Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista de la Sociedad Entomológica Argentina
versión impresa ISSN 0373-5680
Rev. Soc. Entomol. Argent. vol.73 no.1-2 La Plata jun. 2014
TRABAJO CIENTÍFICO
Bionomics of Neolasioptera aculeatae (Diptera: Cecidomyiidae), a promising biological control candidate against Parkinsonia aculeata (Fabaceae)
Bionomía de Neolasioptera aculeatae (Diptera: Cecidomyiidae), un agente promisorio para el control biológico de Parkinsonia aculeata (Fabaceae)
Mc Kay, Fernando1, Alejandro J. Sosa1 & Tim A. Heard2
1Fundación para el Estudio de Especies Invasivas, Bolívar 1559, (B1686EFA), Hurlingham, Buenos Aires, Argentina. E-mail: fmckay@fuedei.org
2CSIRO Ecosystem Sciences, EcoSciences Precinct, GPO Box 2583, Brisbane 4001, Australia.
Recibido: 28-VIII-2013;
Aceptado: 04-XII-2013
ABSTRACT. Field surveys conducted in North-central Argentina on Parkinsonia aculeata L. between 2008 and 2011 revealed the presence of the stem-galling midge Neolasioptera aculeatae Gagné (Diptera: Cecidomyiidae). Bionomical and field host range studies were conducted to determine the insect's suitability for biological control of P . aculeata. Presence of N. aculeatae galls was restricted to the northern distribution of P. aculeata. Larvae and/or pupae were found by dissecting galls collected throughout the year on P. aculeata plants at different phenological stages. Emergence of N. aculeatae adults occurred 13 to 34 days from field collection and over an average period of 22 days. Field host range surveys indicated that of the 11 legume species sampled, N. aculeatae adults only emerged from galls collected on P. aculeata. The biological attributes of N. aculeatae and its restricted field host range suggests that it will be a promising biological control agent for P. aculeata.
KEY WORDS: Field host range; Natural enemies; Weed biological control; Parkinsonia; Cina-cina.
RESUMEN. Inspecciones de campo realizadas sobre Parkinsonia aculeata L. en el Norte-centro de Argentina entre 2008 y 2011 revelaron la presencia del mosquito agallícola Neolasioptera aculeatae Gagné (Diptera: Cecidomyiidae). La presencia de las agallas de N. aculeatae está restringida a la distribución norte de P. aculeata. La disección de agallas recolectadas a lo largo del año, reveló la presencia de larvas y/o pupas en distintos estados fenológicos de P. aculeata. La emergencia de adultos de N. aculeatae tuvo lugar 13 a 34 días desde la recolección en el campo y se extendió por un período promedio de 22 días. Entre once especies de leguminosas inspeccionadas en el campo, adultos de N. aculeatae emergieron únicamente de agallas recolectadas sobre P. aculeata. Los atributos biológicos y el restringido conjunto de plantas hospederas utilizadas en el campo, hacen de N. aculeatae un agente promisorio para el control biológico de P. aculeata.
PALABRAS CLAVE: Inspecciones de campo; Enemigos naturales; Control biológico de malezas; Parkinsonia; Cina-cina.
INTRODUCTION
Parkinsonia aculeata L. ("cina-cina") (Leguminosae: Caesalpinoideae) is a thorny leguminous shrub native to the hot and dry regions of North, Central and South America (Hawkins et al., 2007). This species has a pan-tropical distribution following introduction as an ornamental, hedging, fodder and shade tree (Stewart et al., 1992; Wagner et al., 1999; PIER, 1999; Hawkins, 2001). In Australia, P. aculeata is recognized as one of the 20 worst weeds (Thorp & Lynch, 2000) and considered a weed of national significance due to its impacts on the environment and agricultural production (Heard & Bell, 2009). The detrimental effects include its propensity to form dense, thorny, impenetrable thickets along drainage lines, depressions, ephemeral wetlands and, to a lesser extent, uplands (van Klinken et al., 2009).
