Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista de la Sociedad Entomológica Argentina
versión impresa ISSN 0373-5680
Rev. Soc. Entomol. Argent. vol.71 no.1-2 Mendoza ene./jun. 2012
TRABAJOS CIENTÍFICOS
Differences in wing melanization and pigmentation pattern in Drosophila buzzatii (Diptera: Drosophilidae) under chemical stress
Diferencias en la melanización y el patrón de pigmentación alar en Drosophila buzzatii (Diptera: Drosophilidae) bajo estrés químico
Mongiardino Koch, Nicolás, Esteban Hasson and Ignacio M. Soto
Instituto de Ecología, Genética y Evolución de Buenos Aires (IEGEBA - CONICET). Departamento de Ecología, Genética y Evolución. Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. Ciudad Universitaria, Pabellón II (C1428 EHA). Buenos Aires. Argentina. Tel: +54-011-4576-3348 - Fax: +54-011-4576-3354; e-mail: nmongiar@ege.fcen.uba.ar
Recibido: 14-XII-2011
Aceptado: 9-IV-2012
ABSTRACT. Recently, the genetic basis and developmental mechanisms underlying the process of melanization have become progressively elucidated, allowing us to study the evolutionary processes that govern the huge variability of pigmentation observed in nature. However, environmental factors contributing to such variability have received little attention, even though they might have profound ecological consequences. Here we describe a method for analyzing the wing melanization patterns of drosophilids in both qualitative and quantitative ways. We test this method on wings of Drosophila buzzatii Patterson & Wheeler individuals, reared in control and alkaloid-enriched mediums. The alkaloids were extracted from the secondary host of these flies, Echinopsis terscheckii (Parm. ex Pfeiff.) Friedrich & Rowley, and their effect on wing pigmentation is analyzed, bearing in mind the adverse effects that these compounds have on the development of D. buzzatii. Alkaloid-reared flies were shown to attain a higher degree of wing melanization, accompanied with differences in the spatial distribution of the pigment. Modifications at both metabolic and gene regulatory levels are proposed to explain the changes that chemical stressful conditions are shown to induce in this character. We propose the utilization of this procedure in studies of environmental dependence of pigmentation.
KEYWORDS. Drosophila buzzatii; Melanin. Ecotoxicology; Alkaloids; Stress
RESUMEN. Recientemente, hemos avanzado en nuestra comprensión de las bases genéticas y los mecanismos subyacentes al proceso de melanización durante el desarrollo, lo que permite el estudio de los procesos evolutivos que gobiernan la variabilidad de pigmentación presente en la naturaleza. Sin embargo, los factores ambientales que contribuyen a dicha variabilidad han recibido poca atención, aún cuando pueden conllevar profundas consecuencias a nivel ecológico. En este trabajo, presentamos un método para analizar los patrones de melanización alar de drosofílidos, de forma cualitativa y cuantitativa. Ponemos a prueba esta metodología comparando las alas de Drosophila buzzatii Patterson & Wheeler, criadas en medio control y con el agregado de alcaloides. Los alcaloides fueron extraídos del huésped secundario de estas moscas, Echinopsis terscheckii (Parm. ex Pfeiff.) Friedrich & Rowley, los cuales, se sabe, presentan efectos negativos en el desarrollo de D. buzzatii. Las moscas criadas en un medio enriquecido con alcaloides mostraron una mayor melanización alar, junto con diferencias en la distribución espacial del pigmento. Planteamos modificaciones a nivel metabólico y de expresión génica para explicar los cambios que las condiciones de estrés inducen sobre el carácter. Proponemos la utilización de este procedimiento para el estudio de la dependencia ambiental de la melanización.
PALABRAS CLAVE. Drosophila buzzatii; Melanina; Ecotoxicología; Alcaloides; Stress
INTRODUCTION
Insect melanism has long been a case study in evolutionary biology, providing some of the most conspicuous examples of natural selection (Kettlewell, 1973), genetic regulation (Wittkopp et al., 2002b) and pleiotropic effects (Wittkopp & Beldade, 2009). Melanism is also one of the simplest and most common examples of biodiversity in nature (True, 2003); occuring both as intraspecific polymorphisms and as fixed differences between closely related species, having profound influences on many aspects of insect biology. From an ecological point of view, pigmentation has been proposed to play an important role in thermoregulation, resistance to desiccation, ultraviolet radiation and parasitism, crypsis, aposematism, mate choice and courtship behavior (Wittkopp et al., 2003a; True, 2003). Likewise, the recent effort devoted to elucidate the genetic architecture of pigmentation in Drosophila, revealed the key roles that few genes have on the final body patterning, and showed how small changes in the regulation of such genes can be responsible for the wide array of evolutionary differences within these model organisms (True, 2003).
