Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de la Sociedad Entomológica Argentina
Print version ISSN 0373-5680On-line version ISSN 1851-7471
Rev. Soc. Entomol. Argent. vol.67 no.3-4 Mendoza July/Dec. 2008
Effect of temperature on the development time and survival of preimaginal Culex hepperi (Diptera: Culicidae)
Efecto de la temperatura sobre el tiempo de desarrollo y la supervivencia preimaginal de Culex hepperi (Diptera: Culicidae)
María Verónica Loetti, Nora E. Burroni, Paula Prunella and Nicolás Schweigmann
Grupo de Estudio de Mosquitos, Departamento de Ecología Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Pabellón II, Ciudad Universitaria, (C1428EHA), Ciudad Autónoma de Buenos Aires, Argentina; e-mail: vloetti@ege.fcen.uba.ar
RESUMEN: El objetivo de este trabajo fue analizar el efecto de la temperatura sobre el tiempo de desarrollo y la supervivencia preimaginal de Culex (Culex) hepperi Casal y García, 1967. Los individuos fueron criados en laboratorio, desde el primer estadio larval hasta la emergencia del adulto, a cinco temperaturas constantes: 15, 20, 25, 30 y 33°C. El tiempo total de desarrollo se relacionó de manera inversa con la temperatura entre 15 y 25°C. No se detectaron diferencias entre sexos en el tiempo de desarrollo y ningún individuo alcanzó el estado adulto a 33°C. En los estadios larvales III y IV, el tiempo requerido para mudar al siguiente estadio fue mayor a 30°C. La supervivencia más alta se registró a 20°C. Los estadios más avanzados fueron menos resistentes a las temperaturas por encima y por debajo de los 20°C. De acuerdo con el modelo no lineal de Briére, los umbrales de desarrollo inferior y superior fueron 2.6 y 33°C, respectivamente. Nuestros resultados sugieren que el efecto de la temperatura depende del estadio de desarrollo de C. hepperi.
PALABRAS CLAVE: Mosquito; Phytotelmata; Temperatura constante.
ABSTRACT: The aim of this research was to evaluate the effect of temperature on the development time and survival of Culex (Culex) hepperi Casal and García, 1967. Individuals were reared in the laboratory, from the first larval stage to adult emergence, at five constant temperatures: 15, 20, 25, 30, and 33°C. The total development time was inversely related to temperature between 15 and 25°C. No differences were observed in the development time between sexes and no adults emerged at 33°C. In the larval stages III and IV, the time required for molting to the next stage increased at 30°C. The highest survival was recorded at 20°C. The more developed stages were less resistant to temperatures above and below 20°C. According to the nonlinear model of Briére, the lower and upper development thresholds were 2.6 and 33°C, respectively. Our results suggest that the effect of temperature depends upon the stage of development of C. hepperi.
KEY WORDS: Mosquito; Phytotelmata; Constant temperature.
Recibido: 05-08-2008;
Aceptado: 10-11-2008
INTRODUCTION
The biological performance of preimaginal mosquitoes results from the interaction between the intrinsic attributes of the species and the environmental conditions of the breeding site, with temperature being one of the most important abiotic factors affecting the development, growth, and survival of immatures (Clements, 1992). There is a positive linear relationship between temperature and developmental rate of preimaginal stages of mosquitoes within a certain thermal range, and the relationship becomes sigmoidal when a wider range is considered. Many nonlinear models have been proposed to describe the relationship in the preimaginal stages of arthropods. These models vary with respect to parameter number and basic assumptions about the temperature effect near the threshold. Some of them are descriptive models that allow a descriptive interpretation of parameters (e.g. Logan et al., 1976; Briére et al., 1999); others are biophysical models based on enzyme reaction rates theory (Sharpe & DeMichele, 1977; Schoolfi eld et al., 1981). On the other hand, the optimum temperature for development does not necessarily coincide with that for survival. There are several reports of the effect of breeding temperature on development time and survival of different immature mosquito species (e.g. Rueda et al., 1990; Su & Mulla, 2001). Knowledge of the effect of temperature on the development time and survival of the different mosquito species provides baseline information essential for studies on population dynamics, spatialtemporal distribution and epidemiology.
