Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista de la Sociedad Entomológica Argentina
versión impresa ISSN 0373-5680versión On-line ISSN 1851-7471
Rev. Soc. Entomol. Argent. v.68 n.1-2 Mendoza ene./jun. 2009
Effects of larval crowding on development time, survival and weight at metamorphosis in Aedes aegypti (Diptera: Culicidae)
Efectos del hacinamiento larval en el tiempo de desarrollo, la supervivencia y el peso en la metamorfosis de Aedes aegypti (Diptera: Culicidae)
Maciá, Arnaldo
Centro de Estudios Parasitológicos y de Vectores, CEPAVE, CCT La Plata CONICET-UNLP, 2 Nº 584, 1900 La Plata, Buenos Aires, Argentina; e-mail: amacia@cepave.edu.ar
ABSTRACT. The effects of larval crowding on survival, weight at metamorphosis and development time were assessed in the dengue mosquito, Aedes aegypti L., under a controlled environment. Larval cohorts were bred at 7 different densities (4, 8, 16, 32, 64, 128 and 256 larvae / 175 ml pot), while keeping constant water volume and food amount and quality, under controlled temperature and photoperiod. Natural detritus, mainly leaves, obtained from containers naturally colonized by A. aegypti, were used as a source of nutrients for larvae. Development time, mortality, mass at metamorphosis, and total biomass were recorded for each density. Development time ranged from 4 to 23 days in males, and from 5 to 24 in females, whereby larvae took longer to develop at 64 (females) and 128 (males) larvae per recipient. At high densities there was a male-biased sex proportion. At densities equal to or higher than 0.4 larvae/ml (0.32 larvae/cm2) there was an increase of mortality. An inverse relationship between larval density and pupal weight was detected. Biomass per individual reached asymptotic values of about 1 mg/individual at a density of 128 individuals/pot (0.64 larvae/cm2). This experiment shows that this southern strain of A. aegypti is sensitive to crowding in small containers.
KEY WORDS. Yellow fever mosquito; Intraspecific competition; Larval crowding; Density-dependent development.
RESUMEN. Los efectos del hacinamiento larval sobre el tiempo de desarrollo, la supervivencia y el peso en la metamorfosis fueron estudiados en el mosquito del dengue, Aedes aegypti L., en el laboratorio. Se criaron cohortes de larvas en 7 densidades (4, 8, 16, 32, 64, 128 y 256 larvas/ recipiente de 175 ml) mientras se mantuvo constante el volumen de agua y la calidad y cantidad de alimento, bajo fotoperíodo y temperatura controlados. Se usaron detritos naturales, principalmente hojas, obtenidos de contenedores colonizados naturalmente por A. aegypti como fuente de nutrientes para las larvas. En cada densidad se registraron el tiempo de desarrollo, la mortalidad, el peso en la metamorfosis y la biomasa total. El tiempo de desarrollo varió entre 4 y 23 días en los machos, y 5 a 24 días en hembras; fue más prolongado a la densidad de 64 (en las hembras) y 128 (en los machos) larvas por recipiente. En densidades altas la proporción de sexos favoreció los machos. Hubo un incremento en la mortalidad en densidades iguales o mayores que 0,4 larvas/ ml (0,32 larvas/cm2). Se detectó una relación inversa entre la densidad larval y el peso de las pupas. La biomasa por individuo alcanzó un valor asintótico de aproximadamente 1 mg/individuo en una densidad de 128 individuos/ recipiente (0,64 larvas/cm2). Las poblaciones de A. aegypti, cercanas a su extremo sur de distribución, serían sensibles al hacinamiento en pequeños contenedores de agua.
PALABRAS CLAVE. Mosquito de la fiebre amarilla; Competencia intraespecífica; Hacinamiento larval; Desarrollo densodependiente.
Recibido: 23-12-2008;
Aceptado: 2-04-2009
INTRODUCTION
Aedes aegypti L., the main vector o dengue and urban yellow fever in the Americas, is a cosmopolitan mosquito distributed between latitude 35o N and 35 S (OPS, 1995), with its southern limit of it geographic range included in Argentina (Avilé et al., 1999). The mosquito was eliminated from this country after implementation o an eradication plan by federal authorities in 1963, but infested the territory again in les than two decades (OPS, 1990). Dengue wa reintroduced in Argentina in 1998 (Seijo e al., 2000) and since then there have been some outbreaks (Avilés et al., 1999), including cases in Buenos Aires (Seijo et al., 2000). An autochthonous case in this city confirmed local circulation of serotype DEN-3 (Natiello et al., 2008). Risk associated to dengue i rising mainly due to exchange of people with neighbouring countries where epidemic were recorded (Seijo et al., 2000).
