Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de la Sociedad Entomológica Argentina
Print version ISSN 0373-5680
Rev. Soc. Entomol. Argent. vol.70 no.3-4 Mendoza July/Dec. 2011
TRABAJOS CIENTÍFICOS
Key to the subfamilies, tribes and genera of adult Dytiscidae of Argentina (Coleoptera: Adephaga)
Clave para los adultos de las subfamilias, tribus y géneros de Dytiscidae de la Argentina (Coleoptera: Adephaga)
Libonatti, María L., Mariano C. Michat and Patricia L. M. Torres
CONICET - Laboratorio de Entomología, Dpto. de Biodiversidad y Biología Experimental, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Argentina; e-mail: libonatti.marialaura@gmail.com
Recibido: 8-VIII-2011
Aceptado: 14-X-2011
ABSTRACT. Dytiscids constitute the world's most speciose family of water beetles, whose identifi cation in Argentina is problematic with current keys. In this work, a key (both in English and Spanish) to the eight subfamilies, 16 tribes and 31 genera of adult Dytiscidae of Argentina is presented. The key was constructed using stable qualitative characters of the external morphology and chaetotaxy, easily visualizable and interpretable. Characters such as size and shape of the body, color pattern and geographic distribution were also used. Illustrations of a great number of morphological structures as well as SEM micrographs were included to aid in the interpretation of the text. One subfamily (Hydrodytinae) and fi ve genera (Agaporomorphus Zimmermann, Bidessodes Régimbart, Hydrodytes Miller, Queda Sharp and an unpublished genus of the subfamily Laccophilinae) are cited for the fi rst time for Argentina.
KEY WORDS. Dytiscidae; Diving beetles; Adults; Key; Argentina.
RESUMEN. Los ditíscidos constituyen la familia más numerosa de escarabajos acuáticos a nivel mundial, cuya identifi cación en la Argentina resulta problemática con las claves actuales. En este trabajo, se presenta una clave (en inglés y español) para los adultos de las ocho subfamilias, 16 tribus y 31 géneros de Dytiscidae de la Argentina. La clave fue construida priorizando la inclusión de caracteres cualitativos estables de la morfología externa y quetotaxia, fácilmente visibles e interpretables. También, se utilizaron caracteres como el tamaño y la forma del cuerpo, el patrón de coloración y la distribución geográfi ca. Se incluyeron ilustraciones de un gran número de estructuras morfológicas y fotografías tomadas con el microscopio electrónico, para ayudar a la interpretación del texto. Se citan, por primera vez para la Argentina, una subfamilia (Hydrodytinae) y cinco géneros (Agaporomorphus Zimmermann, Bidessodes Régimbart, Hydrodytes Miller, Queda Sharp y un género inédito de la subfamilia Laccophilinae).
PALABRAS CLAVE. Dytiscidae; Escarabajos buceadores; Adultos; Clave; Argentina.
INTRODUCTION
The family Dytiscidae, commonly known as predaceous diving beetles, belongs to the order Coleoptera, suborder Adephaga. With more than 4,000 described species, it is the world's most speciose family of water beetles (Nilsson, 2001). Although it is cosmopolitan, it displays its greatest diversity in the tropics (Jäch & Balke, 2008). Dytiscidae comprises 10 subfamilies, 27 tribes and 180 genera. Approximately half of the species are included in the subfamily Hydroporinae (nearly 2,000 species) and the rest are distributed in the remaining nine subfamilies, as follows: Agabinae (370 species), Colymbetinae (130 species), Copelatinae (540 species), Coptotominae (5 species), Dytiscinae (380 species), Hydrodytinae (4 species), Laccophilinae (400 species), Lancetinae (22 species), Matinae (8 species) (Nilsson, 2001). Considering the new records given in the present study, the Argentinean fauna of Dytiscidae includes eight subfamilies (only Coptotominae and Matinae are absent), 16 tribes, 31 genera and 119 species (Trémouilles, 1998; Michat et al., 2008).
Eggs, larvae and adults of almost all dytiscid species are aquatic and live in a wide variety of freshwater habitats. They are usually found in lentic water bodies, such as steppe lakes, ponds, forest puddles, large lakes, springs, phytotelmata, hygropetric sites and alpine lakes up to 4,700 m in altitude (Trémouilles, 1995; Balke et al., 2004). In reference to lotic environments, dytiscids are most commonly observed in rivers and streams with a greatly reduced flow rate (Trémouilles, 1995). In general, most species prefer habitats with abundant aquatic vegetation, so meso- and eutrophic sites will usually feature a rich diving-beetle community (Balke et al., 2004). Pupation occurs in cells constructed by the mature larva on land, relatively close to the water (Larson et al., 2000). In some cases, dytiscids are adapted to extreme environments, such as freshwater bodies situated on caves or underground (Trémouilles, 1995). In spite of being one of the most typical aquatic families, Dytiscidae has three genera inhabiting rainforest leaf litter and soils in mountainous areas of the Indian subcontinent and northeastern Australia (Jäch & Balke, 2008; Larson et al., 2000).
Water beetles derive from terrestrial ancestors and have acquired a suite of adaptations that enable an aquatic existence (Larson et al., 2000). The adults have respiratory adaptations that allow them to breathe atmospheric air, such as the presence of hydrofuge hairs covering the last two abdominal tergites (Trémouilles, 1995). Moreover, they have adaptations to the aquatic locomotion, such as an oval and dorsoventrally flattened body shape, and the last pair of legs broadened, flattened and provided with natatory setae that aid in underwater propulsion (Larson et al., 2000; Trémouilles, 1995). Dytiscidae are also known for playing several important roles in aquatic ecosystems, such as preying upon mosquito larvae (Ohba & Takagi, 2010), and being good indicators of biodiversity (Sánchez-Fernández et al., 2006) and contamination (Fernández-Díaz et al., 2008).
Several keys for the identification of adults of the South American genera of Dytiscidae were presented in the past (Trémouilles, 1995; Trémouilles et al., 1995; Benetti et al., 2003 and Archangelsky et al., 2009). Even though they represent important contributions for the identification of the Argentinean genera, all of them have problems that may lead to misidentifications when applied to the Argentinean fauna. Some of these problems are: inclusion of genera that are absent in Argentina, exclusion of genera that are present, use of outdated synonyms and genus-group names, use of genital characters, use of sexually dimorphic characters, and lack of illustrations. To provide a solution to these problems, in this contribution we present a key for the identification of the subfamilies, tribes and genera of adult Dytiscidae of Argentina, including all the genera known so far to be present in our country, using updated nomenclature and avoiding the use of genital and sexually dimorphic characters (or if used, accompanied by other characters). Also, a large number of illustrations is included to assist in the identification process. One subfamily and five genera are cited for the first time for Argentina.
MATERIAL AND METHODS
In total, 73 species were examined, representing all the 31 dytiscid genera present in Argentina. Most of the material was obtained from collecting trips to several Argentinean provinces (Buenos Aires, Chubut, Córdoba, Corrientes, Entre Ríos, Jujuy, Misiones and Tucumán) and Paraguayan departments (Alto Paraguay, Canindeyú and Presidente Hayes). Specimens of Megadytes magnus Trémouilles & Bachmann, M. robustus (Aubé) and Hydaticus tuyuensis Trémouilles were borrowed from the Museo Argentino de Ciencias Naturales Bernardino Rivadavia, and specimens of Neobidessus alvarengai Young and N. pullus (Le Conte) were donated by Dr. G. Challet (Bohart Museum, USA).
Measurements were taken using a micrometer eyepiece mounted on a Leica MZ6 stereoscopic microscope. Total length (TL; = largo total: LT) was measured from the anterior clypeal margin to the elytral apex. Greatest width (GW; = ancho máximo: AM) was measured in the widest part of the specimens. The ratio TL/GW was calculated when shape was important for identification.
