Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Revista de la Sociedad Entomológica Argentina
versão impressa ISSN 0373-5680
Rev. Soc. Entomol. Argent. vol.71 no.1-2 Mendoza jan./jun. 2012
TRABAJOS CIENTÍFICOS
Whitefly species (Hemiptera: Aleyrodidae) on wild and cultivated plants in the horticultural region of Rosario, Santa Fe, Argentina
Especies de moscas blancas (Hemiptera: Aleyrodidae) sobre plantas silvestres y cultivadas en la región hortícola de Rosario, Santa Fe, Argentina
Gonsebatt, Gustavo G.*, Mariana M. Viscarret** and Marcela M. Lietti*
*Universidad Nacional de Rosario, Facultad de Ciencias Agrarias, Cátedra de Zoología Agrícola, C.C. 14, (S2125ZAA), Zavalla, Santa Fe, Argentina; e-mail: ggonseba@hotmail.com, mlietti@fcagr.unr.edu.ar
**INTA, IMYZA-CNIA, Insectario de Investigaciones para Lucha Biológica, C.C. 25, (1712), Castelar, Buenos Aires, Argentina; e-mail: mviscarret@inta.gov.ar
Recibido: 28-II-2012
Aceptado: 2-V-2012
ABSTRACT. Whiteflies of economic importance are polyphagous, being able to develop on a large number of cultivated and spontaneous plants. We recorded the whitefly species on vegetable and flower crops and the wild plants associated, under greenhouse and field conditions, for two years. We observed two species: Trialeurodes vaporariorum (Westwood) and the Bemisia tabaci complex (Gennadius). T vaporariorum was recorded on 24 plant species (11 families), 12 and 8 of which are new hosts in Argentina and in the world, respectively. The B. tabaci complex was recorded only on flower production systems, on 19 plant species (11 families), 14 and 7 of which are new hosts in Argentina and in the world, respectively. The crops Glycine max (L.) and Chrysanthemum morifolium Ramat., the wild species Amaranthus blitum L., Amaranthus quitensis Kunth, Conyza bonariensis (L.), Galinsoga parviflora Cav., Sonchus oleraceus L. and Wedelia glauca (Ortega) O. Hoffm. ex Hicken were hosts of both species. The only parasitoid recorded was Eretmocerus californicus near corni Haldeman (Hymenoptera: Aphelinidae) from T. vaporariorum. This study, which is the first systematic survey of host plants in the region, intends to provide a better knowledge of the range of whiteflies host plants in Argentina.
KEY WORDS. Bemisia tabaci; Trialeurodes vaporariorum; Host plants; Vegetable crops; Flower crops.
RESUMEN. Las moscas blancas de importancia económica son polífagas y capaces de desarrollarse sobre numerosas plantas cultivadas y espontáneas. Registramos las especies de moscas blancas sobre cultivos hortícolas y de flores, y sobre las plantas silvestres asociadas. Observamos dos especies: Trialeurodes vaporariorum (Westwood) y el complejo Bemisia tabaci (Gennadius). T. vaporariorum fue registrada sobre 24 especies de plantas (11 familias), 12 y 8 de las cuales son hospedantes nuevos para Argentina y a nivel mundial, respectivamente. El complejo B. tabaci fue registrado solo en sistemas de producción de flores, sobre 19 especies de plantas (11 familias), 14 y 7 de las cuales son nuevos hospedantes para Argentina y a nivel mundial, respectivamente. Los cultivos Glycine max (L.) y Chrysanthemum morifolium Ramat., las especies silvestres Amaranthus blitum L., Amaranthus quitensis Kunth, Conyza bonariensis (L.), Galinsoga parviflora Cav, Sonchus oleraceus L. y Wedelia glauca (Ortega) O. Hoffm. ex Hicken fueron hospedantes de ambas especies. El único parasitoide registrado fue Eretmocerus californicus cercano a corni Haldeman (Hymenoptera: Aphelinidae) sobre T. vaporariorum. Este estudio que constituye el primer relevamiento sistemático de plantas hospedantes en la región, aporta un mayor conocimiento sobre el rango de plantas hospedantes de las moscas blancas en Argentina.
PALABRAS CLAVE. Bemisia tabaci; Trialeurodes vaporariorum; Plantas hospedantes; Cultivos hortícolas; Cultivos de flores.
INTRODUCTION
Most of the cultivated area in the Province of Santa Fe (Argentina) is occupied by extensive crops, mainly soybean. However, around the most important cities, these crops are being replaced by vegetable and flower intensive crops. The intensive crop productions occupy 22,000 ha and represent around 6% of the Gross Agricultural Income. The horticultural region of Rosario city (Santa Fe) is one of the most important vegetable production areas of Argentina, due both to the quantity and diversity of its production and to the magnitude of its market. The intensive productions cover a surface of 5,462 ha, which include 3,060 ha cultivated with about 30 vegetable crops, mostly in open fields (Ferratto, 2006).