As a particular case of body pigmentation, wing pigmentation has received considerable attention. Wing melanization patterns are established during fly ontogeny by two separate mechanisms acting at different instances in the life cycle (True et al., 1999). The first step involves an enzymatic pre-patterning that occurs before eclosion and during wing development. During this step, the ubiquitous expression of the gene pale is sufficient to produce wing pigmentation patterning in D. melanogaster (a rather unpigmented species), an effect that is enhanced by the co-expression of ddc (True et al., 1999). pale encodes the enzyme tyrosine hydroxylase (TH) that catalyzes the conversion of tyrosine to dopa and ddc encodes dopa decarboxylase (DDC) that is responsible for the conversion of dopa to dopamine (Sugumaran, 1988). Also, the amount of the protein encoded by yellow in various regions of the embryo and the pupa correlates directly with the intensity of melanization over any section of the epidermis (Walter et al., 1991). Moreover, several interspecific differences in the pattern of wing pigmentation have been shown to arise in association with the evolution of cis-regulatory elements that control the expression of the yellow locus (Gompel et al., 2005; Prud'homme et al., 2006; Jeong et al., 2006). A similar regulatory network has been demostrated to modulate the expression of tan (Jeong et al., 2008) and ebony (Hovemann, 1991), genes also encoding enzymes involved in melanin synthesis.
The second step involves the diffusion of melanin precursors from the hemolymph of wing venation system (True et al., 1999).
These precursors are oxidized to melanin in the extracellular matrix, during which cross-linking to proteins and chitin takes place (Hopkins & Kramer, 1992). Therefore, this step is highly susceptible to both vein positioning and hemolymph content. Apparently, the same developmental processes involved in the melanin pigmentation of wings are shared by all drosophilids (True et al., 1999). This profound knowledge of the genetic basis of wing pigmentation contrasts with the scarcity of studies investigating environmental factors that may affect wing pigmentation (Lee et al., 2008), leading to the impression that genetically induced variations are more widespread and of superior biological significance (Karl et al., 2009).
Drosophila buzzatii Patterson and Wheeler is a saprophytofagous insect of the repleta group that uses cactus species of the genus Opuntia Mill. (prickly pears) and columnar cacti of the genera Echinopsis Zucc. and Cereus Mill. as primary and secondary breeding sites, respectively (Hasson et al., 1993). These two types of cacti have been shown to differ in the relative concentration of secondary metabolites; columnar cacti, in general, are richer in toxic compounds such as alkaloids (Fogleman & Danielson, 2001) which are known to obstruct neurotransmission (reviewed in Schoonhoven et al., 2005). Thus, it may be argued that the utilization of these columnar cacti as breeding hosts may constitute an adaptive challenge for the flies, since the presence of alkaloids may generate a stressful environment for the developing larvae and the feeding adults (Corio et al., 2010). Survival under these conditions must involve complex changes in gene regulation and general metabolism both at the larval and adult stages, most likely affecting several aspects of adult fitness. For example, flies reared in an alkaloid enriched medium have lowered viability and exhibit abnormalities in wing morphology and structure (Corio et al., 2010; Padró et al., 2011).
Cuticle pigmentation might be another affected trait in such a chemically adverse environment, first of all due to its dependence on the individual's general metabolism (True et al., 1999). Also, the enzymatic pre-patterning step of wing melanization has been shown to be highly dependent on the expression levels and the regulation network of a handful of genes (Wittkopp et al., 2003a), some of which encode enzymes involved in the synthesis of catecholamines (Nagatsu et al., 1964). These compounds act as neurotransmitters, neuromodulators and regulators of the secretion of other hormones (Brown & Nestler, 1985) and, therefore, their expression levels are expected to vary under stressful conditions. Finally, hemolymph levels of biogenic amines (dopamine included) have been shown to increase under unfavourable conditions in several species of insects (Kozanek et al., 1988; Rauschenbach et al., 1993; Hirashima et al., 1994). This response to the stressor is non-specific, arising under the action of stressors of different origins (Hirashima & Eto, 1993). A differential wing pigmentation pattern should arise as a consequence of any of these changes.
Here we describe a methodological procedure to analyze the impact that host chemistry (especially alkaloids) may have on the amount and allocation of melanin in the wings of D. buzzatii. We test this method by analyzing the different levels of wing pigmentation and the alterations in the spatial pattern of melanin distribution associated with the presence of alkaloids in the rearing medium.
