Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista de la Sociedad Entomológica Argentina
versión impresa ISSN 0373-5680versión On-line ISSN 1851-7471
Rev. Soc. Entomol. Argent. v.67 n.3-4 Mendoza jul./dic. 2008
Livestock grazing, habitat protection and diversity of bees and wasps in the Central Monte desert
Ganadería, protección del hábitat y diversidad de abejas y avispas en el desierto del Monte Central
Diego P. Vázquez*, Valeria Aschero and Erica L. Stevani
Instituto Argentino de Investigaciones de las Zonas Áridas, CCT CONICET Mendoza, CC 507, 5500 Mendoza, Argentina; e-mail: dvazquez@mendoza-conicet.gov.ar, vaschero@mendoza-conicet.gov.ar, elstevani@mendoza-conicet.gov.ar
* Instituto de Ciencias Básicas, Universidad Nacional de Cuyo, Centro Universitario, M5502JMA Mendoza, Argentina
ABSTRACT: Reserves aim mainly at preventing or mitigating human impacts on natural ecosystems. It is important to assess how well reserves meet this goal. We evaluated whether habitat protection offered by Ñacuñán Biosphere Reserve (Central Monte desert, Argentina) results in detectable changes in habitat structure and the species richness and composition of bees and wasps. We conducted pan trap sampling and flower visitor observations in six pairs of protected and unprotected sites. Our results suggest that thirty fi ve years of cattle exclusion in Ñacuñán have had detectable effects on habitat structure. However, these changes in habitat structure translated only into partial and conflicting effects on hymenopteran richness, and did not have detectable effects on hymenopteran composition. Our study should be repeated with a greater sampling effort and throughout multiple years before our results can be applied to guide management decisions.
KEY WORDS: Bees; Wasps; Cattle raising; Species composition and richness; Habitat protection.
RESUMEN: El principal objetivo de las reservas es prevenir o mitigar los impactos humanos sobre los ecosistemas naturales. Es importante evaluar cuán bien las reservas alcanzan este objetivo. Evaluamos si la protección del hábitat que brinda la Reserva de la Biósfera de Ñacuñán (Monte Central, Argentina) resulta en cambios detectables en la estructura del hábitat, y en la riqueza y la composición de especies de abejas y avispas. Realizamos muestreos con trampas bandeja y observaciones de visitantes florales en seis pares de sitios dentro y fuera de la reserva. Nuestros resultados sugieren que los treinta y cinco años de exclusión del ganado vacuno en Ñacuñán han tenido efectos detectables sobre la estructura del hábitat. Sin embargo, estos cambios en el hábitat se tradujeron sólo en efectos parciales y conflictivos sobre la riqueza de himenópteros, y no tuvieron efectos detectables sobre la composición de himenópteros. Nuestro estudio debería repetirse en el futuro, con un mayor esfuerzo de muestreo y a lo largo de varios años antes que estos resultados puedan ser aplicados como guía de decisiones de manejo.
PALABRAS CLAVE: Abejas; Avispas; Ganadería; Composición y riqueza de especies; Protección del hábitat.
Recibido: 03-03-2008;
Aceptado: 12-06-2008
INTRODUCTION
Nature reserves are usually created to prevent or mitigate the widespread impact of human activities such as agriculture, logging and livestock grazing in natural ecosystems. Thus, it is important to evaluate how well reserves meet their goal. However, although reserves may help the conservation of target habitats or species of special concern, they are probably too few, small and isolated to represent a sufficient solution for the preservation of biodiversity, making it necessary to incorporate human-dominated landscapes into conservation strategies.
Under proper management practices, natural habitats under livestock grazing and other types of human impacts may indeed be adequate for an integrative strategy to biodiversity conservation (Gómez-Aparicio et al., 2004; Steffan-Dewenter et al., 2006; Kremen et al., 2007; Winfree et al., 2007). Human-dominated ecosystems may be important for conservation in their own right. In the specific case of cattle raising, there is much controversy around its impact on natural ecosystems, particularly in arid regions (Fleischner, 1994; Noss, 1994; Wuerthner, 1994; Brown & McDonald, 1995; Bestelmeyer & Wiens, 2001; Tabeni& Ojeda, 2005). Most livestock species are exotic to the ecosystems in which they graze, and thus can be seen as a widespread human-caused, chronic disturbance.