The surface cultivated with flowers covers 179,31 ha (60.5% of Santa Fe flower production area), with 89% and 11% of the cultivated area under greenhouse and field conditions, respectively. They are products of high economic value and great economic and social impact because of their high demand for labor. The most cultivated flower species are carnation, chrysanthemum, gypsophila, lisianthus and rose (Zuliani et al., 2007).
Whiteflies are phytophagous insects with several polyphagous pest species which may affect the biochemistry, physiology, anatomy and development of economically important crops, both under greenhouse and in open field crop production systems. Whiteflies cause damage directly by consuming largequantities of phloem sap and inducing physiological disorders. They also inflict damage indirectly through honeydew excretion on the surface of leaves and fruits, which serves as a medium for the development of sooty mold fungi, reduces photosynthesis and stains fruits, increasing post-harvest costs. Besides, a few species transmit plant viral diseases (Morales & Anderson, 2001; Inbar & Gerling, 2008).
Whiteflies of economic importance are polyphagous, being able to colonize and develop on a large number of weeds and spontaneous plants (Attique et al., 2003; Servín, 2004; Gonsebatt & Lietti, 2008). These alternative hosts provide shelter and food for whiteflies when their main host plant is absent from the field or is not in a suitable growth stage for them (Bezerra et al., 2004) and serve as a reservoir of whitefly-transmitted plant viruses (Rodríguez-Pardina et al., 2006). On the other hand, uncultivated plants indirectly provide shelter and food for natural enemies of whiteflies (Coudriet et al., 1986; Naveed et al., 2007; Scotta & Bertolaccini, 2007).
Several whitefly species of economic importance and their parasitoids have been recorded in Argentina (Tapia, 1970; Mound & Halsey, 1978; López & Botto, 1995; Helman et al., 1996; Viscarret et al., 2000; Remes Lenicov, 2004; Martin & Mound, 2007), but the information about their host plants is limited (Muruaga de L'Argentier et al., 1996; Viscarret, unpub.). Moreover, there are no detailed surveys of the whitefly species and their host plants for Santa Fe province or the horticultural region of Rosario. A proper taxonomic identification of the whitefly species and knowledge of the host range constitute indispensable basic information for effective management strategies.
The objective of this research was to survey the host plant-whitefly relationships on vegetable and flower crops and the wild plants associated in the horticultural area of Rosario and thus contribute to a better knowledge of the range of host plants in Argentina.
MATERIAL AND METHODS
The survey was conducted on vegetable and flowering farms located in Rosario, Villa Gobernador Gálvez, Pérez, and Arroyo Seco, in the horticultural region of Rosario (Santa Fe province, Argentina), between February 2005 and April 2007 (Fig 1).

Fig 1. Sampled locations in the horticultural region of Rosario, Santa Fe province, Argentina. Rosario F: Flower farm; Rosario H: Vegetable farm
We selected three plots from open field and three greenhouses from protected production systems, close to each other, with the same crop and presence of wild plants growing within them and at their edges. In each sampling date we chose plant species with presence of whiteflies. Fifteen plants of each species (of both cultivated and wild plants) per plot or greenhouse were selected at random to collect immature stages (nymphs and pupae). Two leaves or leaflets, depending on the leaf morphology of each plant species, from the middle and basal parts of each plant were detached and brought to the laboratory in plastic bags.
The full and empty pupae of the whiteflies collected without signs of parasitism were used for species identification. This material was submerged in alcohol 70% for one week and then mounted in Faure's or Hoyer's solution on a glass microscope slide. Species identification was carried out under a light compound microscope, following the key and work of Martin (1987).
The leaves or leaflets with whitefly pupae which showed signs of parasitism were placed in Petri dishes with a humidified cotton in a controlled environment at 25 ± 1 °C, 70 ± 10% relative humidity and under a photoperiod of 14:10 light:dark and observed twice a week in order to record parasitoid emergence. The adult parasitoids were stored in vials with alcohol 70% and sent to Dra. Silvia López, Insectario de Investigaciones para Lucha Biológica (IILB), IMYZA, INTA Castelar (Buenos Aires, Argentina), for identification. Voucher specimens of parasitoids were kept at the collection of the IILB whereas whiteflies were kept at the entomological collection, Facultad de Ciencias Agrarias, University of Rosario.
RESULTS
During our study period (February 2005 - April 2007) at four localities in the horticultural region of Rosario, we observed immature and adult whiteflies on a total of 35 plant species (14 cultivated and 21 wild species) from 18 plant families. Among the 21 wild species, 12 were native and 9 were naturalized in the region. We accomplished 89 host plant-whitefly relationships observations; five plant species, Sonchus oleraceus L. (16%), Amaranthus quitensis Kunth (10%), Solanum lycopersicum L. (9%), Conyza bonariensis (L.) Cronquist (5%) and Urtica urens L. (5%), included almost half of the whole number of observations. On flower production systems we recorded two whitefly species: the "greenhouse whitefly", Trialeurodes vaporariorum (Westwood), and the "tobacco whitefly", the Bemisia tabaci complex (Gennadius); while on vegetable production systems, we only observed T. vaporariorum (Tables I and II).
