid-infested pepper leaves, B) low aphid-infested pepper leaves, C) healthy pepper leaves and D) control. Locomotor activity was not significantly different among treatments when C. sanguinea was exposed only to the volatiles. However, when adults were placed in direct contact with the evaluated substrates, statistically significant differences were found among treatments. These preliminary results could indicate that the sole presence of an olfactory stimulus could not be sufficient to modulate a different locomotor pattern in C. sanguinea.
KEY WORDS. Foraging behaviour; Capsicum annuum; Cycloneda sanguinea; Myzus persicae; Actometer.
INTRODUCTION
Food sources are distributed along the environment, both in time and space. To forage successfully, predators and parasitoids must face a trade-off between the most proper moments to look for their food and the amount of resources present in a certain volume of space.
Coccinellids are opportunistic polyphagous predators, but their essential food requirements are very specific (Soares et al., 2004). In Argentina, Cycloneda sanguinea (Linnaeus) (Coleoptera: Coccinellidae) is considered an important aphids predator, but its impact in biological control is very variable, particularly due to its polyphagy. When aphids dominate their menu, a complex foraging behaviour representative of a huge range of generalist predators, can be expected (Dicke, 1999; Ninkovic et al., 2001).
According to the Area Concentrated Search (ACS) behaviour model, if predators such as coccinellids detect their preys, which are usually distributed in patches throughout the environment, after feeding, they will explore the prey-patch intensely (Wiens, 1976). They will do this in order to delimit a concentrated area to search for more resources. This area will be slowly swept following sinuous ways and making numerous stops in order to raise the probability of finding more preys. On the other hand, if predators do not find preys, they will undertake a more extensive or less circumscribed search, passing over long straight paths quickly and making just a few stops (Ferren & Dixon, 1993; Carter et al., 1984; Bond, 1980). In this way, they minimize the waste of time on unprofitable areas, avoiding unnecessary energy expenditure (Gendron & Staddon, 1983). ACS foraging behaviour would match to some extent the distribution of resources in a continuous patchy environment.
Some authors consider that coccinellids look randomly for their preys and can detect them only when direct contact is achieved (Ferran et al., 1994). This foraging behaviour has great advantage when the prey lives gregariously, such as aphids. Perhaps this is the reason why coccinellid ovipositions are mostly located in aphid-infested places or near them (Nakamuta, 1982), favoring successful prey foraging when ladybirds larvae hatch. On the other hand, newly emerged adult coccinellids must look for new patches, because usually all the preys around them have been consumed during juvenile development (Obata, 1986).
Other authors (Dicke, 1999; Al Abassi et al., 2000; Ninkovic et al., 2001; Acar et al., 2001) propose that a wide diversity of generalist predators such as ladybirds use different semiochemicals or infochemicals emitted by plants and insects to mediate in a series of key processes during foraging behaviour.
An active movement of predators, characterized by a high locomotor activity, plays an important role in the search for preys (Bell, 1990). However, little research on the effect of volatile semiochemicals in coccinellid locomotor activity has been done.
The aim of the present investigation was to evaluate the locomotor activity of the predator C. sanguinea, exposed to volatile semiochemicals emitted by Capsicum annuum Linnaeus (Solanaceae) plants, healthy or infested with Myzus persicae (Hemiptera: Aphididae), or in direct contact with these materials.
MATERIAL AND METHODS
Rearing of predator and prey. Culture of plant-host
Adults of Cycloneda sanguinea, collected in the campus of FAUBA (Facultad de Agronomía, Universidad de Buenos Aires), were reared in the laboratory. Ladybirds were fed on nymphs and adults of the aphid M. persicae (Hemiptera: Aphididae). Every two days, C. sanguinea eggs were collected and put in a Petri dish until larval emergence. Neonate larvae from each cohort were reared separately in glass flasks closed with gauze. The adults obtained were used for the bioassays.
Myzus persicae colonies were multiplied in the laboratory on pepper plants, Capsicum annuum CV. California Wonder, cultivated in 350 cc polyethylene pots. C. annuum was selected as plant host due to its economic importance as horticultural crop and to its high susceptibility to M. persicae colonization. Predators, prey and host were maintained in standardized conditions (24º C ± 2º C, 65 ± 10% RH, photoperiod: 16:8 h light-darkness).