As a consequence of adaptation to human environments, A. aegypti larvae may develop in man-made containers such a cemetery vases, discarded automobile tires small vials, tanks, flower pots, buckets, and virtually any kind of receptacle able to hold stagnant water. In a previous study carried ou in Florencio Varela city (34o 46' 30" S, 58o 16 04" W), Buenos Aires province, Argentina Maciá (2006) estimated a crowding of abou 90 larvae per liter in automobile tires and 300 larvae per liter in ovitraps using Lloyd' (1967) index of mean crowding. During the course of that study, I found densities ranging from 74 to 460 larvae/l, and an average o 193 larvae/l. This census was carried ou before populations of A. aegypti reached its abundance peak at this latitude (Vezzani et al., 2004), therefore densities could be even higher in the field. These observations suggest that A. aegypti could undergo intraspecific competition in certain scenarios, such as low volume containers, which would promote density-dependent interactions among individuals.
Among mosquitoes, some life history traits depend largely on environmental conditions during their immature stages. Scarcity of larval resources (food, space) gives way to longer development time and little success and smaller size at metamorphosis (Hawley, 1985a; Bradshaw & Holzapfel, 1992; Renshaw et al. 1993). In adults, body size, survival, fecundity,mating success and flight capacity are lowered by a lack of larval resources (Wada, 1965; Steinwascher, 1982; Reisen et al., 1984; Fisher et al., 1990). A vector's capacity to spread a disease depends partly on the factors above mentioned. For instance, DEN-3 virus are more easily transmitted by larger females of A. aegypti (Sumanochitrapon et al., 1998). If resources per individual are insufficient, competition is strong, which in turn affects the capacity of imagoes to transmit diseases. The consequences of density-dependent development have been demonstrated for several traits, but correlations among various densities in the larval stage and pupal mass, survivorship and development time through larval stages are lacking for A. aegypti. Moreover, previous experiments on the relationship between pupal mass and adult fecundity, survivorship (Steinswascher, 1982), larval density and adult body size (Wada, 1965) used artificial food for larvae. In this paper, a natural substrate was used as source of nutrients for the larvae, which allows a more realistic experimental approach than using artificial food (such as food for aquarium fish, liver powder, yeast or grounded dog chow).
I quantified how development time and other life history traits like survival, mass at metamorphosis and biomass change according to various densities ranging from low to high, in a laboratory strain of A. aegypti from Argentina. I hypothesized that populations would show longer development time, lower survival, lower pupal weight and lower achieved biomass as larval density increases, as a consequence of competition among individuals. I also present a prediction of potential weights gained by pupae as a function of their larval density.
MATERIAL AND METHODS
Mosquitoes. Larvae were obtained from a colony maintained at Instituto de Limnología "Dr. R. Ringuelet", ILPLA (UNLP-CONICET), under a L:D 16:8 photoperiod, 80% relative humidity and 26ºC + 2. The colony was originated from wild mosquitoes collected around ILPLA. Adults were maintained in a cage, with free access to a sugar solution. Females were fed with human blood and supplied with two 500 ml black jars, filled with dechlorinated water until a depth of 1 cm, and lined with paper towel. Eggs were collected, air-dried after a 2 -days period in high humidity conditions to allow complete embrionation, and stored in plastic bags until experiments started. Submerging eggs in water with powdered yeast stimulated artificial hatching. Resulting larvae were rinsed once in dechlorinated water before being used in any treatment, and were less than 1 day old when experiment started.
Food source. In October 2000, artificial containers were set outdoors at ILPLA to collect organic matter and detritus falling from surrounding vegetation. Fifty plastic trays (16x12x5 cm) and six halves of automobile tires diametrically cut, were placed under a tree line along a grass field, and then artificially filled with about 250 ml of rainwater. After 10 days, trays and tires were emptied. The entire aqueous contents (10 l obtained from tires and 12 l from trays) plus detritus were transported to the laboratory, mixed in a big container, and distributed in large open pans to allow total evaporation at room temperature. Dry detritus consisting of plant debris such as leaves, seeds and small pieces of bark were mixed and stored in a recipient. Sieving produced thick material (all material retained by a 5 mm-mesh), medium material (retained by a 3 mm-mesh), and fine material (material passed by a 3 mm-mesh). Detritus were weighed with a Sartorius® BP-21 scale to the nearest 0.01 g. This procedure yielded 51.53 g thick material, 25.90 g medium material, and 25.34 g fine material. Then, thick material was cut in fragments <1 cm in order to facilitate handling. All material was distributed in numbered plastic bags, each containing 1.60 g thick detritus, 0.80 g medium detritus and 0.80 g fine detritus, in order to keep the proportion 2:1:1 (thick: medium: fine) found in nature. Each bag with 3.20 g detritus (=food) was assigned to an experimental replicate with a table of random numbers.