Control techniques available to manage P. aculeata include the use of herbicides, machinery, fire, grazing, and classical biological control (Deveze, 2004; van Klinken et al., 2009). Biological control efforts began in the 1980s and resulted in the release of three insect species: Rhinacloa callicrates Herring (Miridae: Hemiptera) (a sap-sucking mirid), Mimosestes ulkei (Horn) (Bruchidae: Coleoptera) from North America and Penthobruchus germaini (Pic) (Bruchidae: Coleoptera) (seed-feeding bruchids) from Argentina (Julien & Griffiths, 1998; Flanagan et al., 1996; Donnelly, 2000; Briano et al., 2002; van Klinken et al., 2008). Existing agents, do not appear to be having a significant impact, and new potential agents are unlikely to be found in the United States or northern Mexico (van Klinken et al., 2009).
Recent genetic studies indicating very old dispersal events of P. aculeata in South America (Hawkins et al., 2007), motivated the search for additional natural enemies in this area. Field surveys conducted recently in Argentina, revealed the presence of Neolasioptera aculeatae Gagné (Diptera: Cecidomyiidae), a gall midge responsible for a common and conspicuous stem gall that stunts the branches and often curbs further axillary growth of P. aculeata (Gagné et al., 2011). It has been proposed as a biological control agent against P. aculeata (van Klinken & Heard, 2012).
Neolasioptera (Alycaulini) is a species-rich genus from the Americas, whose species are primary gall makers on a wide array of plant families and appear to be mostly host specific (Gagné, 1994, 2010). From the 65 described Neotropical Neolasioptera, only five species have been recorded from Argentina, with N. aculeatae being the only one associated with P. aculeata (Gagné, 2010; Gagné et al., 2011). In this paper we present bionomical and field host range data on N. aculeatae, and discuss its potential as a biological control agent against P. aculeata.
MATERIAL AND METHODS
The distribution of N. aculeatae was determined through extensive exploratory trips made along the main roads of North-central Argentina, between 2008 and 2011 (Fig. 1). In total, 20 surveys were conducted with 29 sites inspected, some of which were visited up to four times a year to account for seasonal variations. Surveys generally lasted seven days, and included two to three collectors. Time spent on-site rarely exceeded two hours. P. aculeata plants occurring mainly along the roadsides, rangelands and river beds were visually inspected for the presence of galls. Seasonal occurrence and field host range surveys were conducted at several sites along a longitudinal range of c. 500 km in northern Argentina (Chaco, Formosa and Salta provinces) (Fig. 1). The area has a tropical summer rainfall climate (world climate zone 2, as described by Walter et al., 1975) with a mean annual temperature of 22 °C and annual rainfalls (500-1,000 mm), concentrated in the springsummer months (October-March) (Servicio Meteorológico Nacional, 2000).
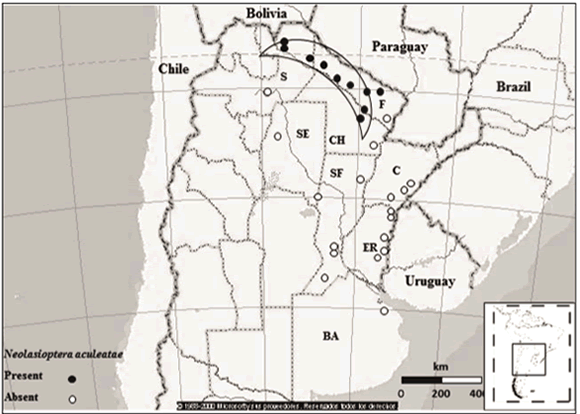
Fig. 1. Main collecting sites of N. aculeatae in Argentina. í—\ Área in which seasonal occurrence and field host range surveys were conducted for this study. BA, Buenos Aires; CA, Catamarca; CH, Chaco; C, Corrientes; ER, Entre Ríos; F, Formosa; S Salta; SE, Santiago del Estero; SF, Santa Fe. This figure was created using Atlas Mundial Encarta 2001 (Microsoft)
Seasonal occurrence
Plants of P. aculeata were sampled four times between March and November 2010 (Table I). For each site, the number of surveyed plants and the number of galls were recorded. Phenological stage of the plants was registered as flowering, fruiting or vegetative. Only fresh active galls with soft green tissue and developing insects were included in the study. Galls were collected by cutting P. aculeata branches with a heavy-duty pruner, kept in plastic containers and transported to the laboratory. A subsample of galls were dissected under a stereo microscope (Olympus SZ61; maximum magnification: 45X) to determine the life stage of specimens inside the galls. The rest of the galls were kept in controlled environmental chambers (25 ± 2 °C: 60-80% RH; 16:8 L:D) for subsequent emergence of adult specimens.