Culex (Culex) hepperi Casal and García, 1967 is a phytotelmic species whose immature stages have been found only in the leaf axils of unidentifi ed species of Eryngium sp. (Belkin et al., 1968; Casal & García, 1967a) and Eryngium pandanifolium Cham. and Schlecht (Campos & Lounibos, 1999). So far, C. hepperi is one of the three species of the genus Culex (Culex) that breed exclusively in the axils of Eryngium species in Argentina (Casal & García, 1967a; Casal & García, 1967b). In Argentina, studies on mosquitoes breeding in phytotelmata are scarce (e.g. Marti et al., 2007; Campos & Lounibos, 1999). In particular for C. hepperi, little is known about its distribution (Mitchell & Darsie, 1985; Campos & Maciá, 1998; Belkin et al., 1968) and ecology (Campos & Lounibos, 1999), and there is no available information on the influence of temperature on the development time and survival of its preimaginal stages.
Consequently, the objective of the present study is to evaluate the effect of the breeding temperature on the development time and survival of immatures of C. hepperi under experimental conditions. The hypothesis tested is that breeding temperature affects development time and survivorship of C. hepperi. It was predicted that within the range of temperatures tested, the immatures of C. hepperi reared at cooler temperatures take longer to emerge as adults than those reared at warmer temperatures. We also expected greater survival at intermediate temperatures than at extreme ones.
MATERIAL AND METHODS
Mosquito collection and rearing. Mosquito eggs used in this study were collected from the leaf axils of Eryngium sp. growing naturally in the Delta of the Paraná River (34º11'S, 58º29'W), Argentina. The region is characterized by a mean annual temperature of 17°C, mean temperatures ranging between 6 and 30°C and a mean annual precipitation of 931 mm. Egg rafts were maintained until eclosion in individual plastic containers with tap water. All eggs were collected between April-June 2003, and during the same period the treatments were conducted. Within 12 h of eclosion, between 10 and 15 first instar larvae were randomly selected from each raft and individually placed in labeled plastic cups (3 cm in diameter, 5 cm high) with tap water. They were reared until adult emergence in thermal baths (Loetti et al., 2001) at one of the following constant temperatures: 15, 20, 25, 30 and 33ºC; the total number of first instar larvae tested in each one was: 65, 48, 37, 68 and 84, respectively. Larvae were kept under a photoperiod of L14:D10 and fed according to Gerber's methodology (1970) for Culex pipiens L.
The presence of exuviae, mortality and date of imago emergence were recorded daily at the same hour of the day to estimate the development time and survival of each immature stage (ecdysis to ecdysis). The adults were killed by freezing (-18ºC), and then sexed under stereoscopic microscope. Fourth-instar exuviae and females were determined using keys to confirm species identity (Darsie, 1985; Rossi et al., 2002) and species description (Casal & García, 1967a).
Data analysis. Differences in the development time (in days) of each immature stage among different experimental temperatures were tested with the nonparametric Kruskal-Wallis test, and the Dunn test was used for post-hoc comparisons (Zar, 1999). For each temperature, the total development time was compared between sexes using the Mann-Whitney U-test (Zar, 1999).
The nonlinear model of Briére (Briére et al., 1999) was used to quantify the relationship between temperature and development rate. The expression of the Briére model is: R(T) = aT(T - T0)(TL - T)1/m
R(T) is the rate of development (1/development time) at temperature T, T = temperature (°C), T0 = low-temperature development threshold (lower threshold), TL = lethal temperature (upper threshold), a = empirical constant and m = shape parameter interpreted as the capacity of an insect species to develop and survive at high temperatures. This capacity is greater when m < 2. The parameters of this model were estimated from the total development rate (the reciprocal of time required for adult emergence) recorded for each experimental temperature.