Drawings were made using a Leica MZ6 stereoscopic microscope or an Olympus CX31 compound microscope, both equipped with a camera lucida. The structures observed with the compound microscope were previously cleared in lactic acid and mounted on glass slides with polyvinyl-lacto-glycerol. Drawings were scanned and digitally edited using a Genius tablet. Micrographs were obtained using a Philips XL30 TMP New Look scanning electron microscope controlled by the software Analysis.
The following abbreviations were used in the figures: Clm (clypeal margin); Cs (cervical stria); CxI (procoxa); CxII (mesocoxa); CxIII (metacoxa); El (elytron); Elas (accessory stria of elytron); Elbs (basal stria of elytron); Epl (epipleuron); Eplc (epipleural carina); M2 (medial vein 2); Msem (mesoepimeron); Mtcxl (metacoxal line); Mtest (metaepisternum); Mtst (metasternum); Mtstk (metasternal keel); Mttac (metatarsal claw); Obc (oblongum cell); Prnt (pronotum); Prpl (propleuron); Prpr (prosternal process); Prst (prosternum); Prta2-4 (protarsomeres 2-4); Sc (scutellum).
The characters used in the key were identified by direct observation of the specimens. Moreover, an exhaustive literature survey was performed in order to find characters that complement those already selected by direct observation. Characters of external morphology and chaetotaxy were privileged, mainly those showing little or null variation within the taxa and also visible at common magnifications. Variable characters were in general accompanied by other characters to assist in the identification process. Morphometry, distribution, and coloration pattern were employed in some cases.
In order to identify the dorsal, ventral, anterior and posterior surfaces, the pro-and mesothoracic legs were considered to be stretched out at right angles to the body. In the metathoracic legs, however, the metacoxae are fused to the metathorax, and the remaining segments are rotated (in different degrees) with respect to their original position. The metafemur and metatibia, for example, are rotated about 90º so that the true anterior surface has become the ventral surface. The metatarsus is further rotated so that its primarily anterior surface is now the dorsal surface. In the present key we follow the criterion of Larson et al. (2000) and use the terms dorsal, ventral, anterior and posterior to refer to the apparent position of the metathoracic leg segments.
RESULTS
Key to subfamilies, tribes and genera of adult Dytiscidae of Argentina
1. Prosternal process in separate (more ventral) plane than prosternum (Fig. 6). Pro-and mesotarsus with fourth tarsomere shorter than third tarsomere and concealed between two lobes of third tarsomere (Figs. 1-2) (except Anodocheilus and Bidessonotus, Figs. 3-4) ...................................... Hydroporinae 2
1'. Prosternal process in approximately same plane as prosternum (Fig. 7). Pro- and mesotarsus with fourth tarsomere almost as long as third tarsomere and not concealed between lobes of third tarsomere (Fig. 5) ............................................................. 18

Figs. 1-15. 1-5, right protarsus: 1, Liodessus sp., dorsal view; 2, Liodessus sp., posterior view; 3, Bidessonotus obtusatus Régimbart, posterodorsal view; 4, Bidessonotus obtusatus, posterior view; 5, Megadytes magnus Trémouilles y Bachmann ♀, posterior view. 6-7, prothorax, lateral view: 6, Laccornellus lugubris (Aubé); 7, Rhantus signatus (Fabricius). 8-9, habitus, dorsal view: 8, Lancetes delkeskampi Ríha; 9, Amarodytes duponti (Aubé). 10-11, thorax, ventral view: 10, Vatellus haagi Wehncke; 11, Laccornellus lugubris. 12-13, pronotum: 12, Vatellus haagi; 13: Derovatellus lentus (Wehncke). 14-15, metacoxae: 14, Vatellus haagi; 15, Derovatellus lentus.
2. Scutellum exposed (Fig. 8) .................................................. Methlini, Celina Aubé
2'. Scutellum not exposed (Fig. 9) .......... 3
3. Mesoepimeron separating metaepisternum from mesocoxal cavity (Fig. 10). Mesocoxae contiguous (Fig. 10) .............................................................................. Vatellini 4
3'. Mesoepimeron not separating metaepisternum from mesocoxal cavity (Fig. 11). Mesocoxae not contiguous (Fig. 11) ............................................................. 5
4. Pronotum with a transverse furrow near posterior margin (Fig. 12). Greatest width of pronotum located anterior to medial transversal line (Fig. 12). Distance separating anterior ends of metacoxal lines approximately twice as long as distance separating posterior ends (Fig. 14). TL more than 5.2 mm .................... Vatellus Aubé
4'. Pronotum without transverse furrow (Fig. 13). Greatest width of pronotum located at or posterior to medial transversal line (Fig. 13). Distance separating anterior ends of metacoxal lines approximately as long as distance separating posterior ends (Fig. 15). TL less than 5.1 mm ................................................................. Derovatellus Sharp
5. Metatarsal claws of unequal length (Fig. 16) .................................... Hyphydrini 6
5'. Metatarsal claws of equal length (Fig. 17) .................................................. 7
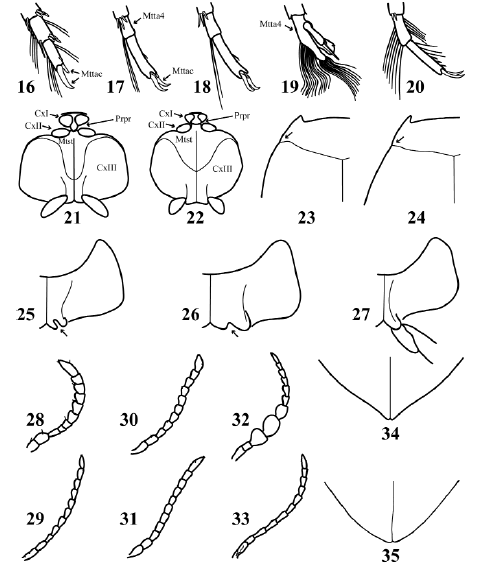
Figs. 16-35. 16-20, left metatarsomeres 4-5, ventral view: 16, Pachydrus obesus Sharp; 17, Hydrovatus caraibus Sharp ♂; 18, Hydrovatus caraibus ♀; 19, Queda youngi Biström ♂; 20, Queda youngi ♀. 21-22, thorax, ventral view: 21, Desmopachria sp.; 22, Pachydrus obesus. 23-24, pronotum and base of elytron: 23, Desmopachria sp.; 24, Pachydrus obesus. 25-26, left metacoxa: 25, Hydrovatus caraibus; 26, Queda youngi. 27, Laccornellus lugubris, left metacoxa, metatrochanter and base of metafemur. 28-33, right antenna: 28, Desmopachria chei Miller 1999; 29, Pachydrus obesus; 30, Hydrovatus caraibus ♂; 31, Hydrovatus caraibus ♀; 32, Queda youngi ♂; 33, Queda youngi ♀. 34-35, apical portion of elytra: 34, Hydrovatus caraibus, 35, Queda youngi.