Table I. Seasonal occurrence of N. aculeatae at selected sites in northern Argentina.

Field host range
During the summer 2009/10 and 2010/11, nine sites with populations of P. aculeata and ten cooccurring legume species were surveyed for the presence of galls (Fig. 1). Plant species were selected for their phylogenetic proximity, ecological/distribution overlap and morphological similarity to P. aculeata (Table II). For each site, the number of surveyed plants and the number of collected galls were recorded. Samples were brought to the laboratory and kept in controlled environmental chambers (25 ± 2 °C: 60-80% RH; 16:8 L:D) for subsequent emergence of adults. Voucher specimens of the plants and insects collected were deposited in collections at the Fundación para el Estudio de Especies Invasivas (FuEDEI), Hurlingham, Buenos Aires.
Table II. Field host range of N. aculeatae in the native range of Argentina.

Laboratory rearing
Preliminary rearing of N. aculeatae was attempted three times. On one occasion in September 2009, 10 adults were confined within a gauze-covered sleeve wrapped around the stem of a potted P. aculeata plant. A piece of cotton soaked in a water-sucrose solution was suspended from the upper access hole of the sleeve as a food source for the adults. A second attempt was conducted in 2011 when a collection of 800 galls rendered 400 adults (67 % female). A balanced number of male and female adults (9-51) were confined in 12 rearing aluminium frame cages lined with gauze and measuring 250 x 250 x 800 mm containing one P. aculeata potted plant. In 2012, a third rearing attempt was conducted using rearing cages upon a collection of 300 galls that rendered 150 adults.
RESULTS
Distribution. Presence of N. aculeatae galls was restricted to the northern distribution area of P. aculeata in Argentina (Fig. 1). Stem galls were collected from sites as far north as Embarcación, Salta (S 23.16°) and south to near Fortín Lavalle, Chaco (S 26.13°).
Galls. N. aculeatae stem galls were woody rounded/ovoid-shaped growths at the junction of the stem and the spines of P. aculeata plants (Fig. 2). Gall size ranged from 3-17 mm in length (9.3 ± 2.3) to 2-13 mm in width (6.6 ± 2.3) (mean ± S.D.; n = 191). Galls were green-purple in colour, similar to P. aculeata branches and young shoots. Inside each gall, 1-5 light orange larvae developed individually inside galleries into yellowish mature larvae which completely filled the cavity. Pupation occurred in the gall after which adults eclosed, leaving the pupal exuviae protruding from the gall.

Fig. 2. Neolasioptera aculeatae galls on Parkinsonia aculeata.
Natural enemies. Hymenopteran parasitoids that emerged from N. aculeatae galls were identified as Platygaster sp. (Platygastridae) (M. Loiácono, Museo de La Plata, pers. comm.).
Seasonal occurrence
Fresh active galls were found on all four of the sampling dates during 2010 (Table I). The highest number of galls per surveyed plant was registered in June, which coincides with the cooldry season, while the lowest number was registered in November, the beginning of the hot-wet season. In March, N. aculeatae galls were mostly recorded to be at an early developmental stage and growing near the shoot-tips of vegetative P. aculeata plants. At some sites, almost all the shoot-tips of plants had galls at high densities (2-3 galls per shoot-tip). In June, most plants were in a vegetative stage, bearing medium- to large-sized galls. In September, small-to medium-sized galls were found near the shoot tips of flowering plants. In November, P. aculeata plants of vegetative/flowering stage bore very few medium-sized galls. Various larval instars were found when dissecting galls collected on each sampling date, while pupae were mostly found on galls collected in March. In addition, adults of N. aculeatae emerged from the remaining galls 13-34 days after collection and continued emerging for an average period of 22 (range: 15-28) days (Table I).