Experimental procedure. I kept a constant food amount across replicates but with increasing numbers of larvae per pot, i.e. varying density. Twenty-eight experimental replicates were set in the laboratory, each consisting of a 250 ml pot (8 cm diameter, 6 cm depth) filled with 175 ml dechlorinated water + 3.2 g food. After 7 days hydration allowed restoration of microorganisms that are the actual food for mosquitoes. The amount of organic matter/ml of water in resulting microcosms was greater than in the natural habitat of the mosquitoes (assessed by eye). On day 8 (= day 0 of the experiment), freshly hatched larvae were added to each recipient, according to the following densities: 4, 8, 16, 32, 64, 128 and 256 larvae per recipient, replicated 4 times. All larvae were added to the replicates in less than 3 hours. Mosquitoes were under the same photoperiod and temperature as previously described. After larval development, all pupae were removed daily, sexed, and weighed to the nearest 0.01 mg. This procedure continued until no pupation was observed for 7 consecutive days in each replica.
Analysis. Response variables were defined as follows: Development time: number of days from first instar to pupation. Survival: percentage of larvae attaining metamorphosis (pooled sexes to indicate percentage of the original population reaching adulthood). Pupal weight: weight of individual live pupae in mg. Biomass: accumulated weight of all individuals reaching the pupal stage in a treatment. The relationship between larval density and weight at metamorphosis and biomass was established using multiple regression analysis. Deviations from a 1:1 relationship between sexes across densities were explored through χ2 tests. Differences in development time, survival, weight at metamorphosis and biomass among experimental treatments (=densities) were subjected to parametric one-way ANOVA. For development time, weight at metamorphosis and biomass, independent ANOVAs were performed for each sex, because protandry and sex-related size dimorphism are features of mosquitoes. In order to meet assumptions of normality and homoscedasticity of data, survival data were transformed using y= arc sin √px, being px= proportion of survivals in cohort x (= experimental replicate x), and then subjected to ANOVA. Procedures ANOVA or GLM of SAS (SAS Institute, 1999) were used for analysis of balanced and unbalanced data, respectively, using type III sums of squares, followed by Tukey´s test (HSD) for comparisons between means, with a 0.05 significance level .
RESULTS
Development time. In both sexes, development time from LI to pupation was nearly constant at low densities (from 4 to 64 larvae/container or 0.02 to 0.32 larvae/ cm2), while at high densities (males: 256 larvae/container or 1.27 larvae/cm2; females: 128 and 256 larvae/recipient or 0.64 and 1.27 larvae/cm2) there was a decrease in development time (Fig. 1a). Development time ranged from 4 to 23 days in males (mean + SE= 7.07 + 0.15, n= 436), and from 5 to 24 days in females (mean + SE= 9.25 + 0.23, n= 331). It was significantly affected by density of those immature (p<0.0001), both for males and females (Table I).
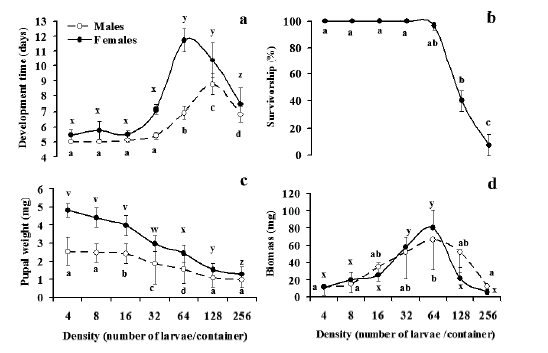
Fig. 1. Relationship larval density and (a) development time, (b) survivorship, (c) pupal weight and (d) biomass produced at metamorphosis in Aedes aegypti in the laboratory. Points show mean + 2 SE in a treatment. Means sharing the same letter are not significantly different after Tukey´s test (p>0.05).
Table I. ANOVA results for development time, survivorship, mean pupal weight and biomass in Aedes aegypti raised at 4, 8, 16, 32, 64, 128 and 256 larvae/container.