6. Distal portion of prosternal process rhomboid-shaped (Fig. 21). Metasternum longer than wide at medial region (Fig. 21). First two antennomeres wider than the others (Fig. 28). Posterolateral angle of pronotum projected backward (Fig. 23). TL less than 3.1 mm ............. Desmopachria Babington
6'. Distal portion of prosternal process triangular-shaped (Fig. 22). Metasternum approximately as long as wide at medial region (Fig. 22). First two antennomeres approximately as wide as the others (Fig. 29). Posterolateral angle of pronotum not projected backward (Fig. 24). TL more than 3.3 mm ......................... Pachydrus Sharp
7. Metacoxal process incised medially (Figs. 25-26) ............................ Hydrovatini 8
7'. Metacoxal process not incised (Fig. 27) ..................................................... 9
8. Metacoxal process incision longer than wide (Fig. 25). Apical portion of elytron acuminate (Fig. 34). Dorsal color reddish-testaceous or yellowish-testaceous. Antennomeres 3-5 (Figs. 30-31) and metatarsomere 4 (Figs. 17-18) not sexually dimorphic. TL less than 3.0 mm .......................................... Hydrovatus Motschulsky
8'. Metacoxal process incision wider than long (Fig. 26). Apical portion of elytron not acuminate (Fig. 35). Dorsal color black to dark-ferrugineous. Antennomeres 3-5 and metatarsomere 4 sexually dimorphic: males with antennomeres 3-5 broader than the rest (Fig. 32) and metatarsomere 4 bilobed (Fig. 19); females with antennomeres 3-5 as broad as the rest (Fig. 33) and metatarsomere 4 not bilobed (Fig. 20). TL more than 4.0 mm ................................................. Queda Sharp
9. Base of metafemur in contact with metacoxal process (Fig. 27). Metatibia not curved (Fig. 36). TL more than 4.1 mm ............................................ Hydroporini, Laccornellus Roughley & Wolfe
9'. Base of metafemur not in contact with metacoxal process (Figs. 47-49). Metatibia curved basally (Fig. 37). Total length less than 4.0 mm ......................... Bidessini 10

Figs. 36-52. 36-37, left metatibia, ventral view: 36, Laccornellus lugubris; 37, Liodessus sp. 38-40, pronotum: 38, Bidessodes sp.; 39, Amarodytes duponti; 40, Hypodessus cruciatus Régimbart. 41-45, left elytron: 41, Hypodessus cruciatus; 42, Liodessus sp.; 43, Liodessus flavofasciatus (Steinheil); 44, Bidessonotus obtusatus; 45, Neobidessus alvarengai Young. 46-49, thorax, ventral view: 46: Amarodytes duponti; 47, Bidessodes sp.; 48, Bidessonotus obtusatus; 49, Liodessus sp. 50, Anodocheilus maculatus Babington, pronotum and elytra. 51-52, right mesotibia, posterior view: 51, Bidessonotus obtusatus ♂; 52, Bidessonotus obtusatus ♀.
10. Head without cervical stria ............ 11
10'. Head with cervical stria (Fig. 93-94) ................................... 13
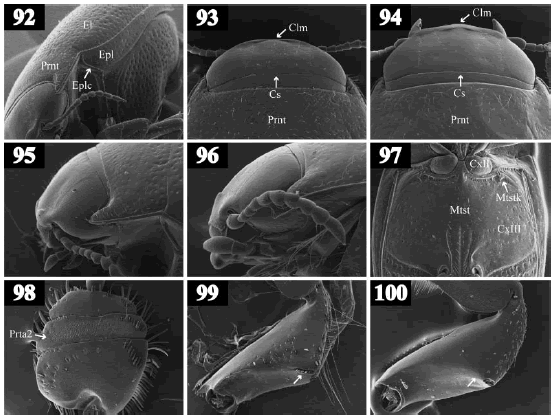
Figs. 92-100. 92, Hemibidessus conicus (Zimmermann), lateroventral view. 93-94, head, dorsal view: 93, Hemibidessus conicus; 94, Brachyvatus acuminatus (Steinheil). 95-96, head, lateral view: 95, Hemibidessus conicus; 96, Brachyvatus acuminatus. 97, Liodessus sp., meso- and metathorax, ventral view. 98, Hydaticus palliatus, right protarsal palette, dorsal view. 99-100, right protibia, posterior view: 99, Hydaticus palliatus; 100, Thermonectus succinctus.
11. Posterior pronotal stria absent or reduced, represented by a few punctures not exceeding posterior third of pronotum (Fig. 40). Elytral color pattern as in Fig. 41 ......................................................... Hypodessus Guignot
11'. Posterior pronotal stria present, exceeding posterior third of pronotum (Figs. 38-39). Elytral color pattern different from Fig. 41 ................................................ 12
12. Posterior pronotal striae connected by an irregular transverse furrow (Fig. 39). Metacoxal lines divergent anteriorly (Fig. 46). Prosternal process with margins convergent posteriorly and ventral surface excavated (Fig. 46) .............. Amarodytes Régimbart
12'. Posterior pronotal striae not connected by a furrow (Fig. 38). Metacoxal lines parallel (Fig. 47). Prosternal process with margins parallel or convergent posteriorly, and ventral surface not excavated (Fig. 47) ................................ Bidessodes Régimbart
13. Posterior pronotal striae present, connected by an irregular transverse furrow (Fig. 50). Elytron with a keel apparently being the extension of the pronotal stria (Fig. 50) ............................... Anodocheilus Babington
13'. Posterior pronotal striae absent or, if present, not connected by a transverse furrow. Elytron without keels ............... 14
14. Epipleura with an oblique carina on humeral angle (Fig. 92). Elytron without basal striae. Ratio TL/GW less than 1.75 .................................................................... 15
14'. Epipleura without carinae on humeral angle. Elytron with a basal stria (Figs. 44-45). Ratio TL/GW more than 1.85 ................ 16
15. Anterior clypeal margin medially broadened, with two tubercles in the middle (Figs. 94, 96). TL less than 1.6 mm ..................... Brachyvatus Zimmermann
15'. Anterior clypeal margin not broadened medially but laterally, without tubercles (Figs. 93, 95). TL more than 2.0 mm ............................................. Hemibidessus Zimmermann
16. Pro- and mesotarsus with fourth tarsomere almost as long as third tarsomere and not concealed between lobes of third tarsomere (Figs. 3-4). Metacoxal lines parallel or slightly convergent anteriorly, reaching apex of prosternal process (Fig. 48). Prosternal process with ventral surface not excavated (Fig. 48). Ventral margin of mesotibia curved in males (Fig. 51), straight in females (Fig. 52) ......................... Bidessonotus Régimbart
16'. Pro- and mesotarsus with fourth tarsomere shorter than third tarsomere and concealed between two lobes of third tarsomere (Figs. 1-2). Metacoxal lines divergent anteriorly, not reaching apex of prosternal process (Fig. 49). Prosternal process with ventral surface excavated (Fig. 49). Ventral margin of mesotibia straight in both sexes (Fig. 52) ...................................................17
17. Elytron without accessory striae between elytral commissure and basal stria (Fig. 44). Metasternum with lateral keels (Fig. 97). Elytral color pattern usually fasciate (Fig. 43), vittate (Fig. 42) or reduced. Pronotum with or without a medial fuscous spot. Clypeal margin not broadened anteriorly, without tubercles above antenna. Distribution: all throughout Argentina ................................................................ Liodessus Guignot
17'. Elytron with an accessory stria between elytral commissure and basal stria (Fig. 45). Metasternum without keels. Elytral color pattern vittate (Fig. 42). Pronotum without spots. Clypeal margin broadened anteriorly, with a tubercle above antenna. Distribution: Salta Province ............ Neobidessus Young
18. Anterior margin of eye rounded, without a notch above base of antenna (Fig. 54) ....................................... Dytiscinae 19
18'. Anterior margin of eye not rounded, with a notch above base of antenna (Fig. 53) ......................................................... 23

Figs. 53-67. 53-54, head, anterior view: 53, Lancetes waterhousei Griffini; 54, Megadytes glaucus (Brullé). 55-57, right metafemur, dorsal view: 55, Megadytes magnus; 56, Hydaticus palliatus Aubé; 57, Thermonectus succinctus (Aubé). 58-59, right protarsal palette, ventral view: 58, Cybister puncticollis (Brullé) ♂; 59, Hydaticus palliatus ♂. 60-62, left metatarsomere 5, ventral view: 60, Megadytes carcharias Griffini ♀; 61, Cybister puncticollis ♂; 62, Megadytes carcharias ♂. 63-65, left metatibia and metatarsus, ventral view: 63, Cybister puncticollis ♂; 64, Hydaticus palliatus ♂; 65, Thermonectus margineguttatus (Aubé). 66-67, right mesotibia and mesotarsus, posterior view: 66, Cybister puncticollis ♂; 67, Megadytes magnus ♀.