MATERIAL AND METHODS
For this study we used an outbred stock of Drosophila buzzatii flies, maintained in the laboratory for approximately two years, which was originally founded with bait-collected individuals from a natural population of these flies in the vicinities of Valle Fértil (San Juan, Argentina: 30° 50' 28" S, 67° 40' 08" W). Fresh tissues of Echinopsis terscheckii (Parm. ex Pfeiff.) Friedrich & Rowley (a columnar cactus known as "Cardón") were also collected in the same locality, where D. buzzatii is known to use it as a secondary host. The sample consisted of the apical part of a lateral branch (10 kg approximately), which was transported refrigerated and maintained freezed for 15 days before performing the alkaloid extraction.
For the alkaloid extraction, we followed the procedure of Obungodede et al., 2010. A sample of aerial parts consisting of the outermost parenchyma and chlorenchyma, the last being the tissue of higher alkaloid concentration within the plant (Ogunbodede et al., 2010), was grinded and blended with EtOH (1L/1kg) and then filtered. The organic extract was concentrated on a rotatory evaporator to an aqueous suspension and 500 mL of 10% HCl were added. The acidic fraction was then extracted three times with CH2Cl2 (500 mL). The acidic fraction was then taken to pH = 8 with NaHCO3 and extracted three more times with CH2Cl2 (500 mL). The organic fraction was taken to dryness to yield a crude alkaloid fraction. This alkaloid fraction was solubilized in dimethyl sulfoxide (DMSO 100 gr/mL).
Several egg-collecting chambers were set up with a petri dish containing egg laying medium (agar 2% + 5 ml of 3 parts of ethanol and 1 part of 60% acetic acid). One hundred pairs of sexually mature flies were released into each chamber. Petri dishes were removed 12 hours later, inspected for the presence of eggs and incubated for another 24 hours to allow larval hatching. Five groups (replicates) of 30 first instar larvae were seeded in vials containing either Instant Laboratory Medium, or Instant Laboratory Medium with the addition of the alkaloid fraction isolated from E. terscheckii, in a final concentration similar to that found in fresh tissues of the cacti in the locality sampled (4.5 mg/gr of dry weight, C. Corio, personal communication). Larvae were reared under a 12:12h light/dark photoperiod, 25 ± 1 °C and 60 ± 10% relative humidity. Newly eclosed adults were kept in vials for 2 h to allow wing extension.
Afterwards, flies were collected, sexed and both wings of each female were removed and mounted on a flat glass slide using DPX clear mounting solution and a glass cover slip. Thirty flies of each treatment were randomly selected, and digital photographs of the right wings were taken using a Leica MZ6 binocular microscope with a Canon PowerShot S50 digital camera attached to a computer, under 2.5x objective and 1.3x camera coupler magnification. In order to minimize the potential biases on wing coloration, photographs were taken under constant ambient illumination and the same white background was used for all pictures.
Photographs were analyzed using Adobe Photoshop CS2, using a modification of the procedure described in Shamble et al. (2009). The polygonal lasso tool was used to delimit a central region of the wing, circumscribed by the lines joining four landmarks (P.1-P.4), as shown in Figure 1. This rendered an area of high wing coverage that had a high repeatability and was almost entirely independent of any abnormality (cracks, folds, etc.) or foreign objects (bubbles, bristles, etc.) that may have originated during wing manipulation. This area was subsequently converted into grey-scale, and values of the mean, median, and standard deviation of the image intensity (a numerical reading where 255 is white and 0 is black) were recorded. The total number of pixels inside the area was also recorded and used as an estimate of total wing area.

Fig. 1. Normal pattern of D. buzzatii wing pigmentation, showing a relatively homogenous and light coloration. Landmarks P.1 to 4 were used to delimit the area of study. For the construction of the composite images, landmarks P.1, 2, 4 and E.1, 2 were used.
To analyse the distribution of pigment in the two experimental groups of flies (Control and Alkaloid-treated flies), the threshold command was used, turning white those pixels with a value of intensity lower to a certain value and black those pixels that exceeded the threshold value, allowing us to transform the pictures into high-contrast, 2-tone images. The threshold was set at one half standard deviation darker than the mean value of intensity, using the values proper to each treatment. Due to the heterogeneity of the wing's structure, with a wide area of lightly pigmented tissue crossed by highly melanized veins, only the latter turned black when the threshold was set at one standard deviation darker than the media, rendering no possible comparison between treatments. We finally settled with half a standard deviation because it maximized the contrast between groups. After the threshold was applied, the number of black pixels was recorded.