For each temperature, the overall survival was calculated as the number of individuals that reached the adult stage divided by the number of larvae I at the beginning of each treatment. The survival of each preimaginal stage was calculated as the number of individuals surviving to the next stage divided by the number of survivors in the previous stage. The results obtained from each trial were compared using the test of proportions for independent samples (Fleiss, 1981).
RESULTS
1. Preimaginal development time. Culex hepperi showed a significant decrease in the number of days required for total development between 15-25°C, but no significant decrease was recorded between treatments of 25 and 30°C (Table I). The relationship between the breeding temperature and the development time varied among the different immature stages. Between 15 and 25°C the time required for molting to the next stage decreased gradually with temperature. Thus, for all stages the development time at 25°C was between one-half and one-fourth that at the lowest temperature (15°C). However, at the warmest temperatures (30 or 33°C) only the development time of first instar larvae continued diminishing, while the time of larvae III and IV showed a signifi cant increase (Table I).
Table I. Duration of the development stages (days) of Culex hepperi at five constant temperatures.
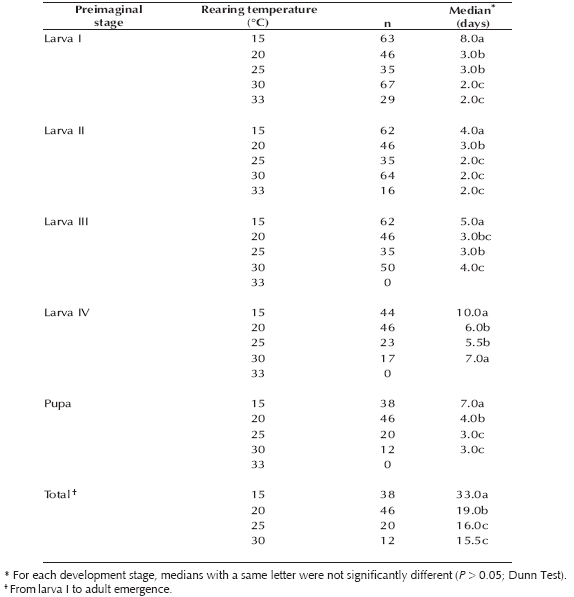
The development time of males and females was similar regardless of the breeding temperature (15°C: U (N: 8, N: 11) = 43.00, 20°C: U (N: 20, N: 26) = 248.50, 25°C: U (N: 10, N: 10) = 31.00, 30°C: U (N: 5, N: 7) = 12.00; P > 0.1).
Fitting to the Briére model. The development rate of C. hepperi as a function of the experimental temperatures (15-33°C) was very well described by the model (Figure 1; R2 = 0.878; P < 0.001). The lethal temperature (T L ) estimated by the model was 33.7°C, which was higher than the temperature to which there was not adults emergency (33ºC). Thus, the model was fitted with the upper threshold temperature (TL) determined empirically (33°C). The lower threshold temperature (T0) estimated by the model was 2.3°C, and values of parameters a and m were 4.5169*10-5 and 2.1957, respectively. The estimated optimum temperature was 27.1°C, with a development time of 14.7 days.
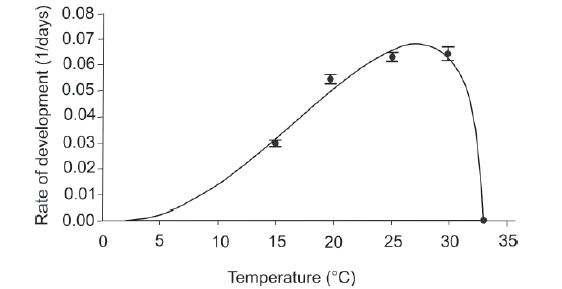
Fig. 1. Temperature-dependent rate (mean±SEM) of development of Culex hepperi from first instar larva to adult emergence: Observed data (•) fitted to the Briére model (solid line).