Alternatively, because many of the original native grazers-under whose influence native species have evolved-have become much rarer than in the past or have gone extinct, the presence of livestock may resemble more the original disturbance regime than their absence. Regardless of our stand in this debate, it is again important to know how human activities and their exclusion influence the structure and dynamics of ecological systems.
Bees and wasps (Hymenoptera) comprise an important group of pollinators and potential enemies of insect pests and are particularly abundant and diverse in arid regions (Michener, 1979, 2000). They perform important community processes and ecosystem services as pollen vectors, predators and parasites. Variation in the diversity of bees and wasps can be related to changes in the structure and abundance of floral and nesting resources (Gess & Gess, 1991; Samejima et al., 2004; Lassau & Hochuli, 2005; Potts et al., 2005; Loyola & Martins, 2008). Browsing and trampling by livestock can contribute to changes in vegetation and habitat structure, and a reduction of grazing pressure through habitat protection can affect the diversity of bees and wasps (Kruess & Tscharntke, 2002; Sjödin, 2007).
Ñacuñán Biosphere Reserve (Mendoza, Argentina) provides an excellent opportunity to evaluate the effectiveness of habitat protection from livestock grazing for biodiversity conservation. Located at the heart of the Central Monte Desert, Ñacuñán is a 12,000 ha rectangular reserve with 35 years of exclusion of human activities, surrounded by active cattle ranches (Boshoven et al., 2001). Here we ask whether habitat protection offered by the reserve results in detectable changes in habitat structure, and whether such changes, if observed, affect in turn the species richness and composition of bees and wasps.
MATERIAL AND METHODS
Study area and sites
Our study region lies within the Central Monte desert of Argentina (Cabrera, 1971; Roig-Juñent et al., 2001). The climate is semi-arid and most rainfall occurs in the spring and summer (October-March). We worked in Ñacuñán Biosphere Reserve (34°02' S, 67°58' W) and surrounding areas. Average annual rainfall in the region is 280 mm (Estrella et al., 2001). Grazing has been excluded from the reserve since 1972, but has continued outside (Boshoven et al., 2001).
Although we have no good estimates of cattle burden in these ranches, we have repeatedly seen cattle and their signs of activity (e.g., feces, tracks) in our non-reserve study sites (see below). Logging of native species has been banned in the whole Province for many decades. The algarrobo woodland is the most representative community, in which Prosopis flexuosa is accompanied by the small tree Geoffrea decorticans and several shrub (Larrea divaricata, Condalia microphylla, Capparis atamisquea, Lycium spp.) and grass species (Pappophorum spp., Trichloris crinita, Digitaria californica; Roig, 1971). Trees and most shrubs in the area are insect-pollinated (D. P. Vázquez and V. Aschero, personal observation). We worked in six pairs of protected and unprotected sites, each pair consisting of two 1-ha plots, one inside and one outside the reserve, at least 1 km away from the fence marking its limit. Distance between paired plots was smaller than distance within pairs.
Habitat structure: plant cover and composition, floral abundance and soil compactness
To characterize the effects of cattle on habitat structure we estimated overall plant cover and composition, floral abundance and soil compactness. The latter is relevant to determine habitat structure because many of the hymenopteran species in our study nest in the ground, and soil compactness could thus affect their nesting success. Vegetation cover is relevant because it may affect soil humidity and temperature (again infl uencing ground-nesting insects) and it may affect nesting sites for wood-nesting insects (e.g., greater cover could mean greater availability of nesting sites). Plant composition could also influence nesting site availability for some species (wood-nesting species). Finally, flower abundance is a measure of resource availability for flower-visiting insects (all bees and some wasps). Although these four components of habitat structure are arguably phenomenological, in the sense that we are not measuring the nesting and feeding requirements of each hymenopteran species in our study, this is the best we can do in a community-wide study involving many species.
In each 1-ha plot we ran two transects crossing each other at the center of the plot. In each transect we estimated percent cover for each plant species and bare ground by direct observation in 20 m × 20 m quadrats every fi ve meters (i.e., a total of 39 quadrats per plot, because the two transects overlapped in the center). We also counted the number of flowering individuals per quadrat of each animal-pollinated plant species as an estimate of overall floral abundance. Floral abundance was estimated periodicaly, at the same dates in which flower visitor sampling was conducted (see below).