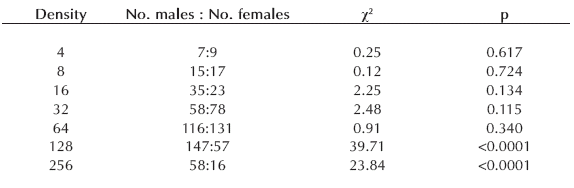
Survival. Almost 40% (767 out of 2032) of mosquito larvae reached metamorphosis. Mortality in larval cohorts developing at low-density was null up to 32 larvae/ recipient (0.16 larvae/cm2), and low at 64 larvae/recipient (0.32 larvae/cm2). At higher densities, survival diminished dramatically (Fig. 1b). Analysis showed that survival was affected by density (p<0.0001) (Table I). Fifty-seven per cent of pupae were male and 43% female, and a deviation from a 1:1 sex rate was significant (χ2= 14.37, df = 1, p<0.001). Significantly more males reached the pupal stage at 124 and 256 larvae per pot (= 0.64 and 1.27 larvae/cm2), while at lower densities there were equal proportions of sexes (Table II).
Table II. Results of chi-square tests under H0: equal proportion of sexes in Aedes aegypti raised at 4, 8, 16, 32, 64, 128 and 256 larvae/container. In all cases, d.f.= 1.
Pupal weight. Male pupal weight ranged from 0.70 to 3.74 mg (mean + SE= 1.49+0.025, n= 436), while female pupae ranged from 0.93 to 5.38 mg (mean + SE= 2.64+0.52, n= 331). Density effect was significant (p<0.0001) for both males and females, showing significant variation of pupal weight among densities (Table I). Therefore, an inverse relationship exists between larval density and pupal weight (Fig. 1c). Regression equations for this relationship are: Pupal weight males= 3.585-1.139 log density, (R2= 0.585, n= 436, p<0.05); pupal weight females= 6.429-2.245 log density, (R2= 0.575, n= 331, p<0.05).
Biomass. Average mosquito biomass from experimental replicates increased with density of individuals per container, up to 64 individuals/replicate (0.32 larvae/cm2). At higher densities there was a decrease in biomass, until reaching similar values to the lowest densities (Fig. 1d). Considering per capita biomass (= sum of pupal weight at a particular treatment / number of pupae surviving at that density), a decreasing function was more evident, reaching asymptotic values of about 1 mg / individual (data not shown). ANOVA showed a highly significant effect of Density (males: p= 0.006, females: p<0.0001) (Table I). Per capita biomass as a function of larval density can be expressed as: Y= 4.939-1.656 log density (R2= 0.76, n= 7, p<0.01).
DISCUSSION
This experiment has shown that crowding in the aquatic habitat of A. aegypti has a drastic effect on fitness components. Results show that even at high temperature, long photoperiod and high food level, which represent a favorable environment for larval development, intraspecific competition can arise promptly as a consequence of high density of those immature. Responses of fitness components studied herein were similar to those found in studies on other species of mosquitoes, i.e., crowding in the habitat generates lighter pupae (Bradshaw & Holzapfel, 1992; Hawley, 1985b), longer time spent as larvae (Reisen et al., 1984), and increased mortality (Renshaw et al. 1993; Gleiser et al. 2000).
The primary cause of immature stress in microcosms cannot be elucidated after this simple experimental design. Stress in crowded larvae is caused by toxic waste released to the environment, larvae that hamper food acquisition by other individuals, direct depletion of nutrients or combinations of these factors (Ikeshoji & Mulla, 1970; Kuno & Moore, 1975; Dye, 1982; Bédhomme et al., 2005). The lowest density at which life history traits were negatively affected was 64 larvae per container, or 0.32 larvae/cm2 (Fig. 1a to 1d). The decrease of biomass starting at that density (Fig. 1d) can be explained by mortality augmented simultaneously, and must not be seen as alleviation in density-dependent effects. Mortality due to competition can also explain the decrease in development time at high densities (256 larvae/container for males and 128 and 256 larvae/container for females). In pots containing high numbers of larvae, it was observed that a low proportion of individuals pupated soon after beginning of the experiment, while the majority remained in fourth stage until their death (after 12 days). Thus, development time could not be computed for most of larvae in high density treatments. This phenomenon could be explained as a larval mechanism favoring some individuals able to develop quickly and avoiding competition with individuals from the same cohort, but being lighter the pupae of those mosquitoes of faster development. As it was shown (Table II), a high proportion of individuals dying at high densities were females. Under larval competition, males maintain development time and females maintain body weight at the expense of other traits, in order to maximize fitness (Bedhomme et al., 2003). Consequently, at high densities, males metamorphose into low-weight pupae and most females die after a long period as larvae and trying to accumulate more reserves in order to attain higher body size.