The threshold action was reversed in order to return to the images' normal contrast levels, and the magnetic lasso tool was used to select exclusively the areas covered by veins. Veins were then eliminated and a new threshold was applied to the image in order to analyze exclusively inter-vein regions. The amount of pixels darker than the mean minus half a standard deviation was recorded once again, allowing us to separate the previously obtained amount of pixels in a vein and a non-vein fraction. The relative proportions of these fractions were obtained by dividing by the total amount of black pixels.
We tested for differences in mean and median values of colour intensity, standard deviation of colour intensity distribution, total number of black pixels, amount of black pixels in veins and in intervein regions. In all cases, one way ANOVAs were performed to analyze differences between control and alkaloid-treated flies. For this and all other statistical analysis we used the package Statistica (Statsoft, 2001).
Finally, a composite image of each treatment was created by superimposing the 30 wings of each group using the TpsSuper tool (Rohlf, 2003). The images were previously digitalized using TPSdig program; the five landmarks used are also shown in Fig. 1. These images represented the average positioning of the darkest regions of pigmented tissue in both conditions, control and alkaloid-reared.
RESULTS
Different types of abnormalities in the pigmentation pattern of the wings were detected (Fig. 2A-B). Abnormalities were exclusively observed in alkaloid-reared flies, and spanned from extreme cases of irregular wing melanization (Fig. 2A), to moderate cases of melanization circumscribed to areas near veins (Fig. 2B).

Fig. 2. Different types of wing melanization patterns. (A) Massive and irregular wing melanization. (B) Moderate melanization, dependent on wing positioning (contrast and brightness level modified for illustrative purposes). (C) Pattern present in more than half of the flies reared under normal conditions, here shown after threshold application.
Among the wings of control flies, more than half showed a distinctive pattern of pigmentation in which the melanized regions of the wing were separated from the veins by a less pigmented area, being restricted to the centre of the intervein regions (Fig. 2C). The remaining control flies showed a more homogenous pigment distribution (Fig. 1).
Differences in the amount of wing pigmentation among flies were analyzed by means of ANOVAs, using either the mean or the median values of images' intensity (Table I). Flies reared in an alkaloid-enriched medium were significantly darker than the ones reared in standard conditions, suggesting that wing pigmentation is quantitatively affected by the presence of alkaloids during larval development.
Table I. Descriptive statistics of wing images intensity distribution, and the inferred wing size and areas of dark pigmentation. The respective F-values, degrees of freedom (DF) and p-values obtained in ANOVAs are also shown. (Lower numbers of image intensity represent darker coloration; significant differences are marked with an asterisk).

In addition, significant differences were detected when contrasting the standard deviation of the image's intensity distribution, with higher values among individuals reared under stressful conditions (Table I). This results shows that there is not only a difference in the amount of dark pigment in the wing's cuticle between alkaloid and control flies, but also that it is distributed along the wing in a different pattern, resulting in a modification of the picture's histogram rather than just a translocation towards darker values. Fig. 3 shows wing photographs representative of the alkaloid (A) and control (B) phenotypes with their respective histograms, showing how flies reared in an alkaloid-enriched medium attain darker mean values and a wider intensity distribution. Therefore, both quantitative and qualitative changes are being induced in wing pigmentation in flies reared in the presence of alkaloids.
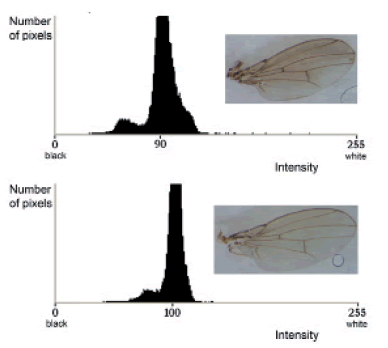
Fig. 3. Representative images of wing phenotypes generated under stressful (A) and control (B) conditions, along the respective histograms showing the distribution of intensity.
In order to further analyze changes in the patterning of pigmentation, we compared the amount of dark pixels in the images after applying the transformation with the threshold tool. The threshold was set at 90 for alkaloid treated flies and 92 for control flies, each value representing the group's media minus half its particular standard deviation. This analysis also revealed significant differences between treated and control flies (Table I). The composite pictures shown in Fig. 4 represent the average position of these pixels in both control and treated conditions, resulting in what could be thought of as the normal pigmentation and the chemically stressed pigmentation patterns (Fig. 4A and B, respectively). As can be seen, alkaloid-reared flies have an increased amount of pixels in the darker fraction of the distribution. Another salient aspect of the distribution of the pigment is that most variations occur in the anterior distal area of the wing, with slight variations in the proximal regions as well. No variation was observed in the posterior distal region, an area that was under-melanized in all individuals, independently of treatment.