To assess soil compactness, we measured soil penetration resistance using a penetrometer device. Penetration resistance was defined as the number of hits needed for the device to penetrate 5 cm belowground. Penetration resistance was measured at each plot at twenty sampling points regularly spaced along 4 equidistant parallel transects.
Pan trap sampling
We conducted pan trap samplings of hymenopterans in four different occassions during the spring of 2005 (late October, early and late November, and mid December).
On each sampling date, nine pan traps were placed in each 1-ha plot to collect bees and wasps. Traps were placed at regular distances along the two transects used for plant sampling, and were exposed approximately from 8 am until 6 pm, when trapped specimens were collected from the traps. Each trap unit consisted of three small blue, white and yellow plastic trays, filled with water with a drop of detergent as surfactant.
Flower visitor sampling
We sampled insects visiting fl owers of the most common entomophilous plant species every 15-20 days throughout the 2005 fl owering season (October-December). Paired sites were sampled simultaneously by different observers in the morning (10:00-13:00 h) and the afternoon (15:00-18:00 h).
Observers were switched between sites in each sampling period to avoid observer bias. At each plot, the observer used an aspirator, a sweep net or a killing jar with ethyl acetate to sample all insects visiting during 5 min a group of flowers or inflorescences of the study plant species: Capparis atamisquea (Capparaceae), Condalia microphylla (Rhamnaceae), Larrea divaricata and L. cuneifolia (Zygophilaceae), and Prosopis flexuosa (Fabaceae); these five species represented the bulk of the insectpollinated flowers available in Ñacuñán during the study period. Individual plants were selected haphazardly for sampling, attempting to sample as many flowering individuals as possible in different locations within each plot, so as to minimize spatial autocorrelation among samples.
Data analyses
To evaluate if floral abundance and soil compactness differed between reserve and non-reserve sites we used generalized linear mixed model analysis, with "protection" as a fixed factor with two levels (reserve and non-reserve), "site" as a random factor with six levels (sites 1-6), a Poisson error distribution and a log link function. Analyses were conducted using the lmer function in the lme4 package of R statistical software, version 2.4.0 (http://www.r-project.org). We used Akaike's information criterion (AIC) to compare the fit of mixed models with and without the interaction between fixed and random factors. The model with the interaction term was considered superior only if its associated AIC value was at least two units lower than the AIC associated to the model without interaction (Burnham& Anderson, 2002). We also checked model deviance for overdispersion, using the "estimated scale" parameter, Φ, returned by lmer (i.e., the model residual deviance over the residual degrees of freedom), which indicates departure from the assumed Poisson distribution of errors (Crawley, 2005). A model is overdispersed if Φ>1, and underdispersed if Φ<1. When for a given model we found a substantial departure from one in the value of Φ, we re-ran the analysis using the quasi-Poisson distribution (by setting family=quasipoisson in the lmer function call).
Cover was compared between reserve and non-reserve sites with a paired t-test; this design is appropriate because there was only one cover estimate per plot. We conducted sample-based rarefaction to evaluate if species richness differed between reserve and non-reserve sites, rescaling richness estimates to individuals (Gotelli & Colwell, 2001). To compare species composition we used non-metric multidimensional scaling (MDS), and evaluated the significance of site ordination with Mantel permutation tests (Legendre & Legendre, 1998; Vázquez & Simberloff, 2003), reporting the Mantel statistic for the original data (ro) and its 95% permutation confidence interval (rp). Rarefaction analyses and Mantel permutations were conducted with ad-hoc functions written in R (http://www.r-project.org) and Octave (http://www.octave.org), respectively, available from the authors upon request. MDS analyses were conducted using the isoMDS in the MASS package of R.
RESULTS
Soil hardness tended to be greater outside than inside the reserve (Fig. 1). The AIC for the model without interaction was 99.52, whereas that for the model with interaction was 99.8. Thus, the inclusion of the interaction term does not improve model fi t, and we can conclude that the effect of the interaction is not statistically significant. The main effect of management in the generalized mixed model without interaction was statistically significant (z=7.003, P<0.0001). The estimated scale for this model was Φ=0.59, thus indicating underdispersion. To account for this departure from the assumption that Φ=1, we ran the model again with the quasi-Poisson distribution; this change in the distribution did not affect parameter estimates and their associated significance, suggesting that the effect of management on soil hardness was indeed significant.