2. Preimaginal survival. The proportion of larvae I of C. hepperi reaching the adult stage varied according to the different experimental temperatures ( 2 = 70.299; d.f. = 3; P < 0.001). The highest survival was recorded at 20°C ( 2 = 44.913; d.f. = 1; P< 0.001), with 96% of larvae I (46/48) reaching the adult stage. Survival was about 55% at 15 and 25°C (38/65 and 20/37, respectively), and this value was significantly higher than the one recorded at 30°C ( 2 = 25.904; d.f. = 1; P < 0.001), with only 18% of larvae I (12/68) reaching the adult stage. No adult emergence was observed at 33°C.
The effect of the breeding temperature on survival varied among different developmental stages. More than 90% of larvae I survived between 15-30°C, and this same pattern was observed for larvae II; these were the only stages that molted at 33°C. The survival of larvae III was high between 15-25°C and decreased significantly at 30°C. The survival of larvae IV and pupae was 100% only at 20°C, whereas it decreased significantly for the rest of the temperatures (Fig. 2)
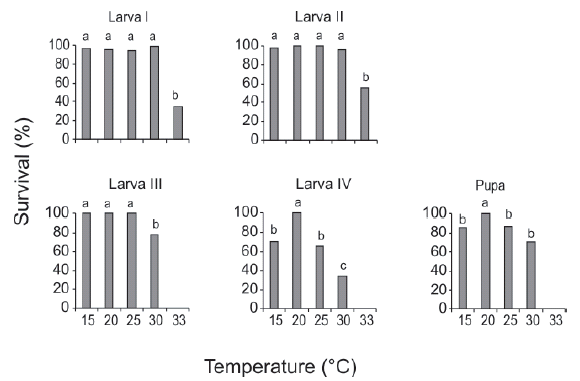
Fig. 2. Effect of temperature on the survival of preimaginal stages of Culex hepperi. The survival was calculated as the number of individuals surviving to the next stage divided by the number of survivors in the previous stage. Different letters indicate significant differences only within the individual plots (P < 0.05).
DISCUSSION
Our results indicate that the time required for C. hepperi to reach the adult stage from larva I is inversely related to water temperature within the range 15-25ºC, but not above that range. A similar relationship has been reported for different mosquito species (e.g. Brust, 1967; Rueda et al., 1990). This pattern of progressive decrease in development time with increasing temperature seems to change for larval stages III and IV, which showed a longer development time at 30°C than at lower temperatures. According to Sharpe & DeMichele (1977), extreme temperatures affect the catalysis of enzymes involved in the development of poikilotherms. In this sense, our results could be interpreted as an indirect evidence of the effect of unsuitable thermal conditions on the enzymatic activity during immature stage development. On the other hand, this laboratory result could indicate a limited adaptation of C. hepperi to zones of very warm temperatures, an assumption that seems to agree with the distribution of this species (Mitchell & Darsie, 1985; Campos & Maciá, 1998).
Both sexes had the same development time within the temperature range studied. In mosquitoes, males usually emerge before females (Christophers, 1960; Clements, 1992), a phenomenon known as protandry. The no observation of protandry in C. hepperi could be either a specific feature like in Culiseta inornata (Williston, 1893) and Aedes nigromaculis (Ludlow, 1906) (Brust, 1967), or simply an artifact resulting from the experimental conditions (constant temperature and photoperiod), as previously observed for Culex apicinus Philippi (Loetti et al., 2007).
The nonlinear model of Briére indicated that the highest development rate occurs between 25-30°C, with the optimum temperature being 27.1°C. According to the values of the lower and upper threshold temperatures (2.3 and 33°C, respectively), this species seems to tolerate a relatively wide thermal range. Our laboratory observations agree with the field observations reported by Campos & Lounibos (1999), who collected immatures of C. hepperi every month for a year in a nearby locality at the south of the Delta of the Paraná River (ca. 150 km).