Fig. 4. Composite images obtained by superimposing the 30 photographs of both control (A) and alkaloid (B) flies. The images represent the average pigmentation pattern in both situations. Note the intense darkening of the anterior distal region in alkaloid-reared flies.
In order to analyze exclusively the inter-vein regions, veins were eliminated from the grey-scale pictures and the threshold command was performed once again, using the same values. The resulting amount of pixels represented exclusively those distributed in the inter-vein regions, effectively partitioning the previous value into vein and non-vein pixels. The repeatability coefficient for the elimination of the venation system using the magnetic lasso tool was calculated (sensu Lessells & Boag, 1987), by making 5 successive measurements on 10 randomly selected individuals. The amount of dark pixels calculated after removal of veins showed a 98% coefficient of repeatability. To further test the effectiveness of this method to partition the amount of black pixels into the aforementioned categories, an ANCOVA was performed on the data, using size (estimated by the total amount of pixels in the study area) as a covariate. An unbiased method should reflect the dependence of the amount of vein black pixels with the wing's total size, while non-vein black pixels should not covariate in such a way. Significant covariation was observed for vein black pixels (F = 12.85; p value = 0.0007) but not for non-vein black pixels (F = 1.01; p = 0.3183), demonstrating the validity of the methodology proposed. Using the same statistical design the amount of vein pixels were shown not to present significant differences among treatments, while the amount of non-vein pixels did (Table I). This allowed us to confirm that a differential pigmentation patterning was being produced beyond any doubt, and that the non-vein tissue fraction of the total dark pixels was solely responsible for the significant differences previously obtained. Finally, we calculated the fraction of dark pixels in inter-vein regions for each treatment, which were 65% in alkaloid treated flies and 55% in controls (Table I).
CONCLUSIONS
In this paper we show that the presence of alkaloids in the rearing medium induces abnormalities in wing pigmentation in the fruit fly Drosophila buzzatii. This effect may be separated in two clearly distinguishable categories (shown in Fig. 2A-B): massive and disorganized cases of melanization (Fig. 2A), and moderate cases of melanization restricted to areas of the wing near veins (Fig. 2B). The latter phenotype is remarkably similar to the one observed in ebony mutants of D. melanogaster, in which an excessive amount of precursor is shunted to the melanin synthesis (Wright, 1987). A similar phenotype can also arise from excessive expression of the enzyme tyrosine hydroxylase (True et al., 1999), the rate-limiting enzyme in the metabolic chain leading to the production of melanin (Neckameyer & Quinn, 1989). However, other studies suggest that TH's activity may be down-regulated under stress conditions in Drosophila (Rauschenbach et al., 1995; Hirashima et al., 2000), and the stressful nature of alkaloids has already been confirmed by the decrease in survival, developmental time and wing size they induce in D. buzzatii (Hasson et al., 2009). Therefore, the increase in concentration of dopamine (the melanin precursor) in hemolymph may be a more plausible explanation for the development of such phenotype, although changes in enzyme activity cannot be discarded. On the other hand, the generation of the phenotype shown in Fig. 2A more likely involves multiple radical changes at both gene expression and metabolism regulation levels, given the substantial modification of both pigmentation intensity and distribution.
Our method also enabled a detailed analysis of the distribution of wing pigmentation under normal conditions. Subsequently, we detected variation in melanin wing patterning with some individuals displaying a homogenous distribution of melanin while others a concentration of melanin in areas away from veins (Fig. 2C). This phenotype was also observed in True et al. (1999) when overexpressing both TH and DDC enzymes, further supporting the aforementioned down-regulation of TH under stressful conditions.
Both qualitative and quantitative modifications of the normal pigmentation pattern were observed in the wings of D. buzzatii treated with the alkaloid fraction extracted from E. terscheckii, a secondary host plant in nature. Moreover, our study not only detected an overall increase in wing melanization, but also that the effect was mainly circumscribed to the distal anterior region of the wing, while the posterior distal region was consistently under-melanized in both conditions. These results are consistent with the expectations of the two-step mechanism of pigmentation (True et al., 1999). Though the biological consequences of these changes remain to be tested, our study demonstrates that the chemistry of the host affects the wing melanization in D. buzzatti. However, this modification is merely the cue that demonstrates that metabolic changes, accompanied by modifications at the gene expression level, are operating on the developing individual that emerges from its secondary host. The pleiotropic effects that these changes might be inducing could prove to be highly significant to the biology of cactophilic flies in general.