Table I. Host plants of Trialeurodes vaporariorum (Westwood) recorded from vegetable farms. Horticultural region of Rosario (Santa Fe province, Argentina).
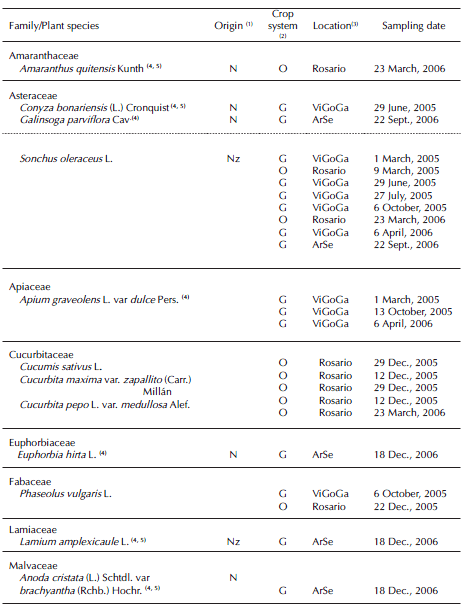
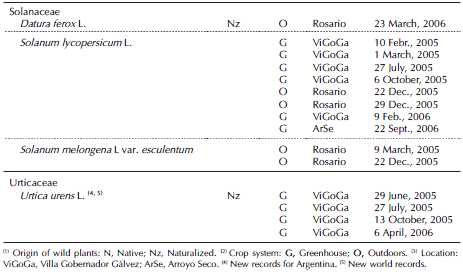
Table II. Host plants of whiteflies recorded from flowering farms under greenhouse conditions. Horticultural region of Rosario (Santa Fe province, Argentina).

T. vaporariorum was recorded on 24 plant species, belonging to 11 families. Among these, 12 plant species were new hosts in Argentina (Tables I and II). Out of these records, we also recorded the occurrence of immature and adults of T. vaporariorum in experimental crops of sunflower (Heliantus annuus L.) (September, 2005) and soybean (Glycine max (L.) Merrill) (March, 2005) under greenhouse conditions at Campo Villarino, Facultad de Ciencias Agrarias, University of Rosario.
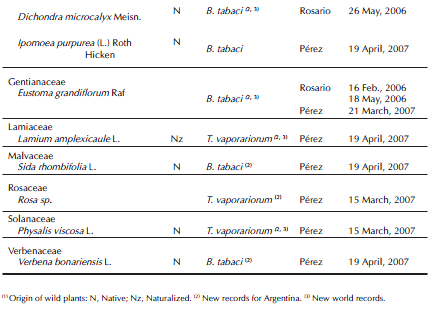
T. vaporariorum was the only whitefly species observed both on protected and field vegetable production systems during the first year of sampling. However, in Rosario locality, the B. tabaci complex was also recorded from February 2006 on Eustoma grandiflorum Raf and the uncultivated plants associated. The B. tabaci complex was observed on 19 plant species belonging to 11 families. Among these, 14 plant species were new hosts in Argentina (Table II). We also recorded immature and adults of the B. tabaci complex on soybean near flower crops in February 2006.
Both whitefly species were simultaneously recorded on Chrysanthemum morifolium Ramat and the wild plant Wedelia glauca (Ortega) O. Hoffm. ex Hicken, associated with gypsophila crops. Among these records, eight plant species were hosts of both species in the region: the crop G. max, and the wild species Amaranthus blitum L., A. quitensis, C. bonariensis, Galinsoga parviflora Cav. and S. oleraceus (Tables I and II).
Only adult whiteflies were found on lettuce (Lactuca sativa L. var. mantecosa) and broccoli (Brassica oleraceae L. var. botrytis subvar. cymosa) crops.
The plant families Asteraceae (seven species), Solanaceae (four species) and Cucurbitaceae (three species) included 58.3% of all T. vaporariorum host plant species recorded. The plant families Amaranthaceae (two species), Asteraceae (six species) and Convolvulaceae (three species) included 57.9% of all B. tabaci complex host plant species recorded.
The only parasitoid species observed was Eretmocerus californicus, near corni Haldeman (Hymenoptera: Aphelinidae), which emerged from pupae of T. vaporariorum on celery (04/27/05) and tomato (10/06/05) protected crops, and on the wild plants U. urens (06/29/05) and C. bonariensis (10/06/05) associated with protected lettuce and tomato crops, respectively, at Villa Gobernador Gálvez. It was recorded in four times out of 89 host plant-whitefly relationships observations.
DISCUSSION
There are 12 species of whiteflies recorded in Argentina: three belonging to the Aleurodicinae family and nine to the Aleyrodinae subfamily. Among these, only the B. tabaci complex and T. vaporariorum have the most extensive list of hosts. In Argentina, the B. tabaci complex has been recorded on 30 plant species (14 botanical families), whereas T. vaporariorum has been recorded on 25 plant species (10 botanical families) (Tapia, 1970; Mound & Halsey, 1978; Muruaga de L'Argentier et al., 1996; Viscarret et al., 2000; Martin & Mound, 2007). The horticultural region of Rosario is characterized by a diversity of vegetable and flower crops, predominantly annual and broadleaf herbaceous plants, which are preferably colonized by these polyphagous species.