Bioassays
Standardized conditions were used for the bioassays (24ºC±2ºC, 65±10% RH and light 60 watts). Every test was performed in the morning (9-12 h) and in the evening (13-16 h). Cycloneda sanguinea individuals from the same cohort, which were food deprived for 24 hours, were tested. The behavioural response, evaluated as the locomotor activity, was estimated according to the number of squares (1.5 cm side) of a squared grid crossed by the insect during its movement for 60 seconds (Tourniaire et al., 1999). Data were analyzed with three way ANOVA and Tukey multiple comparison test (á=0.05). Square root transformations were used to fulfil the assumptions of the test (Sokal & Rohlf, 1995; Zar, 1999). Data were analyzed using InfoStat program.
Two experimental series were done:
First series: exposure to volatile semiochemicals. Locomotor activity of Cycloneda sanguinea was evaluated with an actometer (modified from Manrique et al., 2006). In each test, a male or a female of C. sanguinea, chosen randomly from a randomized sample of 24 h starved individuals, was put in the experimental arena. The actometer (Fig. 1) consisted of an acrylic cylindrical box 12 cm diameter, composed by two horizontal compartments of the same height (2 cm), separated by a wire mesh to allow the diffusion of odour stimulus to avoid direct contact between the insect and the volatiles source. The upper compartment, the arena, where the insects were placed, had a removable and transparent cover on the top, with a squared grid drawn on its surface. The lower compartment, the diffusion volatile chamber, was connected by means of a perforated plate in the centre of its floor, to a stimulus delivery chamber, where the odour sources were placed. After the introduction of the insect into the arena, 4 minutes acclimations were spent until the initiation of the experiment, to allow the uniform diffusion of the volatile chemicals.
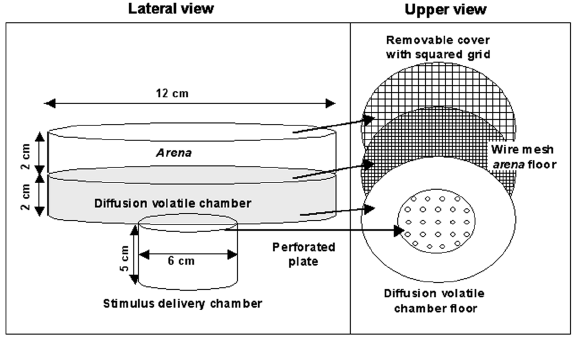
Fig. 1. Actometer scheme. Lateral view shows the disposition and dimensions of the compartments. Upper view shows the removable and transparent cover with a squared grid drawn on its surface, a wire mesh as the arena's floor, and below this, the connection of the diffusion volatile chamber with the stimulus delivery chamber by means of a plate with holes, which allowed the diffusion of volatiles to the arena.
The volatile sources evaluated were: A) high aphid-infested pepper leaves (80 aphids/ replica) on filter paper, infested with Myzus persicae for one month before the experiments, B) low aphid-infested pepper leaves (20 aphids/replica) on filter paper, infested with M. persicae for one week before the experiments, C) healthy pepper leaves on filter paper, and D) control: clean filter paper.
Nine independent replicates resulting from the combination of sex, moment of the day (morning and afternoon) and volatiles source were made for each treatment. The experimental unit consisted of a male or a female of Cycloneda sanguinea.
Second series: Direct contact of predator with the odour source. The experimental arena consisted of a Petri dish, 9 cm diameter, with a squared grid drawn in the upper cover. In each test, a male or a female of Cycloneda sanguinea, chosen randomly from a randomized sample of 24 h starved individuals, was put in the experimental arena, which allowed the contact with the substrate evaluated.
Treatments evaluated were the same as in the first series, but in this case the aphids were removed from the infested leaves with a brush, to avoid fortuitous stops of predators to eat, which could affect the locomotor activity. Eight independent replicates resulting from the same combinations as in the first series of experiments were performed. In the second series, the experimental unit consisted of a male or a female of Cycloneda sanguinea, as well. In both experimental series, 3 gr of pepper leaves were used as the odour source for each bioassay.
RESULTS
When Cycloneda sanguinea was exposed only to the volatiles of the evaluated substrates, its locomotor activity was not significantly different among treatments (F3,113,0.05=1.366, p=0.257). No differences were found between the mean locomotor activity in either female or male adults of this predator measured during the morning and afternoon (F1,113,0.05=3.683, p=0.058) (Fig. 2).