Fig. 1. Soil hardness in each study site. Site pairs 1-6 are shown from left to right. Grey boxes, reserve sites; white boxes, grazed sites outside the reserve.
Floral abundance did not differ significantly between reserve and non-reserve sites (mixed model with interaction: z=0.731, P=0.465, Φ=1.01). Plant cover was significantly greater inside than outside the reserve (paired t-test: t=3.26, P<0.05; Fig. 2). Site ordination in terms of plant composition separated reserve and non-reserve sites, although the trend was non-significant, as the Mantel statistic calculated for the original data falls within the permutation confidence interval (Fig. 3).

Fig. 2. Plant cover in each study site. Site pairs and colors as in Fig. 1.
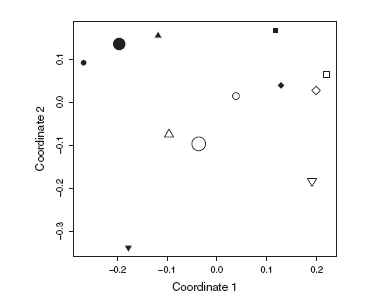
Fig. 3. Reduced-space plot of fi rst two coordinates resulting from nonmetric multidimensional scaling (MDS) of plant species composition. Black symbols, reserve sites; white symbols, non-reserve sites. Symbol shape indicates site pair. Permutation test to evaluate statistical significance of reserve protection on composition: Mantel statistic for observed data: ro=-0.13; permutation confi dence interval: rp=[-0.24,0.24].
Eighty-six species of Hymenoptera were collected in our study sites (Appendices I and II). Rarefied richness of Hymenoptera caught in pan traps inside the reserve was equal or greater than outside the reserve (Fig. 4a). In contrast, rarefied richness of flower visitors in reserve sites was equal or lower than outside the reserve, except for site 1, where richness was greater inside the reserve (Fig. 4b). Species composition of Hymenoptera did not show a consistent, statistically significant pattern between reserve and non-reserve sites, for both Hymenoptera caught in pan traps and flower visitors (Fig. 5).

Fig. 4. Comparison of species richness between reserve (black circles) and non-reserve (white circles) sites. Error bars indicate confidence intervals of rarefied richness for the most abundant sample in each pair (see methods). (a) Hymenoptera from pan traps. (b) Flower visitors.

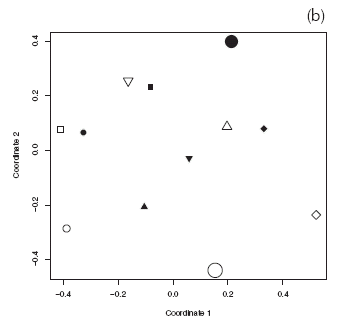
Fig. 5. Reduced-space plot of first two coordinates resulting from nonmetric multidimensional scaling (MDS) of insect species composition. (a) Hymenoptera from pan traps. (b) Flower visiting Hymenoptera. Black symbols, reserve sites; white symbols, non-reserve sites. Symbol shape indicates site pair. Permutation test to evaluate statistical significance of reserve protection on composition: pan traps: ro=0.07; rp=[-0.24,0.24]; flower visitors: ro=0.03; rp=[-0.25,0.24].
DISCUSSION
Our results suggest that thirty five years of cattle exclusion in Ñacuñán Biosphere Reserve have had detectable effects on habitat structure. Soil inside the reserve appears to be less compact and with greater plant cover than in neighboring non-reserve sites. Because many species of bees and wasps nest in the ground, these differences in soil compactness and cover can indeed be interpreted as ecologically meaningful habitat differences. There was also a trend towards differing plant composition which, from the perspective of bees and wasps, could also mean differences in habitat quality and structure, but not in flower abundance.
Changes in habitat structure translated into partial and conflicting effects on hymenopteran richness and did not have detectable effects on hymenopteran composition. Whereas pan trap results suggest that the reserve has led to a modest increase of hymenopteran richness inside the reserve, the flower visitor results suggest the opposite trend. It is reasonable to expect differences in the species composition of samples from multiple sampling methods, given that each has its own bias and works best for specific groups of Hymenoptera (Kearns & Inouye, 1993; Southwood & Henderson, 2000). The latter is likely to be true for our two sampling methods; in fact, only 46% of Hymenoptera visiting flowers were also caught in pan traps.
