Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de la Sociedad Entomológica Argentina
Print version ISSN 0373-5680
Rev. Soc. Entomol. Argent. vol.71 no.1-2 Mendoza Jan./June 2012
TRABAJOS CIENTÍFICOS
First record and ecological features of Goeldichironomus petiolicola (Diptera: Chironomidae) mining Eichhornia crassipes in the Middle Paraná River floodplain, Argentina
Primer registro y aspectos ecológicos de Goeldichironomus petiolicola (Diptera: Chironomidae) minador de Eichhornia crassipes en la llanura aluvial del río Paraná Medio, Argentina
Montalto, Luciana*,**, Soledad Capello* and Analía C. Paggi***
* Instituto Nacional de Limnología (INALI, CCT Santa Fe, CONICET-UNL), Ciudad Universitaria, Paraje El Pozo s/n (3000), Santa Fe, Argentina; e-mail: lmontalto@inali.unl.edu.ar
** Facultad de Humanidades y Ciencias, Universidad Nacional del Litoral-UNL, Ciudad Universitaria, Paraje El Pozo s/n (3000), Santa Fe, Argentina.
*** Instituto de Limnología "Dr. Raúl A. Ringuelet" (ILPLA, CCT La Plata, CONICET), Av. Calchaquí Km 23,5, Florencio Varela, Buenos Aires, Argentina.
Recibido: 08-III-2012
Aceptado: 09-V-2012
ABSTRACT. The first records from Argentina of larvae of Goeldichironomus petiolicola Trivinho-Strixino & Strixino mining in Eichhornia crassipes (Martius) Solms petioles in wetlands of the Paraná River floodplain were analyzed. G petiolicola larvae and pupae were collected seasonally during 2005 from stands of E. crassipes and bottom sediment samples in floodplain habitats. Larvae inside E. crassipes petioles were reared with the purpose of obtaining their complete life cycle. G. petiolicola was the most abundant chironomid species living inside E. crassipes petioles; the III and IV instar larvae and pupae were found during all the seasons while the I and II instar larvae were not found. The highest average densities of G. petiolicola larvae inside the E. crassipes petioles were recorded in Autumn-Winter (588.5 ind.kg-1 dw - 778.7 ind.kg-1 dw). This suggests that they have better conditions for survival, allowing the individuals to develop more slowly until the next flooded period with higher temperatures. The r (Dyar) value obtained was 1.66. We conclude that G. petiolicola mines into E. crassipes petioles mainly when the plants begin their senescence process and does not cause important damage to them.
KEY WORDS. Chironomidae; Eichhornia crassipes miner; Larval ecology; Wetlands.
RESUMEN. Se presenta el primer registro para Argentina de Goeldichironomus petiolicola Trivinho-Strixino & Strixino, especie cuyos estadios inmaduros minan dentro de los pecíolos de Eichhornia crassipes (Martius) Solms, en humedales del río Paraná Medio. Las larvas y pupas de G petiolicola fueron recolectadas estacionalmente durante 2005, de los stands de E. crassipes y en muestras de sedimento en diferentes ambientes de la llanura. Ejemplares de la especie fueron criados en laboratorio a fin de completar su ciclo de vida. G petiolicola fue el quironómido más abundante que habita en el interior de los pecíolos de E. crassipes, se registraron los estadios de larva III y IV y pupa durante todas las estaciones. No fueron registrados los estadios larvales I y II. Las mayores densidades medias de G petiolicola fueron obtenidas en otoño-invierno (588,5 ind.kg1 dw - 778,7 ind.kg1 dw), esto sugiere que presentan mejores condiciones para la supervivencia, lo que permite a los individuos obtener un desarrollo más lento hasta el próximo período de inundación, con mayores temperaturas. El valor calculado de r (Dyar) fue 1,66. G petiolicola mina dentro de los pecíolos de E. crassipes, principalmente, durante el inicio del proceso de senescencia del vegetal, por lo que no provoca daños importantes.
PALABRAS CLAVE. Chironomidae; Minador de Eichhornia crassipes; Ecología larval; Humedales.