Among the 24 plant species recorded for T. vaporariorum in the present survey, 12 were new hosts in Argentina: the crops Apium graveolens L. var. dulce Pers. and Rosasp., and the wild plants A. blitum, A. quitensis, Anoda cristata (L.) Schtdl. var brachyantha (Rchb.) Hochr, C. bonariensis, Euphorbia hirta L., G. parviflora, Lamium amplexicaule L., Physalis viscosa L., U. urens and W. glauca (Muruaga de L'Argentier et al., 1996; Remes Lenicov, 2004; Viscarret et al., 2000; Scotta & Bertolaccini, 2007). Among those new plant species for Argentina, eight had not been previously reported as hosts in the world's whitefly catalogs available (Mound & Halsey, 1978; Vázquez, 2004; Evans, 2008; Martin & Mound, 2007).
Among the 19 plant species recorded in the present study, 14 were new hosts for the B. tabaci complex in Argentina: the crop E. grandiflorum and the wild flora associated A. blitum, C. bonariensis, C. canadensis, Convolvulus arvensis L., Dichondra microcalyx Meisn., G. parviflora and W. glauca, the crop Gypsophila paniculata L. var. paniculata and the wild plants associated, Sida rhombifolia L. and Verbena bonariensis L., and the crop C. morifolium and the uncultivated plants associated Commelina erecta L. and Rapistrum rugosum (L.) Smith (Muruaga de L'Argentier et al., 1996; Remes Lenicov, 2004; Viscarret et al., 2000). Among those plant species cited above as new hosts for Argentina, seven had not been previously reported as hosts in the world's whitefly catalogs available (Mound & Halsey, 1978; Greathead, 1986; McKenzie et al., 2003, Vázquez, 2004; Martin & Mound, 2007; Evans, 2008).
In February 2006, the B. tabaci complex was recorded for the first time in Santa Fe Province (in Rosario) on E. grandiflorum and the wild plants associated (Gonsebatt & Lietti, 2006). This species was not found in any horticultural crop during this study and was localized only on flower crops and the wild plants associated. This species could have been introduced, at least in this flowering farm, by means of the lisianthus plantings brought from the horticultural region of La Plata, Buenos Aires. Interestingly, the global spread of B. tabaci biotype B is linked to trade in ornamentals (De Barro et al., 2011).
Both whitefly species were simultaneously recorded on C. morifolium and the wild plant W. glauca, associated with gypsophila crops. Therefore, both whitefly species coexist in the region; the output of this interspecific competition may be influenced by the host plants and the temperature regimes (Drost et al., 1998; Inbar & Gerling, 2008).
More than 50% of T. vaporariorum host plant species recorded belonged to the botanical families Asteraceae, Solanaceae and Cucurbitaceae. In the world's catalog of Mound & Halsey (1978), these families are also cited as the most numerous. Other researches include Malvaceae and Fabaceae as important host families of T. vaporariorum (Roditakis, 1990; Vázquez, 2004). More than 50% of the plant species surveyed as hosts of the B. tabaci complex belonged to the botanical families Asteraceae, Convolvulaceae and Amaranthaceae. Greathead (1986) and Vázquez (2004) also included Fabaceae, Malvaceae, Solanaceae and Euphorbiaceae and Cucurbitaceae as frequent groups.
The data of the collection sites in Argentina demonstrates that the distribution area of B. tabaci has expanded southward from its earliest location in the northwestern region of the country on wild plants and several crops as cotton, soybean and beans in open fields (Peterlin & Helman, 1995; Muruaga de L`Argentier et al., 1996; Viscarret, unpub.). Viscarret (unpub.) has observed the B. tabaci complex on vegetable, flowers and aromatics species at locations south of Rosario in Buenos Aires province. During the 1990s, T. vaporariorum was the only whitefly species on vegetable crops in the horticultural region of Bella Vista (Corrientes, northeastern Argentina) and La Plata (Buenos Aires), being more detrimental on tomato. The B. tabaci complex has been observed since 2001 and 2004 on pepper and tomato crops in the horticultural areas of Bella Vista and La Plata, respectively. Although pepper was the crop mostly attacked by this species, symptoms of ripening disorders of fruits similar to those caused by the biotype B have been observed on tomato (Cáceres, 2004; Polack, 2005).
B. tabaci is currently considered a complex of well-defined high-level genetically discernible groups containing morphological indistinguishable species, which include populations assigned to different biotypes or host races on the basis of measurable differences in biological and behavioral traits and biochemical profiles (De Barro et al., 2011). The development of biotypes has allowed the colonization of areas with different environmental characteristics than the traditionally inhabited by the B. tabaci complex (tropical, subtropical and fringe temperate regions where winters are mild enough to permit year-round survival). In Argentina, there are reports on the presence of the introduced B biotype, the BR biotype and an indigenous or ‘local' B. tabaci haplotype (AGR1, AGR2/3) (Truol et al., 2003; Viscarret et al., 2003; Cáceres, 2004; Alemandri & Truol, 2009). The presence of begomoviruses in samples from the north of Argentina on tomato, soybean, beans and wild plants has also been recorded (Rodríguez-Pardina et al., 2006).