19. Scutellum not exposed (Fig. 9). TL less than 8.1 mm .................................................... Aubehydrini, Notaticus Zimmermann
19'. Scutellum exposed (Fig. 8). TL more than 8.3 mm ...................................... 20
20. Ventral metatibial spur approximately twice as wide as dorsal metatibial spur (Fig. 63). Anterodorsal margin of metafemur with a row of natatory setae (Fig. 55). Apices of adhesive setae on ventral surface of male protarsomeres 1-3 oval-shaped (Fig. 58). TL more than 15.9 mm ............. Cybistrini 21
20'. Ventral metatibial spur approximately as wide as dorsal metatibial spur (Fig. 64-65). Anterodorsal margin of metafemur without rows of natatory setae (Figs. 56-57). Apices of adhesive setae on ventral surface of male protarsomeres 1-3 round-shaped (Fig. 59). TL less than 15.7 mm ....................... 22
21. Row of setae on posteroapical margin of mesotibia continuous medially (Fig. 66). Posterior surface of male mesotarsomeres and female pro- and mesotarsomeres with two rows of setae near apical margin (Fig. 66). Males with one metatarsal claw (Fig. 61). Females with two unequal metatarsal claws (Fig. 60). TL: 26.0-28.0 mm ................................................... Cybister Curtis
21'. Row of setae on posteroapical margin of mesotibia discontinuous medially (Fig. 67). Posterior surface of male mesotarsomeres and female pro- and mesotarsomeres with one row of setae near apical margin (Fig. 67). Males with two equal metatarsal claws (Fig. 62). Females with two metatarsal claws, either equal (Fig. 62) or unequal (Fig. 60). TL: 16.0-47.0 mm ............... Megadytes Sharp
22. Apices of metatibial spurs simple (Fig. 64). Anterior margin of metasternal wing straight (Fig. 68) or slightly curved (Fig. 69). Male mesotarsus with adhesive setae (Fig. 71). Males with stridulatory apparatus composed of a field of excavations on dorsal surface of protarsomere 2 (file) (Fig. 98) and a row of spines on dorsoposterior margin of protibia (plectrum) (Fig. 99) ......................................... Hydaticini, Hydaticus Leach
22'. Apices of metatibial spurs bifid (Fig. 65). Anterior margin of metasternal wing strongly curved (Fig. 70). Male mesotarsus without adhesive setae (Fig. 72). Males without stridulatory apparatus on prothoracic leg (Fig. 100) ......... Aciliini, Thermonectus Dejean

Figs. 68-80. 68-70, left metasternum and metacoxa: 68, Hydaticus palliatus; 69, Hydaticus tuyuensis Trémouilles; 70, Thermonectus succinctus. 71-72, left mesotarsus, anterior view: 71, Hydaticus palliatus ♂; 72, Thermonectus succinctus ♂. 73-74, left metatibia and metatarsus, ventral view: 73, unpublished genus; 74, Laccophilus sp. 75-76, left wing, ventral view: 75, Laccophilus sp.; 76, Lancetes delkeskampi. 77-78, metacoxae: 77, Copelatus alternatus Sharp; 78, Rhantus calidus (Fabricius). 79-80, right metatarsus, dorsal view: 79, Rhantus signatus (Fabricius); 80, Copelatus alternatus.
23. Scutellum not exposed (Fig. 9). Metatarsus with one claw (Figs. 73-74). Mesoepimeron separating metaepisternum from mesocoxal cavity (Fig. 10). Vein M2 of wing not connected to oblongum (Fig. 75) ................. Laccophilinae, Laccophilini 24
23'. Scutellum exposed (Fig. 8). Metatarsus with two claws (Figs. 79-80). Mesoepimeron not separating metaepisternum from mesocoxal cavity (Fig. 11). Vein M2 of wing connected to oblongum (Fig. 76) .......... 25
24. Apices of metatibial spurs simple (Fig. 73). TL less than 2.6 mm .......................................................... Unpublished genus
24'. Apices of metatibial spurs bifid (Fig. 74). TL more than 2.8 mm .......................................................... Laccophilus Leach
25. Apex of elytron truncate (Fig. 8) ......... Lancetinae, Lancetini, Lancetes Sharp
25'. Apex of elytron not truncate (Fig. 9) .................................................. 26
26. Metacoxal lines strongly approximated in middle region (Fig. 77). Anterodorsal margin of metatarsomeres 1-4 not lobed (Fig. 80) ....................................................... 27
26'. Metacoxal lines not strongly approximated in middle region (Fig. 78). Anterodorsal margin of metatarsomeres 1-4 lobed (Fig. 79) ................................. 29
27. Elytron with long longitudinal striae (Figs. 81-84). Metacoxa with oblique striae (Fig. 77). TL more than 4.0 mm ......................................................... Copelatinae, Copelatini, Copelatus Erichson
27'. Elytron without striae. Metacoxa without striae. TL less than 3.7 mm ............ 28
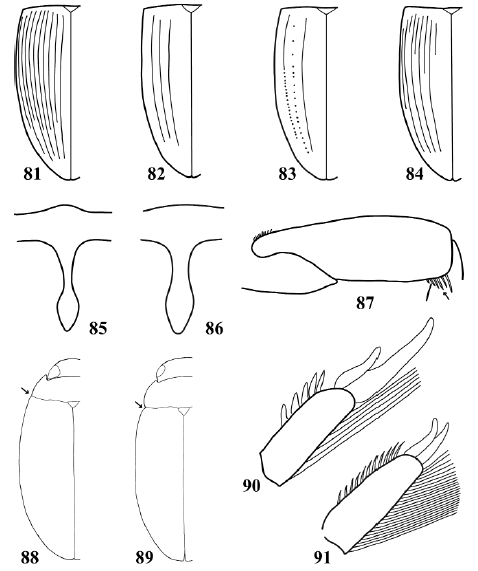
Figs. 81-91. 81-84, left elytron: 81, Copelatus alternatus; 82, Copelatus longicornis Sharp (specimen from Buenos Aires city); 83, Copelatus longicornis (specimen from El Destino natural reserve, Buenos Aires Province); 84, Copelatus sp. 85-86, prosternal process: 85, Agaporomorphus mecolobus Miller; 86, Hydrodytes opalinus (Zimmermann). 87, Leuronectes curtulus Régimbart, metafemur, ventral view. 88-89, habitus, dorsal view: 88, Rhantus calidus; 89, Bunites distigma (Brullé). 90-91, metatarsomere 5, dorsal view: 90, Rhantus signatus; 91, Bunites distigma.