INTRODUCTION
The immature stages of Chironomidae are one of the most abundant and diverse group of insects in freshwater habitats and live associated with benthic, periphyton and macrophyte communities (Coffman & Ferrington, 1984; Fittkau, 1986; Pinder, 1995; Paggi, 2009). They play an important role in the aquatic food webs, in the decomposition processes of organic matter, in nutrient cycling and as bioindicators of environmental conditions (Armitage et al., 1995; Paggi, 1998; Henriques-Oliveira et al., 2003). Despite the diverse ecological roles played by chironomids, as well as their widespread occurrence in freshwater ecosystems, surprisingly few studies have analyzed their ecology and population dynamics (Trivinho-Strixino & Strixino, 1982; Corbi & Trivinho-Strixino, 2006).
Goeldichironomus Fittkau is a predominantly Neotropical genus with 11 species described. Goeldichironomus petiolicola has been recently described by Trivinho-Strixino & Strixino (2005) from Brazil, where most of the species in this genus were reported (Reiss, 1974; Spies & Reiss, 1996; Trivinho-Strixino & Strixino, 1989, 1991, 1998).
The larvae of Goeldichironomus live in drifting vegetation and bottom sediments of small standing water bodies (Pinder & Reiss, 1983). In addition, two species of this genus have been recorded mining inside macrophytes, G. holoprasinus (Goeldi) in E. crassipes (Bennett & Zwolfer, 1968) and G. petiolicola in Eichhornia azurea (Swartz) Kunth and Pontederia cordata L. var. cordata (Trivinho-Strixino & Strixino, 2005).
Eichhornia crassipes is a native of Brazil and is one of the most abundant macrophytes in the Paraná River floodplain, mainly in floodplain lakes, where it also comprises the greatest biomass of these habitats (Lallana, 1980; Sabattini & Lallana, 2007). Due to its adaptability, this species has colonized temperate and tropical water bodies around the world. This is an invasive plant that has generated important economic losses and damages in natural and human-made habitats. Because of this, several studies concerning invertebrates that might be used to control the populations of this plant have been done (Silveira Guido & Perkins, 1975; Deloach & Cordo, 1976; Poi de Neiff et al., 1977; Casco & Poi de Neiff, 1998; Heard & Winterton, 2000; Adis & Junk, 2003; Poi de Neiff & Casco, 2003). In the present study, G. petiolicola is recorded for the first time from Argentina, its larvae mining E. crassipes petioles in floodplain wetlands of the Middle Paraná River system. The immature individuals found inside the plant petioles were measured, reared and some aspects of their life cycle were analyzed with the purpose to improve information about G. petiolicola biology and ecology features that can be useful to evaluate the degree of damage caused in E. crassipes.
MATERIAL AND METHODS
The sampling station selected was a shallow lake of the Middle Paraná River floodplain (31º 43' S - 60º 39' W) (Fig.1). Eichhornia crassipes, Salvinia herzogii de la Sota, Azolla caroliniana Willdenow, Pistia stratiotes L., Echinoclhoa polystachya (Kunth) Hitchcock, Polygonum acuminatum Kunth, Eleocharis R. Brown spp. and Paspalum repens Bergius were the dominant macrophytes in this habitat.

Fig. 1. Study area located in the Paraná River floodplain, Argentina. 1: Paraná City, 2: Santa Fe City.
Stands of E. crassipes and bottom sediment samples were taken seasonally during 2005 to collect G. petiolicola larvae and pupae, including the high water period of the Paraná River in January-February and the low water period in August-September. Three samples of E. crassipes were taken in different stands using a square method (1m2) and three samples of bottom sediment were collected with an Ekman grab of 225 cm2, filtered through a 200 µm sieve and fixed in 10% formaldehyde in the field. Water temperature, pH, conductivity (with water probe Horiba U-10) and depth were measured on each sampling date. Air temperature (standard thermometer) was also measured because the chironomids were sampled inside the aerial plant petioles.
In the laboratory, all chironomid larvae and pupae were removed from the interior of E. crassipes petioles and bottom sediment samples under a stereoscopic microscope (4x) and preserved in alcohol 70%. Total body length (measured from the anterior margin of the cephalic capsule) and cephalic capsule width (maximum ventral width of the cephalic capsule measured transverse to the major axis of the body) of G. petiolicola larvae were measured with a micrometer scale under an optical microscope. The larvae were separated into instars according to the relationship between cephalic capsule width and total body length and Spearman Rhocorrelation was estimated. In order to determine the growth proportion (r) between recorded larval instars, the Dyar proportion was calculated (Dyar, 1890).