The dispersal of the B. tabaci complex in Argentina and, particularly, its occurrence in the horticultural region of Rosario could have also been promoted by the increasing cultivated surface of a suitable reproductive host crop as soybean. In Latin America, the drastic change in traditional cropping systems to non-traditional cash and export crops, such as cotton, soybean and several vegetables, together with cultivation of genetically uniform crop varieties and year-round production practices, are among the most important factors that have contributed to the recent B. tabaci complex outbreaks and subsequent virus epidemics (Morales & Anderson, 2001). Adult whiteflies exhibit passive wind dispersal and active migratory flight behavior over both short and long distances; humans also transport immature and adult stages on plants (Byrne, 1999). Flower and vegetable crops have been substituted by soybean crops in the horticultural region of Rosario since 2002 because of the monetary devaluation and the increase in the input costs (Zuliani et. al., 2007). We observed few immature and adult individuals of the B. tabaci complex on soybean crops near flower crops in the horticultural area of Rosario in February 2006. Moreover, the B. tabaci complex was recorded on soybean crops in the 2005/2006 production campaign in the province of Entre Ríos and in the south of Santa Fe (Molinari et al., 2007). Therefore, soybean crops could impact on the population dynamics of this species, offering a large quantity of food during spring and summer and influencing on the vegetable crops and flowers in the area. However, reciprocally, the year-round production of vegetables and flowers under greenhouse and field conditions could maintain the populations of whiteflies when soybean crops are absent. Although B. tabaci is highly polyphagous, certain host plants affect the rate and success of development (Drost et al., 1998). Soybean has relatively less importance as reproductive host of B. tabaci than cotton or vegetable crops (Anderson et al., 2005).
Vegetable growers regard weeds as a detrimental factor for the crops and they should be eradicated manually or mechanically simultaneously with other management activities in the farm, being scarce the use of herbicides. However, they are interested in the control of weeds that emerge with the crop or during the cycle of the crop, but do not give attention to weeds or spontaneous plants that grow by the harvest time of the crop or during the fallow period. Moreover, uncultivated plants that grow at the edges of fields, greenhouses and trails, fence lines and in channels and ditches for irrigation are not under a permanent control strategy (Portela, 2008). Whiteflies are multivoltine and polyphagous and can survive and develop on a wide variety of alternative plant species in the absence of the main commercial crops. The adults reproduce continually throughout the year by moving sequentially among various crops and non-commercial host plants (Coudriet et al., 1986; Servín, 2004; Naveed et al., 2007). However, the contribution of wild species to the maintenance of whitefly populations depends on the quality of these plants as hosts, the plant life cycle itself and their abundance and distribution in the agroecosystem, among other factors (Coudriet et al., 1986; Calvitti & Remotti, 1998; Attique et al., 2003; Bezerra et al., 2004; Servín, 2004). The spontaneous plant species S. oleraceus, A. quitensis, C. bonariensis and U. urens, which are very common weeds in vegetable and flower production systems in the horticultural region of Rosario, were found in 64%, 68%, 14% and 36%, respectively, of the fields and greenhouses surveyed during this study (Gonsebatt & Lietti, 2008). There are several studies worldwide about the host suitability of some uncultivated plant species, their role as reservoirs for natural enemies and their contribution to the seasonal population dynamics of whiteflies (Coudriet et al., 1986; Roditakis, 1990; Calvitti & Remotti, 1998; Attique et al., 2003; Servín, 2004; Naveed et al., 2007; Scotta & Bertolaccini, 2007).
S. oleraceus has been highly attractive to T. vaporariorum and Bemisia argentifolii (Bellows & Perring) adults in laboratory choice tests (Roditakis, 1990; Calvitti & Remotti, 1998) and maintains high populations of B. argentifolii throughout the year around vegetable farms in Baja California, Mexico (Servín, 2004). Conversely, this species is much appreciated in extensive crops in Argentina because it is colonized by non-pest aphids which provide food for coccinellids, chrysopids and syrphids early in the season (Salto et al., 1993; Salto & Luiselli, 2004). Scotta & Bertolaccini (2007) recorded the parasitoids Eretmocerus spp. attacking T . vaporariorum on this uncultivated plant in the horticultural region of Santa Fe. Hence, the benefits for the agricultural production of particular crop-arthropod-weeds relationships are agroecosystem-specific.