Development time and survival are influenced by abiotic factors such as temperature and quality of water. Hence, both reflect partially increases in density. Biomass is based on the individual weight of pupae, so it can be expected to have a similar response to size at metamorphosis. As pupal weight changes accurately according to density, it can be inferred that pupal weight could be used more confidently as evidence of intraspecific competition in A. aegypti larvae than survivorship or development time. In addition, as body size -measured through pupal weight - is a very reliable predictor of fecundity (Armbruster & Hutchinson, 2002), population replacement rates would be affected by competition as well, an implication that needs confirmation in field populations of A. aegypti.
Maciá (2006) recorded an average of 29 larvae per 150 ml ovitrap in the field, and in a manipulative semi-natural experiment, introduced larvae in control containers in numbers close to those found in nature. In such situation, pupae reached an average weight of 1.5 (males) or 2.3 (females) mg, development time was 6 (males) or 8 (females) days, and survivorship was about 70% with high variability among containers. The treatment of 32 larvae per container (0.16 larvae/cm2) of the present study would promote a similar response in those fitness components. In this paper, pupae reached an average weight of 2 (males) or 3.5 (females) mg, development time was 5 (males) or 7 (females) days, and survivorship was nearly 100%. Results from both studies differ in that the field study was performed under fluctuating temperatures and lower food level, among other factors that can modify the outcome. Nevertheless, the comparison between both experiments suggests that mosquito fitness would be lower in the field than in the laboratory at analogous densities. This in turn supports the concept that populations of A. aegypti experience competition in outdoors,small containers at localities near the southern border of its geographical range. It remains to corroborate if intraspecific competition can regulate the production of adults in the field. In this case, an implication is that biological control of A. aegypti could be less effective if mortality due to the addition of predators or pathogens to breeding sites is compensatory of self-regulation of the population (Juliano, 2007).
ACKNOWLEDGEMENTS
To Raúl Campos for providing mosquito cultures and logistic support. To María C. Tranchida for her help in the preparation of the figure. To Rodolfo De La Sota for help running statistical tests and providing SAS. To Alberto Rodrígues Capítulo and Gustavo Spinelli for providing working space at ILPLA. Two anonymous reviewers greatly improved this paper through their suggestions. This research was supported by Fundación Antorchas (Buenos Aires) through Grant 13783/1-19. AM is a Research Assistant of Comisión de Investigaciones Científicas de la provincia de Buenos Aires.
LITERATURE CITED
1. ARMBRUSTER, P. & R. A. HUTCHINSON. 2002. Pupal mass and wing length as indicators of fecundity in Aedes albopictus and Aedes genicuatus (Diptera: Culicidae). J. Med. Entomol. 39 (4): 699-704. [ Links ]
2. AVILÉS, G., G. RANGEÓN, V. VORNDAM, A. BRIONES, P. BARONI, D. ENRIA & M. S. SABATTINI.1999. Dengue reemergence in Argentina. Emerg. Inf. Dis. 5 (4): 575-578. [ Links ]
3. BÉDHOMME, S., P. AGNEW, C. SIDOBRE & Y. MICHALAKIS. 2003. Sex-specific reaction norms to intraspecific larval competition in the mosquito Aedes aegypti. J. Evol. Biol. 16: 721-730. [ Links ]
4. BÉDHOMME, S., P.AGNEW, C. SIDOBRE & Y. MICHALAKIS. 2005. Pollution by conspecifics as a component of intraspecific competition among Aedes aegypti larvae. Ecol. Entomol. 30: 1-7. [ Links ]