Differences in the final distribution of dark regions in the wings of alkaloid and control individuals were observed (Fig. 4), which we interpreted as the result of modifications in the enzymatic pre-patterning stage of wing pigmentation. This alteration may occur before eclosion of the imago due to changes in gene expression, resulting in the qualitative modifications already described. Candidate genes involved in these patterning differences are those encoding enzymes that intervene in the melanin biosynthesis pathway: pale, Ddc, yellow, ebony and tan (Ng et al., 2008), as well as the transcription factors that regulate their expression (Kopp & Duncan, 1997; Kopp et al., 2000). Differences in the expression of these genes are also involved in interspecific (Llopart et al., 2002; Wittkopp et al., 2002a; 2002b; 2003b) and intraspecific (Lindsley & Zimm, 1992) variations in wing pigmentation. On the other hand, given the wide array of cis-regulatory elements that modulate the expression of ebony, yellow and tan (Hovemann, 1991; Jeong et al., 2006; 2008; Gompel et al., 2005; Prud'homme et al., 2006), these genes possess a high degree of transcriptional plasticity that allows their expression to be modulated in accordance to the insects' environment. Moreover, the fact that they intervene in the synthesis of biogenic amines makes the genes involved in cuticle melanization, and therefore the character itself, an interesting subject for the study of stress response.
The greater mean, median and total amount of black pixels in the images of wings of alkaloid treated flies suggests that the presence of alkaloids in the rearing medium also affects the diffusion stage of pigmentation. Given that incremented catecholamine content in the hemolymph is a well-established characteristic of insect stress response (Kozanek et al., 1988; Rauschenbach et al., 1993; Hirashima et al., 1994), we formulate that the increased wing melanization is due to a higher amount of melanin precursor diffusing from the wing venation system of flies newly emerged from the puparium, as a consequence of the toxic environment produced by the presence of alkaloids in the rearing medium.
The methodology here proposed for the study of wing melanization allowed us to successfully characterize and describe changes in the amount and allocation of pigment in the Drosophila wing in response to chemical stress. To our knowledge, this is the first report of an environmental agent affecting cuticle melanization in Drosophila. The phenotypic changes in pigmentation that arise in response to such external factors may help to elucidate the underlying mechanisms in the evolution of wing pigmentation patterns in Drosophila.
ACKNOWLEDGMENTS
The authors wish to thank the helpful comments of J. Padró in early stages of this investigation. This work was supported by ANPCyT, CONICET and Universidad de Buenos Aires grants. EH and IMS are members of Carrera del Investigador Científico (CONICET).
LITERATURE CITED
1. BROWN, C. S. & C. NESTLER. 1985. Catecholamines and indolalkylamines. En: Kerkut, G.A. & L.I. Gilbert (eds.), Comparative insect physiology, biochemistry and pharmacology, Pergamon Press, Oxford, pp. 435-497. [ Links ]
2. CORIO, C., E. M. SOTO, M. BETTI, I. M. SOTO & E. HASSON. 2010. Efecto de los alcaloides sobre la aptitud en el modelo cactus-Drosophila. En: Actas y Trabajos de la IV Reunión Binacional de Ecología, Buenos Aires, 2010, p. 91. [ Links ]
3. FOGLEMAN, J. C. & P. B. DANIELSON. 2001. Chemical interactions in the cactus-microorganism-Drosophila model system of the Sonoran desert. Integr. Comp. Biol. 41 (4): 877-889. [ Links ]
4. GOMPEL, N., B. PRUD'HOMME, P. J. WITTKOPP, V. A. KASSNER & S. B. CARROLL. 2005. Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila. Nature 433 (3): 481-487. [ Links ]