Although this species could develop allyear-round at least at this latitude (34°S), the estimated value of m parameter (2.20) suggested that low temperatures seem to be the most favorable for immatures of C. hepperi. As for survival, 20°C appears to be the optimum temperature for C. hepperi based on the highest proportion of immatures that reached the adult stage. Survival values of each immature stage showed a significant decrease above and below 20°C for the more advanced stages (larva IV and pupa). In addition, only larvae I and II succeeded in molting at 33°C. The tolerance of mosquitoes to high temperatures tends to decrease with development, and this fact depends on the exposure time and the mosquito species (Forattini, 1962). Our results suggest that the different immature stages tolerate different temperatures, with the more advanced stages showing the lowest tolerance to nonoptimal temperatures. However, we should be cautious about the interpretation of this finding because the effect of exposure time to high temperatures was not evaluated in the present study.
In brief, the most favorable temperatures for C. hepperi immatures in terms of highest survival and development rate seem to be in the range of 20-27.1°C. There were harmful effects at 30°C and lethal at 33°C. Although under natural conditions water temperatures may not remain high (i.e., 30-33°C) for a long period of the day, data obtained from this laboratory study could be used as indicators of the capacity of C. hepperi to tolerate different breeding temperatures. These results can be of interest in the face of a climatic change scenario. Shifts in the physiology, distribution and phenology of some species seem to be explained by the current climatic and atmospheric trends (Hughes, 2000). If the warm temperatures have a negative effect on the preimaginal biology of this species, then an increase of the ambient temperature could cause a displacement of its distribution toward higher latitudes. Clearly, a better knowledge about this phytotelmic mosquito and its habitat is needed to assess these topics.
ACKNOWLEDGEMENTS
We wish to thank Agnes Clarke de Loetti and Silvia Pietrokovsky for their helpful comments on an earlier version of the manuscript. This paper is dedicated to Dra. Alicia B. de Garín, in memoriam. This work was supported by the UBACyT X-187 Project from Universidad de Buenos Aires (Science and Technology Secretary).
LITERATURE CITED
1. BELKIN, J. N., R. X. SCHICK & S. HEINEMANN. 1968. Mosquito studies (Diptera, Culicidae) XI. Mosquitoes originally described from Argentina, Bolivia, Chile, Paraguay, Peru, and Uruguay. Contr. Am. Entomol. Inst. 4: 9-29. [ Links ]
2. BRIÉRE, J. F.; P. PACROS; A. Y. LE ROUX & J. S. PIERRE. 1999. A novel rate of temperature-dependent development for arthropods. Environ. Entomol. 28: 22-29. [ Links ]
3. BRUST, R. A. 1967. Weight and development time of different stadia of mosquitoes reared at various constant temperatures. Can. Entomol. 99: 986-993. [ Links ]
4. CAMPOS, R. E. & L. P. LOUNIBOS. 1999. Eryngium spp. (Umbelliferae) as phytotelmata and their Culex (Culex) inhabitants in temperate Argentina. J. Am. Mosq. Control Assoc. 15: 493-499. [ Links ]
5. CAMPOS, R. E. & A. MACIÁ. 1998. Culicidae. In: Morrone, J. J. & S. Coscarón (eds.), Biodiversidad de artrópodos argentinos. Una perspectiva biotaxonómica, Ediciones Sur, La Plata. pp. 291-303. [ Links ]