Dry weight of petiole biomass was obtained by drying material at 60 ºC down to a constant weight. The larval and pupal densities values were expressed as ind. kg-1 E. crassipes petioles biomass dry weight.
To rear and obtain adult specimens of G. petiolicola, larvae inside E. crassipes petioles were put in separate vials at room temperature during May of 2005 (11.5 °C -23.4 °C) following the procedures described by Paggi (2009). The reared and collected material was mounted on slides with Euparal© or Hoyer´s medium and was measured under an optical microscope following the procedures described by Paggi (2009).
The specimens were identified as G. petiolicola according to Trivinho-Strixino & Strixino (2005). Voucher specimens were deposited at the Instituto de Limnología Dr. Raúl A. Ringuelet (La Plata, Argentina).
RESULTS AND DISCUSSION
Macrophyte substratum
The air temperature in the macrophyte stands in Summer-Autumn was higher than the water temperature. The air and water temperatures were very similar in Spring and Winter. The water depth varied between 2.21 m and 2.70 m according to the hydrologic regime and the water pH and conductivity were similar in all seasons (Table I).
Table I. Environmental parameters recorded in each sampling date.

Goeldichironomus petiolicola was the most abundant chironomid species living inside E. crassipes petioles (86.1% - 98.9%). Endotribelos Grodhaus sp., Asheum Sublette & Sublette sp. and Polypedilum Kieffer sp. were also recorded inside the petioles, but in low densities (Table II).
Table II. Average densities (± SD) (ind.kg-1), seasonal and total percentage frequency of Chironomidae (Diptera) larvae living inside of Eichhornia crassipes petioles from Summer to Spring 2005.

Two clearly separable groups of G. petiolicola (Fig. 2), corresponding to third (III) and fourth (IV) instar larvae (N= 371), were observed when comparing the cephalic capsule width and the total body length. The maximum body length and cephalic capsule width of the III and IV instar larvae were 4.51 mm and 3.22 mm and 9.05 mm and 5.62 mm, respectively (Table III). The Spearman correlation between cephalic capsule width and total body length was Rho: 0.714 (p<0.01).

Fig. 2. Relation between cephalic capsule width (µm) and total body length (µm) of III and IV larval instars of Goeldichironomus petiolicola mining inside Eichhornia crassipes petioles recorded during the annual cycle of 2005.
Table III. Body length minimum, maximum and average (± SD) and head width size recorded for III and IV instar larvae of Goeldichironomus petiolicola. All measurements are in µm.
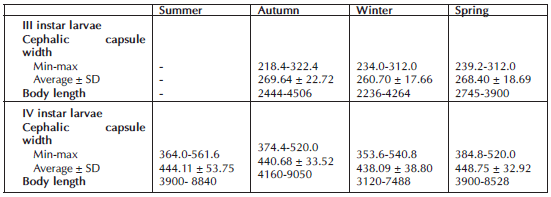
The maximum total body length of the single IV instar larva specimen from Brazil (Trivinho-Strixino & Strixino, 2005) was smaller (8.2 mm) than the specimens from Argentina.
Only III and IV instar larvae of G. petiolicola were found during all the seasons (except only III instar in Summer) mining the petioles of E. crassipes; while I and II instar larvae were not found during the study year. We can assume that they mine the petioles after the II larval instar and they are not easily sampled by ordinary sampling methods because of their small size (Pinder, 1995).
The r (Dyar) value obtained was 1.66. This value was higher than reported for G. holoprasinus (1.64) (Zilli et al., 2009) and G. luridus Trivinho-Strixino & Strixino, 2005 (1.62) and lower than r value reported for G. maculatus Trivinho-Strixino & Strixino, 1991 (1.75) (Corbi & Trivinho-Strixino, 2006). The use of the Dyar r value is important for insects with immature stages on macrophytes like G. petiolicola, because it is very difficult to find the first instars in the field (they are most likely planktonic); the size of I and II instars can be estimated through the r value obtained (Strixino, 1973).
The larvae and pupae of G. petiolicola live in shelters with two openings just below the petiole's epidermis; their sizes are coincident with the maximum body length of approximately 1 cm (Trivinho-Strixino & Strixino, 2005) (Fig. 3). This particular behavior makes this species very difficult to rear under laboratory conditions; for this reason we could not define how many days it takes to complete the life cycle. However, there are data for the life cycles of other Goeldichironomus species. In laboratory conditions, the average life cycle of G. holoprasinus (18 °C-33 °C) was 18 days (Zilli et al., 2009); for G. maculatus and G. luridus (21 ºC-26 ºC) it was 28 and 20 days, respectively (Corbi & Trivinho-Strixino, 2006).