5. HASSON, E., J. J. FANARA, C. RODRIGUEZ, J. C. VILARDI, O. A. REIG & A. FONTDEVILLA. 1993. The evolutionary history of Drosophila buzzatii XXVII. Genetica 91 (1): 61-65. [ Links ]
6. HASSON, E., I. M. SOTO, V. P. CARREIRA, C. CORIO, E. M. SOTO, M. I. BETTI. 2009. Host plants, fitness and developmental instability in a guild of cactophilic species of the genus Drosophila. En: Santos, E. B. (ed), Ecotoxicology Research Developments, Nova Publishers, New York, pp. 89-109. [ Links ]
7. HIRASHIMA, A. & M. ETO. 1993. Effect of stress on levels of octopamine, dopamine and serotonin in the American cockroach (Periplaneta americanaL.). Comp. Biochem. Physiol. C. 105 (2): 279:284. [ Links ]
8. HIRASHIMA, A., T. NAGANO, & M. ETO. 1994. Effect of various insecticides on the larval growth and biogenic amine levels of Tribolium castaneum Herbst. Comp. Biochem. Physiol. C. 107 (3): 393-398. [ Links ]
9. HIRASHIMA, A., M. J. SUKHANOVA & I. Y. RAUSCHENBACH. 2000. Biogenic amines in Drosophila virilis under stress conditions. Biosci. Biotechnol. Biochem. 64 (12): 2625-2630. [ Links ]
10. HOPKINS, T. L. & K. J. KRAMER. 1992. Insect cuticle sclerotization. Annu. Rev. Entomol. 37: 273-302. [ Links ]
11. HOVEMANN, B. 1991. Tissue specific expression of the ebony gene. J. Neurogenet. 7: 128. [ Links ]
12. JEONG, S., M. REBEIZ, P. ANDOLFATTO, T. WERNER, J. TRUE & S. B. CARROLL. 2008. The evolution of gene regulation underlies a morphological difference between two Drosophila sister species. Cell. 132 (5): 783-793. [ Links ]
13. JEONG, S., A. ROKAS & S. B. CARROLL. 2006. Regulation of body pigmentation by the Abdominal-B Hox protein and its gain and loss in Drosophila evolution. Cell. 125 (7): 1387-1399. [ Links ]
14. KARL, I., T. L. GEISTER & K. FISCHER. 2009. Intraspecific variation in wing and pupal melanization in copper butterflies (Lepidoptera: Lycaenidae). Biol. J. Linn. Soc. 98 (2): 301-312. [ Links ]
15. KETTLEWELL, B. 1973. The evolution of melanism, the study of a recurring necessity with special reference to industrial melanism in the lepidoptera. Clarendon, Oxford. [ Links ]
16. KOPP, A. & I. DUNCAN. 2000. Technical tips for analyzing gene expression in the pupal abdomen of Drosophila. Drosophila Inf. Serv. 83: 196-197. [ Links ]
17. KOPP, A., I. DUNCAN & S. B. CARROLL. 2000. Genetic control and evolution of sexually dimorphic characters in Drosophila. Nature 408: 553-559. [ Links ]
18. KOZANEK, M., M. JURANI & E. SOMOGYIOVA. 1988. Effect of long-term stress on monoamine concentration in CNS of cockroach Nauphoeta cinerea. En: Sehnal, F. , A. Zabza & D. L. Denlinger (eds.), Endocrinological frontiers in physiological insect ecology, Wroclaw Technical University Press, Wroclaw, pp. 161-167. [ Links ]
19. LEE, K. P., S. J. SIMPSON & K. WILSON. 2008. Dietary protein-quality influences melanization and immune function in an insect. Funct. Ecol. 22 (6): 1052-1061. [ Links ]
20. LESSELS, C. M. & P. T. BOAG.1987. Unrepeatable repeatabilities: a common mistake. Auk 104 (1): 116-121. [ Links ]
21. LINDSLEY, D. L. & G. G. ZIMM. 1992. The genome of Drosophila melanogaster. Academic Press, San Diego. [ Links ]
22. LLOPART, A., S. ELWYN, D. LACHAISE & J. A. COYNE. 2002. Genetics of a difference in pigmentation between Drosophila yakuba and Drosophila santomea. Evolution 56 (11): 2262-2277. [ Links ]
23. NAGATSU, T., M. LEVITT & S. UDENFRIEND. 1964. Tyrosine hydroxylase: the initial step in norepinephrine biosynthesis. J. Biol. Chem. 239 (9): 2910-2917. [ Links ]
24. NECKAMEYER, W. S. & W. G. QUINN. 1989. Isolation and characterization of the gene for drosophila tyrosine hydroxylase. Neuron. 2 (2): 1167-1175. [ Links ]
25. NG, C. S., A. M. HAMILTON, A. FRANK, O. BARMINA & A. KOPP. 2008. Genetic basis of sex-specific color pattern variation in Drosophila malerkotliana. Genetics 180 (1): 421-429. [ Links ]