However, there is no reason to expect different trends in the responses of these different sets of species to a particular environmental effect, unless the sampling bias correlates with some sort of ecological or life history traits that can influence species' responses. For example, if species trapped with one technique used a different set of resources than those trapped with the other technique, and if resources were differently affected by habitat protection, then different responses of the groups associated to these two methods would be expected. The limited data available on the natural history of the Hymenoptera of the Central Monte do not allow us to assess the validity of these speculations.
Our results should be interpreted with caution if used to guide management decisions. First, we have data for only one year, which may not be representative of what happens in the long term, given the great seasonal and interannual variability of ecological systems. Second, even though we sampled repeatedly throughout the spring, our sampling effort may not have been sufficiently large to detect many rare species, making our richness and composition estimates incomplete. In fact, lack of completeness of the richness estimates is suggested by the rather steep, non-saturating rarefaction curves (not shown). Despite these limitations, it is at least possible that our results are indeed a reflection of reality and that the reserve creation and its associated habitat changes have had little effect on the hymenopteran fauna. Only future studies, conducted over multiple years and with greater sampling effort, will allow this question to be answered.


ACKNOWLEDGEMENTS
This work was supported by a grant from Fundación BBVA (BIOCON03-162), which included a graduate fellowship for VA. Special thanks to Arturo Roig-Alsina for his invaluable help with insect identification. Guillermo Debandi made useful suggestions on the manuscript, and Florencia Fernández Campón, Santiago Cartier, Verónica Herrero, Mariano Mendoza, Emilio Pérez Pascal and Ramiro Ovejero helped with field and laboratory work.
LITERATURE CITED
1. BESTELMEYER, B. & J. WIENS. 2001. Ant biodiversity in semiarid landscape mosaics: The consequences of grazing vs. natural heterogeneity. Ecological Applications 11: 1123-1140. [ Links ]
2. BOSHOVEN, J., M. TOGNELLI, S. CLAVER & S. ROIGJUÑENT (eds.). 2001. El Desierto del Monte: La Reserva de Biósfera de Ñacuñán. IADIZA-CONICET, MAB-UNESCO. Editorial Triunfar, Córdoba. [ Links ]
3. BROWN, J. H. & W. MCDONALD. 1995. Livestock grazing and conservation on southwestern rangelands. Conservation Biology 9: 1644-1647. [ Links ]
4. BURNHAM, K. P. & D. R. ANDERSON. 2002. Model Selectionand Multimodel Inference: A Practical Information-Theoretic Approach. Springer, New York. [ Links ]
5. CABRERA, A. L. 1971. Fitogeografía de la República Argentina. Boletín de la Sociedad Argentina de Botánica 14: 1-42. [ Links ]
6. CRAWLEY, M. J., 2005. Statistics: An Introduction Using R. John Wiley & Sons, Chichester. [ Links ]
7. ESTRELLA, H., J. BOSHOVEN & M. TOGNELLI. 2001. Características del clima regional y de la reserva de Ñacuñán. In: Boshoven, J., M. Tognelli, S. Claver & S. Roig-Juñent (eds.), El Desierto del Monte: La Reserva de Biósfera de Ñacuñán, IADIZA-CONICET, MABUNESCO. Editorial Triunfar, Córdoba, pp. 25-33. [ Links ]
8. FLEISCHNER, T. L. 1994. Ecological costs of livestock grazing in western North America. Conservation Biology 8: 629-644. [ Links ]
9. GESS, F. & S. GESS. 1991. Effects of increasing land utilization on species representation and diversity of aculeate wasps and bees in the semi-arid areas of Southern Africa. In: Lasalle, J. & I. D. Gauld (eds.), Hymenoptera and Biodiversity, CAB International. pp. 83-113. [ Links ]