Fig. 3. Larva of Goeldichironomus petiolicola inside a shelter in Eichhornia crassipes petiole. The arrows indicate the two tube openings.
The presence of larvae and pupae during all seasons indicates that this species can reproduce throughout the year with overlapping generations (Tokeshi, 1995). The highest average densities of G. petiolicola larvae in E. crassipes petioles were recorded in Autumn-Winter (588.5 ind.kg-1 dw - 778.7 ind.kg-1 dw) (Fig. 4), suggesting that they have better conditions to survive allowing the individuals to get slower development until the next flooded period with higher temperatures.
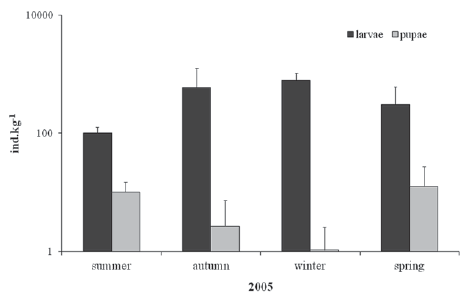
Fig. 4. Density (ind.kg-1) of larvae and pupae of Goeldichironomus petiolicola inside Eichhornia crassipes petioles recorded during all seasons of 2005.
The highest pupal density values were found in Spring-Summer (10.3 ind.kg-1 dw - 12.8 ind.kg-1 dw) (Fig. 4) and were in direct relationship with higher water and air temperatures for the year, similar to Chironomini in temperate zones as stated by Coffman (1973). It is common among multivoltine species that the first Spring emergences are stronger, in terms of growth and development, than later emergences which coincide with rising temperatures and increasing photoperiod in Spring (Tokeshi, 1995).
Goeldichironomus petiolicola has been described as a facultative phytophage, feeding on seston and using plant material only as a substratum for mining. Thus, the larvae consume plant tissue incidentally when building their shelters (Trivinho-Strixino & Strixino, 2005). We observed that G. petiolicola mines into E. crassipes petioles mainly when the plants begin their senescence and decomposition processes (Autumn-Winter) and the water content (%) in vegetal tissues is higher (Lallana, 1980), but we couldn't find signs of serious damage produced by chironomids.
Bottom sediments
In the present study larvae and pupae of G. petiolicola were not found in bottom sediment samples. There was a record of specimens of G. petiolicola in bottom sediment, but they were inside decomposing tissues of E. crassipes petioles, in the marginal fluvial wetland of the Middle Paraná secondary channel (Montalto, unpub.). The average density of G. petiolicola larvae in decomposing tissues of E. crassipes petioles in marginal fluvial wetland was 254 ind.m-2 (Montalto, unpub.). This density was lower than the ones found for other Goeldichironomus species that live in bottom sediments of freshwater habitats; G. maculatus had reported densities of 475-483 ind.m-2 in sandy lentic habitats from Brazil (Corbi & Trivinho-Strixino, 2006).
According to Montalto & Paggi (2006) and Montalto (unpub.), marginal wetlands are subject to fluctuating conditions. The invertebrates that live in these temporary wetlands are adapted to withstand such fluctuating environments, featuring rapid changes and periodical situations of desiccation. Thus, during low water levels when E. crassipes plants drop down to the bottom sediment, immature individuals that live inside petioles are able to survive desiccation periods and emerge when their development is completed. The fact that G. petiolicola larvae and pupae were not found in bottom sediment samples free of decomposing macrophytes leads us to assume that this species is exclusively associated with macrophytes. Nevertheless, more studies are needed.
ACKNOWLEDGEMENTS
This work was supported by PIP-CONICET 2010 Nº 132 and PICT Bicentenario 2010 N° 1965 projects. This paper is the Scientific Contribution N° 829 of the Instituto de Limnología "R. A. Ringuelet" (ILPLA) La Plata, Argentina. We thank RSEA Editors and anonymous referees for helpful suggestions.