28. Dorsal surface not iridescent. Basal portion of pronotum and elytra of same color as other areas of dorsal surface. Middle portion of prosternal process narrowing up to half the maximum width of posterior portion (Fig. 85). TL more than 3.3 mm ......................................... Copelatinae, Copelatini, Agaporomorphus Zimmermann
28'. Dorsal surface iridescent. Basal portion of pronotum and elytra lighter in color than other areas of dorsal surface. Middle portion of prosternal process not narrowing up to half the maximum width of posterior portion (). TL less than 3.2 mm ...................................................... Hydrodytinae, Hydrodytini, Hydrodytes Miller
29. Posteroapical angle of metafemur with a row of setae (Fig. 87). Metatarsal claws equal in length (Fig. 91). TL less than 9.0 mm......................................................... Agabinae, Agabini, Leuronectes Sharp
29'. Posteroapical angle of metafemur without a row of setae. Metatarsal claws equal (Fig. 91) or unequal (Fig. 90) in length. TL more than 10.0 mm .......................................................... Colymbetinae, Colymbetini 30
30. Body outline discontinuous in dorsal view, with a visible angle between pronotum and elytron (Fig. 89). Metatarsal claws equal in length (Fig. 91). TL: 13.0-15.4 mm. Distribution: Jujuy and Tucumán Provinces ............................. Bunites Spangler
30'. Body outline continuous in dorsal view, without a visible angle between pronotum and elytron (Fig. 88). Metatarsal claws unequal in length (Fig. 90). TL: 10.4-17.0 mm. Distribution: all throughout Argentina .................................... Rhantus Dejean
Clave para los adultos de las subfamilias, tribus y géneros de Dytiscidae de la Argentina
1. Proceso prosternal en un plano distinto (más ventral) que la región media del prosterno (Fig. 6). Pro y mesotarso con el cuarto tarsómero más corto que el tercero y oculto entre los lóbulos de éste (Figs. 1-2) (excepto Anodocheilus y Bidessonotus, Figs. 3-4) ............................. Hydroporinae 2
1'. Proceso prosternal aproximadamente en el mismo plano que la región media del prosterno (Fig. 7). Pro y mesotarso con el cuarto tarsómero casi tan largo como el tercero y no oculto entre lóbulos de éste (Fig. 5) ................................................ 18
2. Escutelo expuesto (Fig. 8) .................... Methlini, Celina Aubé
2'. Escutelo no expuesto (Fig. 9) ...................................................... 3
3. Mesoepímero que separa el metaepisterno de la cavidad mesocoxal (Fig. 10). Mesocoxas contiguas (Fig. 10) ...........................................Vatellini 4
3'. Mesoepímero que no separa el metaepisterno de la cavidad mesocoxal (Fig. 11). Mesocoxas no contiguas (Fig. 11) ............ 5
4. Pronoto con un surco transversal cercano al margen posterior (Fig. 12). Ancho máximo del pronoto ubicado anteriormente a la línea media transversal (Fig. 12). Distancia entre los extremos anteriores de las líneas metacoxales aproximadamente el doble de la distancia entre los extremos posteriores (Fig. 14). LT mayor que 5,2 mm .......................................Vatellus Aubé
4'. Pronoto sin surco transversal (Fig. 13). Ancho máximo del pronoto ubicado en o posteriormente a la línea media transversal (Fig. 13). Distancia entre los extremos anteriores de las líneas metacoxales aproximadamente igual que la distancia entre los extremos posteriores (Fig. 15). LT menor que 5,1 mm ............................... Derovatellus Sharp
5. Uñas metatarsales desiguales en longitud (Fig. 16) ......................... Hyphydrini 6
5'. Uñas metatarsales iguales en longitud (Fig. 17) ........................................... 7
6. Porción distal del proceso prosternal romboidal (Fig. 21). Metasterno más largo que ancho en su región media (Fig. 21). Antenómeros 1-2 más anchos que los restantes (Fig. 28). Ángulo posterolateral del pronoto prolongado posteriormente (Fig. 23). LT menor que 3,1 mm ..................................................... Desmopachria Babington
6'. Porción distal del proceso prosternal triangular (Fig. 22). Metasterno tan largo como ancho en su región media (Fig. 22). Antenómeros 1-2 aproximadamente del mismo ancho que los restantes (Fig. 29). Ángulo posterolateral del pronoto no prolongado posteriormente (Fig. 24). LT mayor que 3,3 mm ........ Pachydrus Sharp
7. Proceso metacoxal con una incisión en la región media (Figs. 25-26) ...................................... Hydrovatini 8
7'. Proceso metacoxal sin incisión en la región media (Fig. 27) ....................... 9
8. Incisión metacoxal más larga que ancha (Fig. 25). Porción apical del élitro acuminada (Fig. 34). Coloración dorsal testácea-rojiza o testácea-amarillenta. Sin dimorfismo sexual en los antenómeros 3-5 (Figs. 30-31), ni en el metatarsómero 4 (Figs. 17-18). LT menor que 3,0 mm ...................... Hydrovatus Motschulsky
8'. Incisión metacoxal más ancha que larga (Fig. 26). Porción apical del élitro no acuminada (Fig. 35). Coloración dorsal negra a ferrugínea oscura. Machos con los antenómeros 3-5 más anchos que el resto (Fig. 32) y con el metatarsómero 4 bilobulado (Fig. 19). Hembras con los antenómeros 3-5 del mismo ancho que el resto (Fig. 33) y con el metatarsómero 4 no bilobulado (Fig. 20). LT mayor que 4,0 mm ............................................................ Queda Sharp
9. Base del metafémur en contacto con el proceso metacoxal (Fig. 27). Metatibia no arqueada en la base (Fig. 36). LT mayor que 4,1 mm .................................................... Hydroporini, Laccornellus Roughley y Wolfe
9'. Base del metafémur sin contacto con el proceso metacoxal (Figs. 47-49). Metatibia arqueada en la base (Fig. 37). LT menor que 4,0 mm ............................. Bidessini 10
10. Cabeza sin estría cervical...... ............................................. 11
10'. Cabeza con estría cervical (Figs. 93-94) ................................................ 13
11. Estría pronotal posterior ausente o reducida, representada por algunos puntos impresos que no sobrepasan un tercio de la longitud del pronoto (Fig. 40). Patrón de color elitral como en la Fig. 41 ............................. Hypodessus Guignot
11'. Estría pronotal posterior bien desarrollada, sobrepasa un tercio de la longitud del pronoto (Figs. 38-39). Patrón de color elitral distinto de la Fig. 41 ............................... 12
12. Estrías pronotales posteriores conectadas por un surco transversal irregular (Fig. 39). Líneas metacoxales divergentes anteriormente (Fig. 46). Proceso prosternal de lados convergentes posteriormente, con la superficie ventral excavada (Fig. 46) ......................... Amarodytes Régimbart
12'. Estrías pronotales posteriores no conectadas por un surco transversal (Fig. 38). Líneas metacoxales paralelas (Fig. 47). Proceso prosternal de lados paralelos o convergentes posteriormente, con la superficie ventral no excavada (Fig. 47) ........................... Bidessodes Régimbart
13. Estrías pronotales posteriores presentes, conectadas por un surco transversal irregular (Fig. 50). Élitro con una quilla que parece continuarse con la estría pronotal posterior (Fig. 50) ............. Anodocheilus Babington
13'. Estrías pronotales posteriores ausentes o, si presentes, no conectadas por un surco transversal. Élitro sin quillas ................................................. 14
14. Epipleura con una carena oblicua en el ángulo humeral (Fig. 92). Élitro sin estría basal. Cociente LT/AM menor que 1,75.................................................. 15
14'. Epipleura sin carenas en el ángulo humeral. Élitro con estría basal (Figs. 44-45). Cociente LT/AM mayor que 1,85.................................................................16
15. Clípeo con el margen anterior engrosado medialmente y dos tubérculos en el centro (Figs. 94, 96). LT menor que 1,6 mm ..................... Brachyvatus Zimmermann
15'. Clípeo con el margen anterior no engrosado medialmente sino lateralmente y sin tubérculos (Figs. 93, 95). LT mayor que 2,0 mm ............ Hemibidessus Zimmermann
16. Pro y mesotarso con el cuarto tarsómero casi tan largo como el tercero y no oculto entre los lóbulos de este (Figs. 3-4). Líneas metacoxales paralelas o ligeramente convergentes anteriormente, continúan hasta el ápice del proceso prosternal (Fig. 48). Proceso prosternal con la superficie ventral no excavada (Fig. 48). Margen ventral de la mesotibia curvado en los machos (Fig. 51), recto en las hembras (Fig. 52) .......................... Bidessonotus Régimbart