The only parasitoid species observed was Eretmocerus californicus, near corni Haldeman (Hymenoptera: Aphelinidae), which emerged from pupae of T . vaporariorum. This species has also been previously recorded on protected tomato and mint in Buenos Aires (Argentina) (López & Evans, 2008). Other known T. vaporariorum parasitoids recorded in Argentina are, Eretmocerus corni Haldeman, Er. paulistus Hempel, Encarsia lycopersici De Santis, En. porteri (Mercet) and En. formosa Gahan, on protected tomato crops with regular pesticide applications (López & Botto, 1995; Viscarret et al., 2000, Scotta et al., 2006; Castresana & Paz, 2007). T. vaporariorum has been observed to be parasitized by Encarsia spp. and Eretmocerus spp. on Conyza bonariensis and Urtica urens, respectively, at vegetable farms in the horticultural region of Santa Fe (Scotta & Bertolaccini, 2007). We surveyed commercial crops with regular non-selective insecticide applications that could affect the development of parasitoids and thus explain their scarce presence during this study.
CONCLUSIONS
- This study is the first systematic survey of host plants in the region and expands the host range of T. vaporariorum and the B. tabaci complex in Argentina.
- We recorded uncultivated plants with spring-summer and autumn-winter growing life cycles as hosts of whiteflies in both production systems, in open fields and under greenhouses that offer a continuous source of food and ensure the presence of these species throughout the year in the region.
- However, some wild plants also allowed the survival of a parasitoid of whiteflies in crop production systems with regular spraying of insecticides.
- This study provides a better knowledge on the role of uncultivated plants as hosts for whiteflies and as reservoirs of parasitoids in a vegetable and flower agroecosystem with regular applications of insecticides.
ACKNOWLEDGEMENTS
To Lic. Vanesa Saucedo (vanesasaucedo@yahoo.com.ar) for designing Figure 1. To entomologist student Silvia Salate† for collaborating in the field sampling. To weed science researcher Dr. Eduardo Puricelli for collaborating in the identification of the spontaneous plant species. This investigation was partially supported by grants of the Secretaría de Ciencia y Tecnología de la Universidad Nacional de Rosario.
LITERATURE CITED
1. ALEMANDRI, V. & G. TRUOL. 2009. Identificación de biotipos de Bemisia tabaci (Gennadius), vector de virus en hortalizas. En: Resúmenes XIII Jornadas Fitosanitarias Argentinas, Termas de Río Hondo, Santiago del Estero, Argentina, Trabajo Z 001, 5p. [ Links ]
2. ANDERSON, P. K., A.HAMON, P. HERNÁNDEZ & J. MARTIN. 2005. Reproductive Crop Hosts of Bemisia tabaci (Gennadius) in Latin America and the Caribbean. En: Anderson, P. K. & F. J . Morales (eds.), Whitefly and whitefly-borne viruses in the tropics: building a knowledge base of global action, Tropical Whitefly IPM project, pp. 243-250. [ Links ]
3. ATTIQUE, M. R., M. RAFIQ, A. GHAFFAR, Z. AHMAD & A. I. MOHYUDDIN. 2003. Hosts of Bemisia tabaci (Genn.) (Homoptera: Aleyrodidae) in cotton areas of Punjab, Pakistan. Crop Protection 22: 715-720. [ Links ]
4. BEZERRA, M. A. S., M. R. V. DE OLIVEIRA & S. D. VASCONCELOS. 2004. Does the presence of weeds affect Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) infestation on tomato plants in a semi-arid agro-ecosystem?. Neotropical Entomology 3(6): 69-775. [ Links ]
5. BYRNE, D. N. 1999. Migration and dispersal by the sweet potato whitefly, Bemisia tabaci. Agricultural and ForestMeteorology 97: 309-316. [ Links ]
6. CÁCERES, S. 2004. Moscas blancas del complejo Bemisia tabaci en cultivos hortícolas de Corrientes. Estrategias de Manejo. En: Jornada de Actualización, La Plata, Buenos Aires, 2004, pp. 9-13. [ Links ]
7. CALVITTI, M. & P. REMOTTI. 1998. Host preference and performance of Bemisia argentifolli (Homoptera: Aleyrodidae) on weeds in central Italy. Environmental Entomology 27(6): 1350-1356. [ Links ]
8. CASTRESANA, J. & M. R. PAZ. 2007. Parasitoides de Trialeurodes vaporariorum (Westwood) (Hemiptera: Aleyrodidae) en cultivos de Capsicum annum y Lycopersicum esculentum en el área de Concordia, Entre Ríos. Horticultura Argentina 26(61): 37. [ Links ]