LITERATURE CITED
1. ADIS, J. A. & W. J. JUNK. 2003. Feeding impact and bionomics of grasshopper Cornops aquaticum on the water hyacinth Eichhornia crassipes in the Central Amazonian floodplains. Stud. Neotrop. Fauna Environ. 38: 245-249. [ Links ]
2. ARMITAGE, P., P. S. CRANSTON & L. C. V. PINDER. 1995. The Chironomidae. The biology and ecology of non-biting midges. Chapman & Hall, New York. [ Links ]
3. BENNETT, F. D. & H. ZWOLFER. 1968. Exploration for natural enemies of the water hyacinth in northern South America and Trinidad. Hyac. Cont. J. 7: 44-52. [ Links ]
4. CASCO, S. L. & A. POI DE NEIFF. 1998. Daño ocasionado por adultos de Neochetina spp. (Coleoptera: Curculionidae) a Eichhornia crassipes en la planicie de inundación del río Paraná. Facena 14: 31-43. [ Links ]
5. COFFMAN, W. P. 1973. Energy flow in a woodland stream ecosystem. II. The taxonomic composition and phenology of the Chironomidae as determine by the collection of pupal exuviae. Arch. Hydrobiol. 71: 281-322. [ Links ]
6. COFFMAN, W. P. & L. C. FERRINGTON. 1984. Chironomidae. En: Merritt, R. W. & K. W. Cummins (eds.), An Introduction to the Aquatic Insects of North America, Kendall/Hunt Publishers, Iowa, pp. 551-652. [ Links ]
7. CORBI, J. J. & S. TRIVINHO-STRIXINO. 2006. Ciclo de vida de duas espécies de Goeldichironomus (Diptera, Chironomidae). Rev. Bras. Entomol. 50: 72-75. [ Links ]
8. DANK, H. V. 2006. Short life cycle in insects and mites. Can.Entomol. 138: 407-463. [ Links ]
9. DELOACH, C. J. & H. A. CORDO. 1976. Biological studies of Neochetina bruchi and N. eichhorniae on waterhyacinth in Argentina. J. Aquat. Plant Manage. 14: 53-59. [ Links ]
10. DYAR, H. G. 1890. The number of molts of Lepidopterus larvae. Psyche 5: 420-422. [ Links ]
11. FITTKAU, E. J. 1986. Conocimiento actual sobre la colonización de la región tropical sudamericana por insectos acuáticos y su historia evolutiva, con especial referencia a los quironómidos. Ann. Mus. Hist. Nat. Val. 17: 97-103. [ Links ]
12. HEARD, T. A. & S. L. WINTERTON. 2000. Interactions between nutrient status and weevil herbivory in the biological control of water hyacinth. J. Appl. Ecol. 37: 117-127 [ Links ]
13. HENRIQUES-OLIVEIRA, A. L., J. L. NESSIMIAN & L. F. M. DORVILLE. 2003. Feeding habits of Chironomids larvae (Insecta: Diptera) from a stream in the Floresta da Tijuca, Rio de Janeiro, Brazil. Braz. J. Biol. 63: 269-281. [ Links ]
14. LALLANA, V. H. 1980. Productividad de Eichhornia crassipes (Mart.) Solms. en una laguna isleña de la cuenca del río Paraná Medio. II. Biomasa y dinámica de población. Ecología 5: 1-16. [ Links ]
15. MONTALTO, L. Unpub. Dinámica espacio-temporal de asociaciones de invertebrados en un humedal marginal fluvial de la llanura aluvial del río Paraná Medio. Tesis Doctoral, Universidad de Buenos Aires, 2008, 215 pp. [ Links ]
16. MONTALTO, L. & A. C. PAGGI.2006. Diversity of chironomids larvae in a marginal fluvial wetland of Middle Paraná River floodplain, Argentina. Wetlands 42: 289-300. [ Links ]
17. PAGGI, A. C. 1998. Chironomidae. En: Morrone, J. J. & S. Coscarón, Biodiversidad de Artrópodos argentinos. Una perspectiva biotaxonómica, Ediciones Sur, La Plata, pp. 327-337. [ Links ]
18. PAGGI, A. C. 2009. Diptera: Chironomidae. En: Dominguez, E. & H. R. Fernandez (eds.), Macroinvertebrados bentónicos sudamericanos. Sistemática y biología, Fundación Miguel Lillo, Tucumán, pp. 383-409. [ Links ]