16'. Pro y mesotarso con el cuarto tarsómero más corto que el tercero y oculto entre los lóbulos de este (Figs. 1-2). Líneas metacoxales divergentes anteriormente, no continúan hasta el ápice del proceso prosternal (Fig. 49). Proceso prosternal con la superficie ventral excavada (Fig. 49). Margen ventral de la mesotibia recto en ambos sexos (Fig. 52) ................................................. 17
17. Élitro sin estría accesoria entre la comisura elitral y la estría basal (Fig. 44). Metasterno con quillas laterales (Fig. 97). Patrón de color elitral usualmente fasciado (Fig. 43), vittado (Fig. 42) o reducido. Pronoto con o sin una mancha oscura central. Margen clipeal no engrosado anteriormente, sin tubérculos por encima de las antenas. Distribución: toda la Argentina ................... Liodessus Guignot
17'. Élitro con estría accesoria entre la comisura elitral y la estría basal (Fig. 45). Metasterno sin quillas laterales. Patrón de color elitral vittado (Fig. 42). Pronoto sin manchas. Margen clipeal engrosado anteriormente, con un tubérculo por encima de la antena. Distribución: provincia de Salta ................................ Neobidessus Young
18. Margen anterior del ojo redondeado, sin muesca sobre la base de la antena (Fig. 54) .................................... Dytiscinae 19
18'. Margen anterior del ojo no redondeado, con una muesca sobre la base de la antena (Figs. 53) ......................................... 23
19. Escutelo no expuesto (Fig. 9). LT menor que 8,1 mm ....................................... Aubehydrini, Notaticus Zimmermann
19'. Escutelo expuesto (Figs. 8). LT mayor que 8,3 mm .................................... 20
20. Espolón metatibial ventral aproximadamente el doble de ancho que el espolón metatibial dorsal (Fig. 63). Margen anterodorsal del metafémur con una hilera de setas natatorias (Fig. 55). Ápices de las setas adhesivas ventrales de los protarsómeros 1-3 del macho ovalados (Fig. 58). LT mayor que 15,9 mm .................. Cybistrini 21
20'. Espolón metatibial ventral de ancho aproximadamente igual que el espolón metatibial dorsal (Figs. 64-65). Margen anterodorsal del metafémur sin hilera de setas natatorias (Figs. 56-57). Ápices de las setas adhesivas ventrales de los protarsómeros 1-3 del macho redondeados (Fig. 59). LT menor que 15,7 mm ................................ 22
21. Margen posteroapical de la mesotibia con una hilera continua de setas (Fig. 66). Superficie posterior de los mesotarsómeros de los machos y de los pro y mesotarsómeros de las hembras con dos hileras de setas cerca del margen apical (Fig. 66). Machos con una uña metatarsal (Fig. 61). Hembras con dos uñas metatarsales desiguales (Fig. 60). LT: 26-28 mm .......................... Cybister Curtis
21'. Margen posteroapical de la mesotibia con una hilera de setas discontinua en la región central (Fig. 67). Superficie posterior de los mesotarsómeros de los machos y de los pro y mesotarsómeros de las hembras con una hilera de setas cerca del margen apical (Fig. 67). Machos con dos uñas metatarsales iguales (Fig. 62). Hembras con dos uñas metatarsales iguales (Fig. 62) o desiguales (Figs. 60). LT: 16-47 mm .................................. Megadytes Sharp
22. Ápices de ambos espolones metatibiales simples (Fig. 64). Margen anterior del ala metasternal recto (Fig. 68) o levemente curvado (Fig. 69). Mesotarso de los machos con ventosas (Fig. 71). Machos con aparato estridulador conformado por un grupo de excavaciones en la cara dorsal del protarsómero 2 (lima) (Fig. 98) y una hilera de espinas en el margen dorsoposterior de la protibia (plectrum) (Fig. 99) ..................... Hydaticini, Hydaticus Leach
22'. Ápices de ambos espolones metatibiales bífidos (Fig. 65). Margen anterior del ala metasternal marcadamente curvado (Fig. 70). Mesotarso de los machos sin ventosas (Fig. 72). Machos sin aparato estridulador en la pata protorácica (Fig. 100) ................ Aciliini, Thermonectus Dejean
23. Escutelo no expuesto (Fig. 9). Metatarso con una uña (Figs. 73-74). Mesoepímero que separa el metaepisterno de la cavidad mesocoxal (Fig. 10). Vena M2 del ala no conectada al oblongum (Fig. 75) ................ Laccophilinae, Laccophilini 24
23'. Escutelo expuesto (Fig. 8). Metatarso con dos uñas (Figs. 79-80). Mesoepímero que no separa el metaepisterno de la cavidad mesocoxal (Fig. 11). Vena M2 del ala conectada al oblongum (Fig. 76) .................................................... 25
24. Ápices de ambos espolones metatibiales simples (Fig. 73). LT menor que 2,6 mm ...................................... Género inédito
24'. Ápices de ambos espolones metatibiales bífidos (Fig. 74). LT mayor que 2,8 mm ............................... Laccophilus Leach
25. Ápice elitral truncado (Fig. 8) ............. Lancetinae, Lancetini, Lancetes Sharp
25'. Ápice elitral no truncado (Fig. 9) .................................................... 26
26. Líneas metacoxales muy próximas en su región media (Fig. 77). Margen anterodorsal de los metatarsómeros 1-4 no lobulado (Fig. 80) ........... ......................... 27
26'. Líneas metacoxales no muy próximas en su región media (Fig. 78). Margen anterodorsal de los metatarsómeros 1-4 lobulado (Fig. 79) ............................. 29
27. Élitro con estrías longitudinales largas (Figs. 81-84). Metacoxa con estrías oblicuas (Fig. 77). LT mayor que 4,0 mm................................. Copelatinae, Copelatini, Copelatus Erichson
27'. Élitro sin estrías. Metacoxa sin estrías. LT menor que 3,7 mm .................... 28
28. Superficie dorsal no iridiscente. Porción basal de pronoto y élitros del mismo color que el resto de la superficie dorsal. Región media del proceso prosternal que se angosta hasta la mitad del ancho máximo de la región posterior (Fig. 85). LT mayor que 3,3 mm ...................................... Copelatinae, Copelatini, Agaporomorphus Zimmermann
28'. Superficie dorsal iridiscente. Porción basal de pronoto y élitros de color más claro que el resto de la superficie dorsal. Región media del proceso prosternal que no se angosta hasta la mitad del ancho máximo de la región posterior (Fig. 86). LT menor que 3,2 mm ............................... Hydrodytinae, Hydrodytini, Hydrodytes Miller
29. Ángulo posteroapical del metafémur con un grupo de setas (Fig. 87). Uñas metatarsales iguales en longitud (Fig. 91). LT menor que 9,0 mm .................................................. Agabinae, Agabini, Leuronectes Sharp
29'. Ángulo posteroapical del metafémur sin setas. Uñas metatarsales iguales (Fig. 91) o desiguales (Fig. 90) en longitud. LT mayor que 10,0 mm .................................................. Colymbetinae, Colymbetini 30
30. Contorno del cuerpo discontinuo en vista dorsal, con un ángulo visible entre pronoto y élitro. (Fig. 89). Uñas metatarsales iguales en longitud (Fig. 91). LT: 13,0-15,4 mm. Distribución: provincias de Jujuy y Tucumán ................................... Bunites Spangler
30'. Contorno del cuerpo continuo en vista dorsal, sin ángulo visible entre pronoto y élitro (Fig. 88). Uñas metatarsales desiguales en longitud (Fig.90). LT: 10,4-17,0 mm. Distribución: toda la Argentina ..................................... Rhantus Dejean
DISCUSSION
The key presented here has several advantages with respect to previous keys used for the identification of adult Dytiscidae of Argentina. As mentioned above (see Introduction), existing keys have several problems when applied to the Argentinean fauna. A first advantage derives from the study of specimens of all the dytiscid genera of Argentina, which allowed us to perform an extensive search for characters that complement the (traditional) characters obtained from the literature. The utility of the traditional characters was also tested and only those that proved to be useful were kept. An emphasis was given to include stable and qualitative characters of the external morphology (to avoid the dissection of the specimens), and to chose characters easily seen at common magnifications. The use of sexually dimorphic characters was restricted as much as possible, and in the cases in which these characters were included, they were accompanied by other (not sexually dimorphic) characters. In the case of quantitative characters we avoided the use of vague terminology and privileged the use of precise measurements and ratios. Finally, we included illustrations of a great number of morphological structures, tending to facilitate the interpretation of the text, and added SEM micrographs to illustrate very small structures.