26. OGUNBODEDE, O., D. MCCOMBS, K. TROUT, P. DALEY & M. TERRY. 2010. New mescaline concentrations from 14 taxa/cultivars of Echinopsis spp. (Cactaceae) ("San Pedro") and their relevance to shamanic practice. J. Ethnopharmacol. 131: 356-362. [ Links ]
27. PADRÓ, J., N. MONGIARDINO KOCH, P. FONTANARROSA, V. CARREIRA, C. CORIO, E. HASSON & I. M. SOTO. 2011. Ecotoxicología evolutiva: efectos letales y teratogénicos de alcaloides naturales presentes en el hospedador secundario de moscas cactófilas. Acta Toxicol. Argent. 19 (Suplem.): 30-116. [ Links ]
28. PRUD'HOMME, B., N. GOMPEL, A. ROKAS, V. A. KASSNER, T. M. WILLIAMS, S. D. YEH, J. R. TRUE & S. B. CARROLL. 2006. Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature 440: 1050-1053. [ Links ]
29. RAUSCHENBACH, I. Y., L. I. SEROVA, I. S. TIMOCHINA, N. A. CHENTSOVA & I. V. SCHUMNAJA. 1993. Analysis of differences in dopamine content between two lines of Drosophila virilis in response to heat stress. J. Insect Physiol. 39 (9): 761-767. [ Links ]
30. RAUSCHENBACH, I. Y., L. V. SCHUMNAJA, T. M. KHLEBODAROVA, N. A CHENTSOVA & L. G. GRENBACK. 1995. Role of phenol oxydases and tyrosine hydroxylase in control of dopamine content in Drosophila virilis under normal conditions and heat stress. J. Insect Physiol. 41 (3): 279-286. [ Links ]
31. ROHLF, F. J. 2003. TpsSuper ver. 1.38. Available at http://morph.bio.sunysb.edu/morph/index.html. Department of Ecology and Evolution, State University of New York. [ Links ]
32. SCHOONHOVEN, L. M., J. J. A. VAN LOON & M. DICKE. 2005. Insect-plant biology, 2nd ed. Oxford University Press, Oxford. [ Links ]
33. SHAMBLE, P. S., D. J. WILGERS, K. A. SWOBODA & E. A. HEBETS. 2009. Courtship effort is a better predictor of mating success than ornamentation for male wolf spiders. Behav. Ecol. 20 (6): 1242-1251. [ Links ]
34. StatSoft, Inc. STATISTICA (data analysis software system), version 6. 2001. www.statsoft.com [ Links ]
35. SUGUMARAN, M. 1988. Molecular mechanisms of cuticular sclerotization. Adv. Insect Physiol. 21: 179-231. [ Links ]
36. TRUE, J. R. 2003. Insect melanism: the molecules matter. Trends Ecol. Evol. 18 (12): 640-647. [ Links ]
37. TRUE, J. R., K. A. EDWARDS, D. YAMAMOTO & S. B. CARROLL. 1999. Drosophila wing melanin patterns form by vein-dependent elaboration of enzymatic prepatterns. Curr. Biol. 9 (23): 1382-1391. [ Links ]
38. WALTER, M. F., B. C. BLACK, G. AFSHAR, A. Y. KERMABON, T. R. F WRIGHT & H. BIESSMAN. 1991. Temporal and spatial expression of the yellow gene in correlation with cuticle formation and dopa decarboxylase activity in Drosophila development. Dev. Biol. 147 (1): 32-45. [ Links ]
39. WITTKOPP, P. J. & P. BELDADE. 2009. Development and evolution of insect pigmentation: genetic mechanisms and the potential consequences of pleiotropy. Semin. Cell Dev. Biol. 20 (1): 65-71. [ Links ]
40. WITTKOPP, P. J., J. R. TRUE & S. B. CARROLL. 2002a. Reciprocal functions of the Drosophila yellow and ebony proteins in the development and evolution of pigmentation patterns. Development 129: 1849-1858. [ Links ]
41. WITTKOPP, P. J., K. VACCARO & S. B. CARROLL. 2002b. Evolution of yellow gene regulation and pigmentation in Drosophila. Curr. Biol. 12 (18): 1547-1556. [ Links ]
42. WITTKOPP, P. J., S. B. CARROLL & A. KOPP. 2003a. Evolution in black and white: genetic control of pigment patterns in Drosophila. Trends Genet. 19 (9): 495-504. [ Links ]
43. WITTKOPP, P. J., B. L. WILLIAMS, J. E. SELEGUE & S. B. CARROLL. 2003b. Drosophila pigmentation evolution: divergent genotypes underlying convergent phenotypes. P. Natl. Acad. Sci. USA 100 (4): 1808-1813. [ Links ]
44. WRIGHT, T. R. F. 1987. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv. Genet. 24: 127-222. [ Links ]