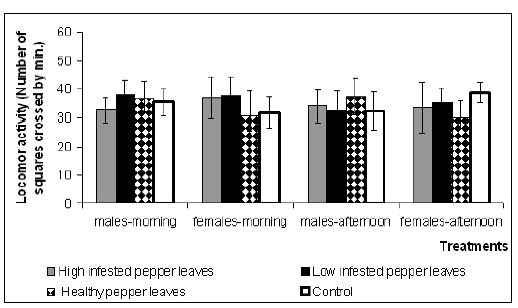
Fig. 2. Locomotor activity of Cycloneda sanguinea adults (male and female), exposed to several substrate volatiles, evaluated during morning or afternoon hours. Locomotor activity was estimated according to the number of squares crossed by the insect during 60 seconds. Substrates: A) High-infested pepper leaves, B) Low-infested pepper leaves, C) Healthy pepper leaves, D) Control. Mean locomotor activity and standard error are shown.
However, when adults of the coccinellid were placed in direct contact with the evaluated substrates, statistically significant differences were found among treatments (F3,112,0.05=73,62, p<0,0001) and between both moments of the day (F1,112,0.05=9.54, p=0.0025) (Fig. 3).
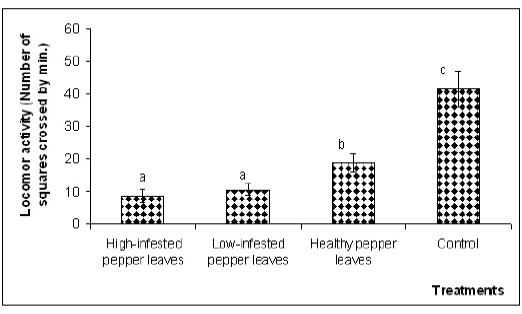
Fig. 3. Locomotor activity of Cycloneda sanguinea adults (male and female), in contact with several substrates, evaluated during morning or afternoon hours. Locomotor activity was estimated according to the number of squares crossed by the insect during 60 seconds. Substrates: A) High-infested pepper leaves (aphids removed), B) Low-infested pepper leaves (aphids removed), C) Healthy pepper leaves, D) Control. Mean locomotor activity and standard error are shown.
The mean locomotor activity of Cycloneda sanguinea measured in contact with pepper leaves, with either high or low density of infestation by Myzus persicae, was significantly lower than that recorded in contact with uninfested pepper leaves or clean filter paper. Furthermore, C. sanguinea was significantly more active on filter paper than on healthy pepper leaves (p<0.05).
On the other hand, the mean locomotor activity non discriminating among treatments neither between sexes resulted statistically lower ( p=0.0025) in the morning (mean=3.87;S.E.=0.24) than in the afternoon (mean=4.44; S.E.=0.19).
No significant differences between sexes were observed neither during the volatile- only-exposure series of bioassays ( F1,113,0.05=0.0004, p=0.983) nor during those of direct contact with the odour source (F1,112,0.05=1.025, p=0.313).
DISCUSSION
Coccinellids are important natural enemies of aphids and have often been used as a tool for biological control of several plagues (Obrycki & Kring, 1998). These predators are constantly monitoring the environment in search for food by means of different visual and olfactory cues for orientation (Nakamuta, 1980; Ferren & Dixon, 1993; Lambin et al., 1996). Olfactory cues such as volatile semiochemicals emitted by the aphids, the host or their interaction are chemical messages essential for survival of these species.
Some authors (Dicke, 1999; Al Abassi et al., 2000; Ninkovic et al., 2001; Acar et al., 2001; Heit et al., 2005) consider that coccinellids would be able to use olfactory and/ or visual cues to optimise their foraging behaviour. In this case, a specific stimulus linked to the presence of the prey or pepper leaves could trigger a distinctive locomotor activity pattern in the predator, although there may not be any contact with the aphids or the host plant. However, according to our results (Fig. 3), once Cycloneda sanguinea was in contact with a patch-prey on pepper leaves infested with Myzus persicae (regardless of prey density), it followed a behaviour similar to the one described by the Area Concentrated Search model (ACS) and moved slowly scanning exhaustively the area around. These results agree with those of Ferran et al. (1994), who pointed out that coccinellids could only detect their preys when they become in contact with them. On the other hand, when C. sanguinea did not detect a feeding resource, i.e. in contact with uninfested pepper leaves or clean filter paper (control), it moved significantly faster, monitoring very superficially the area devoid of food.