5. BRADSHAW, W. E. & C. M. HOLZAPFEL.1992. Reproductive consequences of density-dependent size variation in the pitcher plant mosquito, Wyeomyia smithii (Diptera: Culicidae). Ann. Entomol. Soc. Am. 85: 274-281. [ Links ]
6. DYE, C. 1982. Intraspecific competition amongst larval Aedesaegypti: food exploitation or chemical interference? Ecol. Entomol. 7 (1): 39-46. [ Links ]
7. FISHER, I. J., W. E. BRADSHAW & C. KAMMEYER. 1990. Fitness and its correlates assessed by intra- and interspecific interactions among tree-hole mosquitoes. J. Animal Ecol. 59: 819-829. [ Links ]
8. GLEISER, R. M., J. URRUTIA & D. E. GORLA. 2000. Effects of crowding on populations of Aedes albifasciatus larvae under laboratory conditions. Ent. Exp. Appl. 95: 135-140. [ Links ]
9. HAWLEY, W. A. 1985a. A high fecundity Aedine: factors affecting egg production of the western tree-hole mosquito, Aedes sierrensis (Diptera: Culicidae). J. Med. Entomol. 22: 220-225. [ Links ]
10. HAWLEY, W. A. 1985b. The effect of larval density on adult longevity of a mosquito, Aedes sierrensis: epidemiological consequences. J. Animal Ecol. 54: 955-964. [ Links ]
11. IKESHOJI, T. & M. S. MULLA. 1970. Overcrowding factors of mosquito larvae. 2. Growth retarding and bacteriostatic effects of the overcrowding factors of mosquito larvae. J. Econ. Entomol. 63: 1737-1743. [ Links ]
12. JULIANO, S. A. 2007. Population dynamics. In: Floore, T. G. (ed.), Biorational Control of Mosquitoes. J. Am. Mosq. Control Assoc. 23, Bulletin No. 7, pp. 265-275. [ Links ]
13. KUNO, G. & C. G. MOORE. 1975. Production of larval growth retardant in axenic cultures of Aedes aegypti. Mosq. News 35: 199-201. [ Links ]
14. LLOYD, M. 1967. "Mean crowding". J. Animal Ecol. 36: 1-30. [ Links ]
15. MACIÁ, A. 2006. Differences in performance of Aedesaegypti larvae raised at different densities in tires and ovitraps under field conditions in Argentina. J. Vector Ecol. 31 (2): 371-377. [ Links ]
16. NATIELLO, M., V. RITACCO, M. A. MORALES, B. DEODATO, M. PICOLLO, E. DINERSTEIN & D. ENRIA. 2008. Indigenous dengue fever, Buenos Aires, Argentina. Emerg. Inf. Dis. 14: 1498-1499. [ Links ]
17. OPS. 1990. Las condiciones de la salud en las Américas, Vol. 1. Organización Panamericana de la Salud, Geneve. [ Links ]
18. OPS. 1995. Dengue y dengue hemorrágico en las Américas:Guías para su prevención y control. Organización Panamericana de la Salud, Publ. Cient. No 548, Washington. [ Links ]
19. REISEN, W. K., M. M. MILBY & M. E. BOCK. 1984. The effects of immature stress on selected events in the life history of Culex tarsalis. Mosq. News 44 (3): 385-395. [ Links ]
20. RENSHAW, M., M. W. SERVICE & M. H. BIRLEY. 1993. Density-dependent regulation of Aedes cantans (Diptera: Culicidae) in natural and artificial populations. Ecol. Entomol. 18: 223-233. [ Links ]
21. SAS INSTITUTE. 1999. SAS user's guide: statistics. Version 8.0. SAS Institute, Cary. [ Links ]
22. SEIJO A., D. CURCIO, G. AVILÉS, B. CERNIGOI, B. DEODATO & S. LLOVERAS. 2000. Imported dengue in Buenos Aires, Argentina. Emerg. Inf. Dis. 6: 655-656. [ Links ]
23. STEINWASCHER, K. 1982. Relation between pupal mass and adult survivorship and fecundity for Aedes aegypti. Environ. Entomol. 11 (1): 150-153. [ Links ]
24. SUMANOCHITRAPON, W., D. STRICKMAN, R. SITHIPRASASNA, P. KITTAYAPONG, & B. INNIS. 1998. Effect of size and geographic origin of Aedes aegypti on oral infection with dengue-2 virus. Am. J. Trop. Med. Hyg. 58 (3): 283-286. [ Links ]
25. VEZZANI, D., S. M. VELÁSQUEZ & N. SCHWEIGMANN. 2004. Seasonal pattern of abundance of Aedes aegypti (Diptera: Culicidae) in Buenos Aires city, Argentina. Mem. Inst. Oswaldo Cruz 99 (4): 351-356. [ Links ]
26. WADA, Y. 1965. Effect of larval density on the development of Aedes aegypti (L.) and the size of adults. Quaest. Entomol. 1: 223-249. [ Links ]