9. COUDRIET, D. L., D. E. MEYERDIRK, N. PRABHAKER & A. N. KISHABA. 1986. Bionomics of sweetpotato whitefly (Homoptera: Aleyrodidae) on weeds hosts in the Imperial Valley, California. Environmental Entomology 15: 1179-1183. [ Links ]
10. DE BARRO, P. J., S. S. LIU, L. M. BOYKIN & A. B. DINSDALE. 2011. Bemisia tabaci: A statement of species status. Annual Review of Entomololgy 56: 1-19. [ Links ]
11. DROST, Y. C., J. C. VAN LENTEREN & H. J. W. VAN ROERMUND. 1998. Life-history parameters of different biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae) in relation to temperature and host plant: a selective review. Bulletin of Entomological Research 88: 219-229. [ Links ]
12. EVANS, G. A. 2008. The whiteflies (Hemiptera: Aleyrodidae) of the world and their host plants and natural enemies. http://www.sel.barc.usda.gov.591/1WF/world-whitefly-Catalog.pdf, 23/09/2008. [ Links ]
13. FERRATTO, J. (ed). 2006. El sector frutihortícola regional, aspectos que contribuyen a su desarrollo. UNR Editora, Rosario. 104 pp. [ Links ]
14. GONSEBATT G. & M. LIETTI. 2006. Presencia de Bemisia tabaci en el cinturón hortícola de Rosario: Primeros Registros. Revista Agromensajes Facultad Ciencias Agrarias (UNR) 20: 37-38. [ Links ]
15. GONSEBATT, G. & M. LIETTI. 2008. Importancia relativa de las malezas como hospedantes de moscas blancas en el Cinturón Hortícola de Rosario. Horticultura Argentina 27(64): 64. [ Links ]
16. GREATHEAD, A. H. 1986. Host plants. En: Cock, M. J. W. (ed.), Bemisia tabaci: a literature survey on the cotton whitefly with an annotated bibliography, CAB International Biological Control, Ascot, UK, FAO UN, pp. 17-25. [ Links ]
17. HELMAN, S., O. PETERLIN & M. CONTRERAS. 1996. Parasitoids of Bemisia tabaci in Santiago del Estero, Northwest Argentina. En: Gerling, D. & R. T. Mayer (eds.), Bemisia: 1995 Taxonomy, biology, damage, control and management, Intercept Ltd., Andover, Hants, U.K., pp. 527-529. [ Links ]
18. INBAR, M. & D. GERLING. 2008. Plant-mediated interactions between whiteflies, herbivores, and natural enemies. Annual Review of Entomology 53: 431-448. [ Links ]
19. LÓPEZ, S. N. & E. N. BOTTO. 1995. Control biológico de Trialeurodes vaporariorum (Westwood) (Homoptera: Aleyrodidae) por entomófagos parasitoides (Hymenoptera: Aphelinidae) en cultivos protegidos. En: Resúmenes III Congreso Argentino de Entomología, 1995, Mendoza, Argentina, pág. 177. [ Links ]
20. LÓPEZ, S. N. & G. A. EVANS. 2008. Nuevos registros y distribuciones de especies del género Eretmocerus (Hymenoptera: Aleyrodidae), parasitoides de Trialeurodes vaporariorum y el complejo Bemisia tabaci (Hemiptera: Aleyrodidae) en Argentina. Revista de la Sociedad Entomológica Argentina 67: 185-187. [ Links ]
21. MARTIN, J. H. 1987. An identification guide to common whitefly pest species of the world (Homoptera: Aleyrodidae). Tropical Pest Management 33: 298-322. [ Links ]
22. MARTIN, J. H. & L. A. MOUND. 2007. An annotated check list of the world's whiteflies (Insecta: Hemiptera: Aleyrodidae). Zootaxa 1492: 1-84. [ Links ]
23. MACKENZIE, C. L., P. K. ANDERSON & N. VILLARREAL. 2003. An extensive survey of Bemisia tabaci (Homoptera: Aleyrodidae) in agricultural ecosystems in Florida. Florida Entomologist 87(3): 403-407. [ Links ]
24. MOLINARI, A. M., G. GONSEBATT, M. F. DAVID & E. PEROTTI. 2007. Mosca blanca (Bemisia tabaci Gennadius) en cultivos de soja. Revista Soja, para mejorar la producción, EEA INTA Oliveros 36: 109-111 . [ Links ]
25. MORALES, F. J. & P. K. ANDERSON. 2001. The emergence and dissemination of whitefly-transmitted geminiviruses in Latin America. Archives of Virology 146: 415-441. [ Links ]
26. MOUND, L. A. & S. H. HALSEY. 1978. Whitefly of the world: A systematic catalogue of the Aleyrodidae (Homoptera) with host plants and natural enemy data. John Wiley & Sons, Chichester, British Museum (Natural History), London, UK. 340 pp. [ Links ]
27. MURUAGA DE L`ARGENTIER, S., E. A. DE MANERO & A. LOIÁCONO. 1996. Especies de Aleyrodidae (Homoptera) presentes en poroto (Phaseolus vulgaris L.) y en las malezas asociadas al cultivo en las provincias de Jujuy y Salta, República Argentina. Revista de Investigación CIRPON 10: 25-31. [ Links ]
28. NAVEED, M., A. SALAM & M. A. SALEEM. 2007. Contribution of cultivated crops, vegetables, weeds and ornamental plants in harboring of Bemisia tabaci (Homoptera: Aleyrodidae) and associated parasitoids (Hymenoptera: Aphelinidae) in cotton agroecosystem in Pakistan. Journal of Pest Science 80: 191-197. [ Links ]