These results could have also been affected by the presence of honeydew, a sugar-rich sticky substance released by aphids as they feed, which could act as an additional patch cue in a similar way as other sugars that are phagostimulants for phytophagous insects do (Mitchell & Gregory, 1979; Mitchell & Harrison 1984; Blaney et al., 1990; Nagnan-Le Meillour et al., 2000). It has been reported that honeydew could influence the behaviour of syrphids. Budenberg & Powell (1992) point out that females of Episyrphus balteatus (De Geer) (Diptera: Syrphidae) landed more frequently on ears contaminated with honeydew than on clean ears, suggesting a response to honeydew volatiles. Additional experiments could be useful to evaluate the effect of honeydew on foraging activities of Cycloneda sanguinea adults.
Our results indicate that the sole presence of an olfactory stimulus could not be sufficient to modulate a different locomotor pattern in Cycloneda sanguinea. Perhaps the lack of response to volatiles could be due to a short acclimation time to the odour sources (4 min). New assays with longer acclimation periods would be useful.
The p value 0.058 for the mean locomotor activity of Cycloneda sanguinea (both sexes) exposed to volatiles and measured during the morning and afternoon is very close to the limit of significance (α=0.05), which suggests that additional replicates should be added in order to obtain more conclusive results. Instead, this activity in males and females put on direct contact with the substrates was significantly higher during the afternoon than during the morning hours (p=0.0025). These results agree with Elliott et al (2000), who found that searching activity of Hippodamia convergens Guerin-Meneville, Hippodamia tredecimpunctata tibialis (Say), and Coleomegilla maculata (De Geer) (Coleoptera: Coccinellidae) adults is influenced positively as time of day and temperature increase in spring cereal fields.
The above mentioned could point out to the presence of a rhythm in the foraging activity of Cycloneda sanguinea (Mishra & Omkar, 2004). Foraging behaviour is one of the main adaptive functions which allow predator insects to find their preys. To be successful, the search for food must be done when and where the preys are more available. In this sense, volatile semiochemicals and/or tactile cues from phytosuccivorous preys, the host plant or their interaction, must be necessary. The increase of the locomotor activity in the afternoon suggests an adaptive behavioural response of this predator.
The prey searching behaviour did not differ between sexes in both experimental series during morning and afternoon. Probably, foraging in this predator is the main diurnal activity in both sexes. Later, in the evening or night, foraging would stop and sexual activities would start, and then, male and female locomotor activity could differ. Mishra and Omkar (2004) observed that the major foraging activity and field presence levels of Propylea dissecta (Mulsant) (Coleoptera: Coccinellidae) occur in the photophase while peaks of rhythm of mating, oviposition, hatching and moult occur in scotophase, then supposing that the concentration of life events other than foraging in the same moments could be a survival strategy of evolutionary significance.
ACKNOWLEDGEMENTS
We wish to thank the University of Buenos Aires (Project UBACyT G062) for the financial support, and the referees for their useful comments, which improved the manuscript.
LITERATURE CITED
1. ACAR, E., J. MEDINA, M. LEE & G. BOOTH. 2001. Olfactory behaviour of convergent lady beetles (Coleoptera: Coccinellidae) to alarm pheromone of green peach aphid (Hemiptera: Aphididae). Can. Entomol. 133: 389-397.
2. AL ABASSI, S., M. BIRKETT, J. PETTERSSON, J. PICKETT, L. WADHAMS & C. WOODCOCK. 2000. Response of the seven-spot ladybird to an aphid alarm pheromone and an alarm pheromone inhibitor is mediated by paired olfactory cells. J. Chem. Ecol. 26: 1765-1771.
3. BELL, W. J. 1990. Searching Behavior Patterns in Insects. Annu. Rev. Entomol. 35: 447-467.
4. BLANEY, W. M., M. S. SIMMONDS & S. J. SIMPSON. 1990. Dietary selection behaviour: comparisons between locusts and caterpillars. Symp. Biol. Hung. 39: 47-52.
5. BOND, A. B. 1980. Optimal foraging in a uniform habitat: the search mechanism of the green lacewing. Anim. Behav. 28: 10-19
6. BUDENBERG, W. J. & W. POWELL. 1992. The role of honeydew as an ovipositional stimulant for two species of syrphids. Entomol. Exp. Appl. 64: 57-61.
7. CARTER, M. C., D. SUTHERLAND & A. F. DIXON. 1984. Plant structure and the searching efficiency of coccinellid larvae. Oecologia 63: 394-397.
8. DICKE, M. 1999. Are herbivore-induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods. Entomol. Exp. Appl. 91: 131-142.