19. PINDER, L. C. V. 1995. The habitats of chironomid larvae. En: Armitage, P. D., P. S. Cranston & L. C. V. Pinder (eds.), The Chironomidae. Biology and ecology of non-biting midges, Chapman & Hall, New York, pp. 107-135. [ Links ]
20. PINDER, L. C. V. & F. REISS. 1983. The larvae of Chironominae (Diptera: Chironomidae) of the Holarctic region. Keys and Diagnosis. En: Wiederholm, T. (ed.). Chironomidae of the Holarctic region: Keys and diagnoses. Entomol. Scand. Suppl. 19, pp. 293-435. [ Links ]
21. POI DE NEIFF, A. & S. L. CASCO. 2003. Biological agents that accelerate Winter decay of Eichhornia crassipes Mart. Solms. in northeast Argentina. En: Thomaz, S. M. & L. M. Bini, (eds.), Ecologia e manejo de macrófitas aquáticas, Eduem, Maringa, pp. 127-144. [ Links ]
22. POI DE NEIFF, A., J. J. NEIFF & A. A. BONETTO. 1977. Enemigos naturales de Eichhornia crassipes en el Nordeste argentino y sus posibilidades de aplicación al control biológico. Ecosur 4: 137-156. [ Links ]
23. REISS, F. 1974. Die in stehenden Gewässern der Neotropis verbreitete Chironomidengattung Goeldichironomus Fittkau (Diptera, Insecta). Stud. Neotrop. Fauna Environ. 9: 85-122. [ Links ]
24. SABATTINI, R. A. & V. H. LALLANA. 2007. Aquatic Macrophytes. En: Iriondo M. H., Paggi J. C. & M. J. Parma (eds), The Middle Paraná River: Limnology of a Subtropical Wetland, Springer, Berlin Heidelberg, New York, pp. 205-226. [ Links ]
25. SILVEIRA GUIDO, A. & B. D. PERKINS. 1975. Biology and host specificity of Cornops aquaticum (Bruner) (Orthoptera: Acrididae), a potential biological control agent for waterhyacinth. Environ. Entomol. 4: 400-404. [ Links ]
26. SPIES, M. & F. REISS. 1996. Catalog and bibliography of Neotropical and Mexican Chironomidae (Insecta, Diptera). Spixiana Suppl. 22: 61-119. [ Links ]
27. STRIXINO, S. Inéd. A largura da cabeça na determinação das fases larvais de Chironomidae na Represa do Lobo. Tesis Doctoral, Universidade Federal de São Carlos, Brazil, 1973, 167 pp. [ Links ]
28. TOKESHI, M. 1995. Life cycles and population dynamics. En: Armitage, P. D., Cranston, P. S. & L. C. V. Pinder (eds.), The Chironomidae. Biology and ecology of non-biting midges, Chapman & Hall, New York, pp. 225- 268. [ Links ]
29. TRIVINHO-STRIXINO, S. & G. STRIXINO. 1982. Ciclo de vida de Chironomus sancticaroli Strixino and Strixino, (Diptera, Chironomidae). Rev. Bras. Entomol. 26: 183-189. [ Links ]
30. TRIVINHO-STRIXINO, S. & G. STRIXINO. 1989. Observações sobre a biologia da reprodução de um quironomídeo da Região Neotropical (Diptera: Chironomidae). Rev. Bras. Entomol. 33: 207-216. [ Links ]
31. TRIVINHO-STRIXINO, S. & G. STRIXINO. 1991. Nova espécie de Goeldichironomus Fittkau (Diptera, Chironomidae) do Brazil. Rev. Bras. Entomol. 35: 593-602. [ Links ]
32. TRIVINHO-STRIXINO, S. & G. STRIXINO. 1998. Goeldichironomus neopictus, a new species from the southeast of Brazil: Description and bionomic information (Insecta, Diptera, Chironomidae). Spixiana 21: 271-278. [ Links ]
33. TRIVINHO-STRIXINO, S. & G. STRIXINO. 2005. Two new species of Goeldichironomus Fittkau from southeast Brazil (Diptera, Chironomidae). Rev. Bras. Entomol. 49: 441-445. [ Links ]
34. ZILLI, F., M. MARCHESE & A. PAGGI. 2009. Life cycle of Goeldichironomus holoprasinus Goeldi (Diptera: Chironomidae) in laboratory. Neotrop. Entomol. 38 (4): 472-476. [ Links ]