Field host range
From the nine sites visited, a total of 919 galls were collected from 416 P. aculeata plants. Adults of N. aculeatae emerged only from P. aculeata stem galls (Table II). N. aculeatae was not even found on the conspecific Parkinsonia praecox. However, different stem galls were found on A. aroma and P. alba species. Cecidomyiidae larvae dissected from galls on Acacia aroma were identified as "not Neolasioptera" (R. J. Gagné, USDA-ARS Systematic Entomology Laboratory pers. comm.).
Gracillariidae moths emerging from P. alba and P. fiebrigii were identified as members of the Neurobathra group (unknown genus and species) (Don Davis, USDA-ARS Systematic Entomology Laboratory pers. comm.).
Laboratory rearing
Preliminary rearing of N. aculeatae using rearing sleeves produced two immature stem galls. However, neither full gall development nor adult emergence was recorded. Rearing attempts using cages proved unsuccessful.
DISCUSSION
Galls may act as physiological nutrient sinks on their host plants, (McCrea et al., 1985; Larson & Whitham, 1991; Harris & Shorthouse, 1996; Goolsby et al., 2000), and thus the impact of gall-forming insects on their host plants is not necessarily centered on the attacked host plant organ or tissue (Hartnett & Abrahamson, 1979; Sacchi et al., 1988; Fernandes et al., 1992). Therefore, the formation of stem galls by N. aculeatae, may be acting as a metabolic sink, indirectly reducing the potential of P. aculeata plants to flower and set mature, viable seeds.
Biological traits of gall-forming insects that increase their potential as biocontrol agents have been described by several authors. Based on studies conducted on the hymenopteran gall-maker Trichilogaster acaciaelongifoliae on A. longifolia in South Africa, Dennill (1988) indicated different biological attributes that appear to increase the potential of gall-forming insects as biocontrol agents. Among these, the agent must live within the tissue that is galled, and the gall development must span the entire reproductive and/or growth phase of the plant. This was the case for N. aculeatae, where larvae and or pupae were recorded in dissected P. aculeata galls throughout the year on plants at different phenological stages. This could be regarded as an attribute that would increase the potential effectiveness of N. aculeatae as biocontrol agent of P. aculeata.
Surveying closely related cooccurring species in the native range of the target contributes useful host-specificity data to prioritize agents for biocontrol (Witt, 2004; Goolsby et al., 2006). Surveys of host plant use in northern Argentina indicated that N. aculeatae galls were found exclusively on P. aculeata, thus constituting it's only known natural host. Although data could only be obtained from a small number of species, the fact that N. aculeatae galls were not found on the conspecific P. praecox constitutes strong evidence of a restricted host range. Given the potential of N. aculeatae as biocontrol agent, we consider that further investigations, especially to improve rearing methods of N. aculeatae, should be pursued.
ACKNOWLEDGEMENTS
We would like to thank Marcelo Parisi and Natalia Cuadra for field work technical assistance, Dr. Ulibarri (Instituto Darwinion, Buenos Aires, Argentina) for the identification of Senna, Sesbania and Neptunia species; Dr. Marta Loiácono (Museo de La Plata, Buenos Aires, Argentina) for the identification of Platygastridae; Don Davis (USDA-ARS Systematic Entomology Laboratory) for the identification of Gracillariidae (Lepidoptera) species, and Juan Briano (FuEDEI) for valuable comments and suggestions on the manuscript.