9. ELLIOTT, N., KIECKHEFER & D. BECK. 2000. Adult coccinellid activity and predation on aphids in spring cereals. Biol. Control 17: 218-226.
10. FERRAN, A., M. ETTIFOURI, P. CLEMENT & W. J. BELL. 1994. Sources of variability in the transition from extensive to intensive search in coccinellid predators (Homoptera: Coccinellidae). J. Insect Behav. 7: 633-647.
11. FERREN, A. & F. G. DIXON. 1993. Foraging behaviour of ladybirds larvae (Coleoptera: Coccinellidae). Eur. J. Entomol. 90: 383-402.
12. GENDRON, R. P. & J. E. R. STADDON. 1983. Searching for Cryptic Prey: The Effect of Search Rate. Am. Nat. 121: 172-186.
13. HEIT, G., J. CASTRESANA, L. PUHL y G. MAREGGIANI. 2005. Rol de los volátiles emitidos por solanáceas cultivadas y silvestres en el comportamiento de búsqueda de coccinélidos predadores. Idesia 23 (3): 13-20
14. LAMBIN, M., A. FERRAN & K. MAUGAN. 1996. Perception visual information in the ladybird Harmonia axyridis Pallas. Entomol. Exp. Appl. 79: 121-130.
15. MANRIQUE, G., A. VITTA, R. FERREIRA, C. ZANI, C. UNELIUS, C. LAZZARI, L. DIOTAIUTI & M. LORENZO. 2006. Chemical communication in Chagas disease vectors. Source, identity, and potential function of volatiles released by the metasternal and Brindley's glands of Triatoma infestans adults. J. Chem. Ecol. 32: 2035-2052.
16. MISHRA G. & OMKAR. 2004. Diel rhythmicity in certain life events of ladybird, Propylea dissecta (Mulsant). Biol. Rhythm Res. 35: 269-276.
17. MITCHELL, B. K. & P. GREGORY. 1979. Physiology of the maxillary sugar sensitive cell in the red turnip beetle, Entomoscelis americana. J. Comp. Physiol. 132:167-178.
18. MITCHELL, B. K. & G. D. HARRISON. 1984. Characterization of galeal chemosensilla in the adult Colorado beetle, Leptinotarsa decemlineata. Physiol. Entomol. 9: 49-56.
19. NAGNAN-LE MEILLOUR, P., A. H. CAIN, E. JACQUIN-JOLY, M. C. FRANCOIS & S. RAMACHANDRAN. 2000. Chemosensory proteins from the proboscis of Mamestra brassicae. Chem. Senses 25: 541-53.
20. NAKAMUTA, K. 1982. Switchover in searching behavior of Coccinella septempunctata L. (Coleoptera:Coccinellidae) caused by prey consumption. Appl. Entomol. Zool. 17: 501-506.
21. NINKOVIC, V., S. AL ABASSI & J. PETTERSSON. 2001. The influence of aphid-induced plant volatiles on ladybirds Beetle searching behavior. Biol. Control 21: 191-195.
22. OBATA, S. 1986. Mechanism of prey finding in the aphidophagous ladybird beetle, Harmonia axyridis (Coleoptera: Coccinellidae). Entomophaga 31: 303-311.
23. OBRYCKY, J. J. & T. J. KRING. 1998. Predaceus Coccinellidae in Biological Control. Annu. Rev. Entomol. 43: 295-321.
24. SOARES, A., D. CODERRE & H. SCHENDERL. 2004. Dietary self-selection behaviour by the adults of the aphidophagous ladybeetle Harmonia axyridis (Coleptera: Coccinellidae). J. Anim. Ecol. 73: 478-486.
25. SOKAL, R. & F. ROHLF. 1995. Biometry. Third edition. W. H. Friedman and Company, USA.
26. TOURNIAIRE, R., A. FERRAN, J. GAMBIER, L. GIUGE & F. BOUFFAULT. 1999. Locomotor Behaviour of Flightless Harmonia axyridis Pallas (Coleoptera: Coccinellidae). J. Insect Behav. 12: 545-558.
27. WIENS, J.A. 1976. Population Responses to Patchy Environments. Annu. Rev. Ecol. Syst. 7: 81-120.
28. ZAR, J. 1999. Biostatistical Analysis. Fourth Edition, Prentice Hall, New Jersey, USA. [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
Recibido: 29-05-2007;
aceptado: 26-09-2007