6. CASAL, O. H. & M. GARCÍA. 1967a. Culex (Culex) hepperi, nueva especie del Delta Bonaerense del Río Paraná (Diptera, Culicidae). Physis 27: 87-94. [ Links ]
7. CASAL, O. H. & M. GARCÍA. 1967b. Culex (Culex) castroi, nueva especie de la República Argentina (Diptera, Culicidae). Physis 26: 451-457. [ Links ]
8. CHRISTOPHERS, S. R. 1960. Aedes aegypti (L.) - the yellow fever mosquito. Its life history, bionomics and structure. Cambridge University Press, Cambridge, U.K. [ Links ]
9. CLEMENTS, A. N. 1992. The Biology of Mosquitoes. Vol. I. Development, Nutrition and Reproduction. Chapman and Hall, London. [ Links ]
10. DARSIE, R. F. Jr. 1985. Mosquitoes of Argentina. Part I. Keys for identification of adult females and fourth stage larvae in English and Spanish (Diptera: Culicidae). Mosq. Syst.17: 153-253. [ Links ]
11. FLEISS, J. L. 1981. Statistical Methods for Rates and Proportions. John Wiley and Sons, New York. [ Links ]
12. FORATTINI, O. P. 1962. Entomologia Médica. Vol. I. Parte Geral, Diptera, Anophelini. Faculdade de Higiene e Saúde Pública - Departamento de Parasitología, São Paulo, Brasil. [ Links ]
13. GERBER, E. J. 1970. Manual for mosquito rearing and experimental techniques. Am. Mosq. Control Assoc. Bull. 5: 1-109. [ Links ]
14. HUGHES, L. 2000. Biological consequences of global warming: is the signal already. Tree 15: 56-61. [ Links ]
15. LANE, J. & G. E. RAMALHO. 1960. A new Neotropical Culex. Rev. Bras. Ent. 9: 173-176. [ Links ]
16. LOETTI, V., A. de GARÍN & N. SCHWEIGMANN. 2001. Un aparato económico para la cría simultánea de mosquitos a distintas temperaturas controladas. Actas de las II Jornadas Regionales sobre Mosquitos - Publicación Especial Nº 2 de la Sociedad Entomológica Argentina, pág 46. [ Links ]
17. LOETTI, M. V., N. E. BURRONI, N. SCHWEIGMANN & A. de GARÍN. 2007. Effect of different thermal conditions on the pre-imaginal biology of. Culex apicinus (Philippi, 1865) (Diptera: Culicidae). J. Vector Ecol. 32: 106-111. [ Links ]
18. MARTI, G. A., M. V. MICIELI, A. MACIÁ, L. P. LOUNIBOS & J. J. GARCÍA. 2007. Seasonality and abundance of the mosquito Isostomyia paranensis from phytotelmata in temperate Argentina. J. Am. Mosq. Control Assoc. 23: 252-258. [ Links ]
19. MITCHELL, C. J. & R. F. DARSIE Jr. 1985. Mosquitoes of Argentina. Part II. Geographic distribution and bibliography (Diptera: Culicidae). Mosq. Syst. 17: 279-360. [ Links ]
20. ROSSI, G. C., J. MARILUIS, J. A. SCHNACK & G. R. SPINELLI. 2002. Dípteros Vectores (Culicidae y Calliphoridae) de la Provincia de Buenos Aires. Secretaría de Política Ambiental y Universidad de la Plata, Buenos Aires. [ Links ]
21. RUEDA, L., K. PATEL, R. AXTELL & R. STINNER. 1990. Temperature-dependent development and survival rates of Culex quinquefasciatus Say and Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 27: 892-898. [ Links ]
22. SHARPE, P. J. H. & D. W. DEMICHELE. 1977. Reaction kinetics of poikilotherm development. J. Theor. Biol. 64: 649-670. [ Links ]
23. SU, T. & M. S. MULLA. 2001. Effects of temperature on development, mortality, mating and blood feeding behavior of Culiseta incidens (Diptera: Culicidae). J. Vector Ecol. 26: 83-92. [ Links ]
24. ZAR, J. H. 1999. Biostatistical Analysis. Prentice Hall, Upper Saddle River, New Jersey. [ Links ]