29. PETERLIN, O. & S. HELMAN. 1995. Some aspects of the population dynamics of Bemisia tabaci as a cotton plague in Santiago del Estero, northwestern Argentina. En: Gerling, D. & R. T. Mayer (eds.), Bemisia: 1995 Taxonomy, biology, damage, control and management, Intercept Ltd., Andover, Hants, UK, pp. 133-141. [ Links ]
30. POLACK, L. A. 2005. Manejo Integrado de Moscas Blancas. Boletín Hortícola, EEA INTA San Pedro 10: 1-5. [ Links ]
31. PORTELA, J. A. 2008. Control de malezas en cultivos hortícolas: ¿una cuestión de factores o de procesos? Horticultura Argentina 27(62): 28-34. [ Links ]
32. REMES LENICOV, A. M., S. PARADELL & E. VIRLA. 2004. Homoptera. Aleyrodidae. En: Cordo, H., G. Logarzo, K. Braun & O. Di Iorio (Directores), Catálogo de Insectos Fitófagos de la Argentina, Sociedad Entomológica Argentina Ediciones, Buenos Aires, Argentina, pp. 284-268. [ Links ]
33. RODITAKIS, N. E. 1990. Host plants of greenhouse whitefly Trialeurodes vaporariorum Weestwood (Homoptera: Aleyrodidae) in Crete. Attractiveness and impact on whitefly life stages. Agriculture, Ecosystems and Environment 31: 217-224. [ Links ]
34. RODRÍGUEZ-PARDINA, P. E., F. MURILO ZERBINI & D. A. DUCASSE. 2006. Genetic diversity of begomoviruses infecting soybean, bean and associated weeds in northwestern Argentina. Fitopatologia Brasileira 31(4): 342-348. [ Links ]
35. SALTO, C. E, J. A. LÓPEZ, I. BERTOLACCINI & J. M. IMWINKELRIED. 1993 Observaciones preliminares de las interacciones malezas-fitófagos-enemigos naturales en el área de la provincia de Santa Fe. Gaceta Agronómica 12: 21-30. [ Links ]
36. SALTO, C. & S. LUISELLI. 2004. La utilidad de la vegetación espontánea para el manejo integrado de plagas. INTA Informa 285. En: http://www.inta.gov.ar/info/intainfo/ ant/2004/285.htm [ Links ]
37. SCOTTA, R. & I. BERTOLACCINI. 2007. Plantas silvestres hospederas de mosca blanca (Trialeurodes vaporariorum) y de sus enemigos naturales en el cinturón hortícola santafesino. En: Resúmenes XXX Congreso Argentino de Horticultura, La Plata, Buenos Aires, Argentina, 2007, pág. 381. [ Links ]
38. SERVÍN, R. 2004. Especies de mosquita blanca en agroecosistemas desérticos de baja California sur, México, y fenología de sus hospederos. Revista Biología 18: 57-64. [ Links ]
39. TAPIA, E. A. 1970. Estudio preliminar de los aleirodídeos hallados en la Argentina (Homoptera). En: Actas del IV Congreso Latinoamericano de Zoología I, Caracas, Venezuela, pp. 219-234. [ Links ]
40. TRUOL, G., W. N. M. LAGO, P. R. QUEIROZ, L. H. C. LIMA, M. R. V. DE OLIVEIRA, G. LAGUNA, W. M. PAIVA & A. M. R. ALMEIDA. 2003. Variabilidade genética determinada por RAPD-PCR de populações de Bemisia tabaci provenientes do Brasil, Argentina e Paraguai. Boletim de Pesquisa e Desenvolvimento. Embrapa Recursos Genéticos e Biotecnologia, Brasilia 54. 19 pp. [ Links ]
41. VÁZQUEZ, L. L. 2004. Lista de moscas blancas (Hemiptera: Auchenorryncha: Aleyrodidae) y sus plantas hospedantes en el Caribe. Fitosanidad 8: 7-18. [ Links ]
42. VISCARRET, M. Unpub. Estudios biológicos sobre Aleyrodidae de importancia económica (Insecta: Hemiptera) con énfasis en el complejo Bemisia tabaci (Gennadius) y su posible control biológico. Tesis Doctoral, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Buenos Aires, 2000, 131 pp. [ Links ]
43. VISCARRET, M., E. N. BOTTO & A. POLASZEK. 2000. Whiteflies (Hemiptera: Aleyrodidae) of economic importance and their natural enemies (Hymenoptera: Aphelinidae, Signiphoridae) in Argentina. Revista Chilena de Entomología 26: 5-11. [ Links ]
44. VISCARRET, M. M., I. TORRES-JEREZ, E. AGOSTINI DE MANERO, S. N. LÓPEZ, E. N. BOTTO & J. K. BROWN. 2003. Mitochondrial DNA evidence for a distinct New World group of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) indigenous to Argentina and Bolivia, and presence of the Old World B Biotype in Argentina. Annals of Entomological Society of America 96: 65-72. [ Links ]
45. ZULIANI, S., C. SEVERÍN, M. V. ROMAGNOLI & E. CASELLA. 2007. Estrategias productivas y socioeconómicas de las Pymes florícolas del Gran Rosario. Revista Agromensajes Facultad Ciencias Agrarias (UNR) 21: 22-23. [ Links ]