The subfamily Hydrodytinae and five genera are cited for the first time for Argentina: Agaporomorphus (Copelatinae), Bidessodes (Hydroporinae: Bidessini), Hydrodytes (Hydrodytinae), Queda (Hydroporinae: Hydrovatini) and an unpublished genus of the subfamily Laccophilinae. Moreover, new records are presented for the following provinces: Chaco (Rhantus Dejean, Brachyvatus Zimmermann, Unpublished genus); Corrientes (Agaporomorphus Zimmermann, Anodocheilus Babington, Bidessonotus Régimbart, Derovatellus Sharp, Desmopachria Babington, Hemibidessus Zimmermann, Hydrodytes Miller, Pachydrus Sharp, Queda Sharp, Unpublished genus); Entre Ríos (Bidessodes Régimbart, Bidessonotus, Unpublished genus); Mendoza (Celina Aubé, Rhantus); Misiones (Agaporomorphus, Celina, Hydrodytes); Santa Fe (Lancetes Sharp).
The finding of Hydrodytes opalinus (Zimmermann) in Argentina (recorded in Corrientes and Misiones Provinces) represents a great expansion of the distributional range of the genus Hydrodytes and of the subfamily Hydrodytinae. Previously, the southern limit of Hydrodytes was northern South America (Miller, 2002). On the other hand, the presence of Queda in Argentina was expected since members of this genus were recorded recently in southern Paraguay (Trémouilles et al., 2004). The specimens of Q. youngi Biström were collected in Corrientes Province, so it is probable that this genus is also present in Chaco and Formosa Provinces. Agaporomorphus and Bidessodes also had their distributional limits near Argentina, so their presence in our country corroborates previous hypotheses stating that these genera were probably to be found here (Miller, 2001; Young, 1986).
Anodocheilus maculatus Babington was cited from Buenos Aires and Entre Ríos Provinces (Trémouilles, 1995, 1998; Michat & Torres, 2006). This paper incorporates Corrientes Province to the distribution area of the species and the genus. The first mention of Bidessonotus for our country corresponds to Torres et al. (2008), who found an unidentified species in Jujuy Province. Here, we report the finding of specimens of Bidessonotus in Corrientes and Entre Ríos Provinces. In our country Brachyvatus acuminatus (Steinheil) was known from Buenos Aires and Entre Ríos Provinces (Torres et al., 2007). In the present paper the genus and species are mentioned for the first time for Chaco Province. Hemibidessus was recorded for Buenos Aires and Santa Fe Provinces (Trémouilles, 1995, 1998; Miller, 2000). We report two new species for Argentina (H. conicus (Zimmermann) and H. spiroductus Miller) in Corrientes Province. Desmopachria was known from Buenos Aires, Chaco, Chubut, Córdoba, Entre Ríos, Formosa, Jujuy, Mendoza, Misiones, Neuquén, Salta, San Luis, Santa Fe and Santiago del Estero Provinces (Michat & Archangelsky, 2007; Trémouilles, 1995; Torres et al., 2008). On the other hand, the distributional range of Pachydrus included Buenos Aires, Chaco, Entre Ríos, Misiones and Jujuy Provinces (Trémouilles, 1995; Torres et al., 2008), and that of Derovatellus included Tucumán, Santa Fe, Entre Ríos and Buenos Aires Provinces (Miller, 2005; Torres et al., 2007; Trémouilles, 1995). This paper reports the presence of Desmopachria, Pachydrus and Derovatellus in Corrientes Province. Celina distributes in Formosa, Chaco, Tucumán, Corrientes, Santa Fe, Entre Ríos and Buenos Aires Provinces (Torres et al., 2007; Trémouilles, 1995). This paper presents the first record of this genus for Misiones Province. Finally, Rhantus was cited for all Argentinean provinces except Chaco, Formosa, Mendoza and Santiago del Estero (Archangelsky, 2004; Balke, 1992; Trémouilles, 1984, 1995) and Lancetes was cited for all Argentinean provinces except Chaco, Entre Ríos, Formosa, Santa Fe and Santiago del Estero (Bachmann & Trémouilles, 1981; Nilsson, 2001; Torres et al., 2008). In this paper Chaco and Mendoza Provinces are added to the distribution of Rhantus and Santa Fe Province to the distribution of Lancetes.
Appendix 1. Glossary of the terms used in the key
Acuminate: with an acute extreme.
Accessory stria of elytron: short longitudinal groove situated near the basal margin of the elytron, between the elytral commissure and the basal stria.
Antennomere: antennal article.
Basal stria of elytron: short longitudinal groove situated near the anterior margin of the elytron.
Cervical stria: transverse groove crossing the dorsal surface of the head posterior to the eyes.
Clypeus: anterior portion of the head, to which the labrum is attached anteriorly.
Coxal cavity: more or less rounded area surrounded by the sternal and pleural sclerites, in which the coxa articulates.
Elytral apex: distal (posterior) portion of the elytron.
Elytral commisure: straight line along which the elytra meet medially.
Elytral stria: longitudinal groove of the elytron.
Epipleuron: ventral portion of the elytron, situated external to the lateral margins of mesothorax, metathorax and abdomen.
Fasciate: coloration pattern composed of bands whose main axes are perpendicular to the longitudinal axis of the body.
Ferrugineous: reddish-brown color.
Humeral angle: anterolateral angle of the elytron, situated posterior to the pronotum.
Iridescent: reflecting the colors of the rainbow.
Mesoepimeron: posterior region of the mesothoracic pleuron, situated anteriorly to the metaepisternum.
Metacoxa: first segment of the metathoracic leg, fused to the metasternum.
Metacoxal line: longitudinal groove dividing the metacoxa in two; homologous to the basicostal sulcus of other insects.
Metacoxal process: portion of the metacoxa situated posteriorly between the dividing line of the coxae and the coxa-trochanter articulation.
Metaepisternum: anterior region of the metathoracic pleuron, triangular-shaped, situated posteriorly to the mesoepimeron and between the metasternal wing and the epipleuron.
Metasternal wing: leaf-like portion of the metasternum expanding between the metaepisternum and the metacoxa.
Metasternum: ventral sclerite of metathorax, surrounded by the posterior margin of the mesocoxae and the anterior margin of the metacoxae.
Metatibial spur: spine-shaped and mobile process situated distally on the metatibia.
Oblongum cell: a closed cell in the metathoracic wing.
Posterior pronotal stria: short longitudinal groove situated near the posterior margin of the pronotum.
Pronotum: dorsal portion of the prothorax.
Prosternal process: portion of the prosternum extending posteriorly between the procoxae.
Prosternum: ventral portion of the prothorax, situated posterior to the head.
Scutellum: triangular area of the mesonotum, situated posterior to the pronotum and between the bases of the elytra; it may be covered by the posterior margin of the pronotum resulting in a not exposed scutellum.
Sexual dimorphism: presence of morphological characters particular to one sex, that are absent in the other; it does not correspond to the sexual organs.
Stridulatory apparatus: sound-producing organ consisting of an active structure (plectrum) and a passive structure (file).
Tarsomere: tarsal article.
Testaceous: brownish-yellow color.
Truncate: apparently incomplete, with the aspect of having been cut or interrupted abruptly.