10. GÓMEZ-APARICIO, L., R. ZAMORA, J. M. GÓMEZ, J. A. HÓDAR, J. CASTRO, & E. BARAZA. 2004. Applying plant facilitation to forest restoration: A meta-analysis of the use of shrubs as nurse plants. Ecological Applications 14: 1128-1138. [ Links ]
11. GOTELLI, N. J. & R. K. COLWELL. 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379-391. [ Links ]
12. KEARNS, C. A. & D. W. INOUYE. 1993. Techniques for pollination biologists. University Press of Colorado, Niwot, Colorado. [ Links ]
13. KREMEN, C., N. M. WILLIAMS, M. AIZEN, B. GEMMILLHERREN, G. LEBUHN, R. MINCKLEY, L. PACKER, S. G. POTTS, T. ROULSTON, I. STEFFAN-DEWENTER, D. P. VÁZQUEZ, R. WINFREE, L. ADAMS, E. E. CRONE, S. S. GREENLEAF, T. H. KEITT, A. KLEIN, J. REGETZ, & T. H. RICKETTS. 2007. Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land use change. Ecology Letters 10: 299-314. [ Links ]
14. KRUESS, A. & T. TSCHARNTKE. 2002. Grazing intensity and the diversity of grasshoppers, butterflies, and trapnesting bees and wasps. Conservation Biology 16: 1570-1580. [ Links ]
15. LASSAU, S. & D. HOCHULI. 2005. Wasp community responses to habitat complexity in Sydney sandstone forests. Austral Ecology 30: 179-187. [ Links ]
16. LEGENDRE, P. & L. LEGENDRE. 1998. Numerical Ecology. Elsevier, Amsterdam, 2nd ed. [ Links ]
17. LOYOLA, R. D. & R. P. MARTINS. 2008. Habitat structure components are effective predictors of trap-nesting Hymenoptera diversity. Basic and Applied Ecology, in press. [ Links ]
18. MICHENER, C. D. 1979. Biogeography of bees. Annals of the Missouri Botanical Garden 66: 277-347. [ Links ]
19. MICHENER, C. D. 2000. The Bees of the World. Johns Hopkins University Press, Baltimore, Maryland [ Links ]
20. NOSS, R. F., 1994. Cows and conservation biology. Conservation Biology 8: 613-616. [ Links ]
21. POTTS, S., B. VULLIAMY, S. ROBERTS, C. O'TOOLE, A. DAFNI, G. NE'EMAN, & P. WILLMER. 2005. Role of nesting resources in organising diverse bee communities in a mediterranean landscape. Ecological Entomology 30: 78-85. [ Links ]
22. ROIG, F. A., 1971. Flora y vegetación de la reserva forestal de Ñacuñán. Deserta 1: 21-239. [ Links ]
23. ROIG-JUÑENT, S., G. FLORES, S. CLAVER, G. DEBANDI, & A. MARVALDI. 2001. Monte Desert (Argentina): insect biodiversity and natural areas. Journal of Arid Environments 47: 77-94. [ Links ]
24. SAMEJIMA, H., M. MARZUKI, T. NAGAMITSU,& T. NAKASIZUKA. 2004. The effects of human disturbance on a stingless bee community in a tropical rainforest. Biological Conservation 120: 577-587. [ Links ]
25. SJÖDIN, N. E. 2007. Pollinator behavioural responses to grazing intensity. Biodiversity and Conservation 16: 2103-2121. [ Links ]
26. SOUTHWOOD, T. E. & P. A. HENDERSON. 2000. Ecological Methods. Blackwell, Oxford. [ Links ]
27. STEFFAN-DEWENTER, I., A.-M. KLEIN, V. GAEBELE, T. ALFERT, & T. TSCHARNTKE. 2006. Bee diversity and plant-pollinator interactions in fragmented landscapes. In: Waser, N. M. & J. Ollerton (eds.), Plant-pollinator interactions: from specialization to generalization, University of Chicago Press, pp. 387-407. [ Links ]
28. TABENI, S. & R. OJEDA. 2005. Ecology of the Monte desert small mammals in disturbed and undisturbed habitats. Journal of Arid Environments 63: 244-255. [ Links ]
29. VÁZQUEZ, D. P. & D. SIMBERLOFF. 2003. Changes in interaction biodiversity induced by an introduced ungulate. Ecology Letters 6: 1077-1083. [ Links ]
30. WINFREE, R., T. GRISWOLD, & C. KREMEN. 2007. Effect of human disturbance on bee communities in a forested ecosystem. Conservation Biology 21: 213-223. [ Links ]
31. WUERTHNER, G. 1994. Subdivisions versus agriculture. Conservation Biology 8: 905-908. [ Links ]