Vittate: coloration pattern composed of bands whose main axes are parallel to the longitudinal axis of the body.
ACKNOWLEDGEMENTS
We thank two anonymous referees for their helpful comments on the manuscript. This project was funded by an undergraduate scholarship from the University of Buenos Aires, and by the following institutions: National Scientific and Technical Research Council (CONICET PIP 112-200801-02759), National Agency for Scientific and Technological Promotion (ANPCyT PICT-2007-01438 and PICT-2010-0526), and University of Buenos Aires (UBACyT-20020090300135).
LITERATURE CITED
1. ARCHANGELSKY, M. 2004. Nuevas citas de Coleoptera acuáticos y Megaloptera para la provincia de Chubut (Argentina). Rev. Soc. Entomol. Argent. 63(3-4): 66-68. [ Links ]
2. ARCHANGELSKY, M., V. MANZO, M. C. MICHAT & P. L. M. TORRES. 2009. Coleoptera. In: Domínguez, E. & H. R. Fernández (eds.), Macroinvertebrados bentónicos sudamericanos: sistemática y biología, Fundación Miguel Lillo, Tucumán, pp. 411-468. [ Links ]
3. BACHMANN, A. O. & E. R. TRÉMOUILLES. 1981. El género Lancetes en la Argentina continental (Coleoptera, Dytiscidae). Physis 39(97): 103-118. [ Links ]
4. BALKE, M. 1992. Taxonomische Untersuchungen an neotropischen Wasserkäfern der Gattung Rhantus Dejean (Insecta, Coleoptera: Dytiscidae). Reinchenbachia 29(6): 27-39. [ Links ]
5. BALKE, M., M. A. JÄCH & L. HENDRICH. 2004. Insecta: Coleoptera. In: Yule, C. M. & H. S. Yong (eds.), Freshwater invertebrates of the Malaysian Region, Academy of Sciences Malaysia, Kuala Lumpur, pp. 555-609. [ Links ]
6. BENETTI, C. J., J. A. RÉGIL CUETO & G. L. FIORENTIN. 2003. Gêneros de Hydradephaga (Coleoptera: Dytiscidae, Gyrinidae, Haliplidae, Noteridae) citados para o Brasil, com chaves para identificação. Biota Neotropica 3(1): 1-20. [ Links ]
7. FERNÁNDEZ-DÍAZ, M, C. J. BENETTI & J. GARRIDO. 2008. Influence of iron and nitrate concentration in water on aquatic Coleoptera community structure: Application to the Avia River (Ourense, NW Spain). Limnetica 27(2): 285-298. [ Links ]
8. JÄCH, M. A. & M. BALKE. 2008. Global diversity of water beetles (Coleoptera) in freshwater. Hydrobiologia 595: 419-442. [ Links ]
9. LARSON, D. J., Y. ALARIE & R. E. ROUGHLEY. 2000. Predaceous Diving Beetles (Coleoptera: Dytiscidae) of the Nearctic Region, with emphasis on the fauna of Canada and Alaska. NRC Research Press, Ottawa. [ Links ]
10. MICHAT, M. C. & ARCHANGELSKY, M. 2007. Descriptions of larvae of Desmopachria Babington (Coleoptera: Dytiscidae: Hydroporinae): the D. vicina Sharp species group. Coleopt. Bull. 61(2): 264-276. [ Links ]
11. MICHAT, M. C., M. ARCHANGELSKY & A. O. BACHMANN. 2008. Generic keys for the identification of larval Dytiscidae from Argentina (Coleoptera: Adephaga). Rev. Soc. Entomol. Argent. 67: 17-36. [ Links ]
12. MICHAT, M. C. & P. L. M. TORRES. 2006. The unknown larva of Anodocheilus Babington (Coleoptera: Dytiscidae: Hydroporinae: Bidessini): description of A. maculatus Babington and chaetotaxic considerations. Trans. Am. Entomol. Soc. 132(3-4): 431-444. [ Links ]
13. MILLER, K. B. 2000. Revision of the Neotropical genus Hemibidessus Zimmermann (Coleoptera: Dytiscidae: Hydroporinae: Bidessini). Aquat. Insects 23(4): 253-275. [ Links ]
14. MILLER, K. B. 2001. Revision of the genus Agaporomorphus Zimmermann (Coleoptera: Dytiscidae). Ann. Entomol. Soc. Am. 94(4): 520-529. [ Links ]
15. MILLER, K. B. 2002. Revision of the subfamily Hydrodytinae Miller (Coleoptera: Dytiscidae) with description of a new genus. Insect Syst. Evol. 33: 1-8. [ Links ]
16. MILLER, K. B. 2005. Revision of the New World and south-east Asian Vatellini (Coleoptera: Dytiscidae: Hydroporinae) and phylogenetic analysis of the tribe. Zool. J. Linn. Soc. 144: 415-510. [ Links ]
17. NILSSON, A. N. 2001. World Catalogue of Insects, volume 3: Dytiscidae (Coleoptera). Apollo Books, Stenstrup. [ Links ]
18. OHBA, S. & M. TAKAGI. 2010. Predatory ability of adult diving beetles on the Japanese encephalitis vector Culex tritaeniorhynchus. J. Am. Mosquito Control Assoc. 26(1): 32-36. [ Links ]
19. SÁNCHEZ-FERNÁNDEZ, D, P. ABELLÁN, A. MELLADO, J. VELASCO & A. MILLÁN. 2006. Are water beetles good indicators of biodiversity in Mediterranean aquatic ecosystems? The case of the Segura river basin (SE Spain). Biodiversity Conserv. 15: 4507-4520. [ Links ]
20. TORRES, P. L. M., S. A. MAZZUCCONI & M. C. MICHAT. 2007. Los coleópteros y heterópteros acuáticos del Parque Nacional El Palmar (Provincia de Entre Ríos, Argentina): lista faunística, diversidad y distribución. Rev. Soc. Entomol. Argent. 66(3-4): 127-154. [ Links ]
21. TORRES, P. L. M, S. A. MAZZUCCONI, M. C. MICHAT & A. O. BACHMANN. 2008. Los coleópteros y heterópteros acuáticos del Parque Nacional Calilegua (Provincia de Jujuy, Argentina). Rev. Soc. Entomol. Argent. 67: 127-144. [ Links ]
22. TRÉMOUILLES, E. R. 1984. El género Rhantus Dejean en la Argentina (Coleoptera, Dytiscidae). Physis 42(102): 9-24. [ Links ]
23. TRÉMOUILLES, E. R. 1995. Insecta, Coleoptera, Dytiscidae. Fascículo 1. Dytiscidae: Methlinae-Hydroporinae. Fauna de Agua Dulce de la República Argentina37: 1-82. [ Links ]
24. TRÉMOUILLES, E.R. 1998. Dytiscidae. In: Morrone, J. J. & S. Coscarón (eds.), Biodiversidad de artrópodos argentinos. Una perspectiva biotaxonómica, Ediciones Sur, La Plata, pp. 210-217. [ Links ]
25. TRÉMOUILLES, E. R., A. OLIVA & A. O. BACHMANN. 1995. Insecta Coleoptera. In Lopretto, E. C. & G. Tell (eds.), Ecosistemas de aguas continentales, Metodologías para su estudio, tomo III, Ediciones Sur, La Plata, pp. 1133-1197. [ Links ]
26. TRÉMOUILLES, E. R., P. L. M. TORRES & M. C. MICHAT. 2004. New distributional records and comments for the species of the genus Queda (Coleoptera: Dytiscidae). Rev. Soc. Entomol. Argent. 63(1-2): 38-40. [ Links ]
27. YOUNG, F. N. 1986. Review of the water beetles of the genus Bidessodes Régimbart (Coleoptera, Dytiscidae). Entomol. Basil. 11: 203-220. [ Links ]