LITERATURE CITED
1. BRIANO, J. A., H. A. CORDO & C. J. DELOACH. 2002. Biology and field observations of Penthobruchus germaini (Coleoptera: Bruchidae), a biological control agent for Parkinsonia aculeata (Leguminosae: Caesalpinioideae). Biological Control 24: 292-9. [ Links ]
2. DENNILL, G. B. 1988. Why a gall former can be a good biological control agent: the gall wasp Trichilogaster acaciaelongifoliae and the weed Acacia longifolia. Ecological Entomology 13: 1-9. [ Links ]
3. DEVEZE, M. 2004. Parkinsonia. Approaches to the management of Parkinsonia in Australia. National Case Studies Manual. Queensland Department of Natural Resources, Mines and Energy, Brisbane. [ Links ]
4. DONNELLY, G. P. 2000. Biology and host specificity of Rhinocloa callicrates Herring (Hemiptera: Miridae) and its introduction and establishment as a biological control agent of Parkinsonia aculeata L. (Caesalpiniaceae) in Australia. Australian Journal of Entomology 39: 89-94. [ Links ]
5. FERNANDES, G. W., A. L. D. SOUZA & C. F. SACCHI. 1992. Impact of a Neolasioptera (Diptera: Cecidomyiidae) stem galler on its host plant, Mirabilis linearis (Nyctaginaceae). Phytophaga 5: 1-6. [ Links ]
6. FLANAGAN, G. J., D. S. VAN RANGELROOY & S. KERIN. 1996. Integrated management of Parkinsonia aculeata on the Roper River, Northern Territory, Australia. In: Proceedings of the IX International Symposium on Biological Control of Weeds, Stellenbosch, 1996, pp. 441-443. [ Links ]
7. GAGNÉ, R. J. 1994. The Gall Midges of the Neotropical Region. Cornell University Press, New York. [ Links ]
8. GAGNÉ, R. J. 2010. Avalaible at http://www.ars.usda.gov/SP2UserFiles/Place/12754100/Gagne_2010_World_Catalog_Cecidomyiidae.pdf [Acceso: 8 May 2013]. [ Links ]
9. GAGNÉ, R. J., F. MC KAY & T. A. HEARD. 2011. A new species of Neolasioptera (Diptera: Cecidomyiidae) from Parkinsonia aculeata (Leguminosae) in Argentina for possible use in biological control in Australia, with a key to Neotropical species of Neolasioptera. Zootaxa 2866: 61-68. [ Links ]
10. GOOLSBY, J. A., J. MAKINSON & M. PURCELL. 2000. Seasonal phenology of the gall-making fly Fergusonina sp. (Diptera: Fergusoninidae) and its implications for biological control of Melaleuca quinquenervia. Australian Journal of Entomology 39: 336-343. [ Links ]
11. GOOLSBY, J. A., R. D. VAN KLINKEN & W. A. PALMER. 2006. Maximising the contribution of native-range studies towards the identification and prioritization of biocontrol agents. Australian Journal of Entomology 45: 276-286. [ Links ]
12. HARRIS, P. & J. D. SHORTHOUSE. 1996. Effectiveness of gall inducers in weed biological control. Canadian Entomologist 128: 1021-1055. [ Links ]
13. HARTNETT, D. C. & W. G. ABRAHAMSON. 1979. The effects of stem gall insects on life history in Solidago canadensis. Ecology 60: 910-917. [ Links ]
14. HAWKINS, J. A. 2001. Parkinsonia aculeata (Mexican palo-verde). In: Beverley, C., V. Bonham & L. Charles (eds.), Forestry Compendium, CABI Publishing, Wallingford, UK. [ Links ]
15. HAWKINS, J. A., N. BOUTAOUI, K. Y. CHEUNG, R. D. VAN KLINKEN & C. E. HUGHES. 2007. Intercontinental dispersal prior to human translocations revealed in a cryptogenic invasive plant. New Phytologist 175: 575-87. [ Links ]
16. HEARD, T. & K. BELL. 2009. Development of new biocontrol agents for Parkinsonia. Land and Water Australia, Canberra, ACT. [ Links ]
17. JULIEN, J. H. & M. W. GRIFFITHS. 1998. Biological Control of Weeds: a world catalogue of agents and their target weeds. CABI Publishing, Wallingford. [ Links ]
18. LARSON, K. C. & T. G. WHITHAM. 1991. Manipulation of food resources by a gall-forming aphid: the physiology of sink-source interactions. Oecologia 88: 15-21. [ Links ]
19. MCCREA, K. D., W. G. ABRAHAMSON & A. E. WEIS. 1985. Goldenrod ball gall effects on Solidago altissima: 14C translocation and growth. Ecology 66: 1902-1901. [ Links ]
20. PIER (Pacific Islands Ecosystems at Risk). 1999. Invasive Plant Species: Parkinsonia aculeata. (última actualización: 2 Nov 2011). http://www.hear.org/pier. [Acceso: 18 Jun 2013]. [ Links ]
21. SACCHI, C. F., P. W. PRICE, T. P. CRAIG & J. K. ITARNI. 1988. Impact of the shoot galler attack on sexual reproduction in the arroyo willow. Ecology 69: 2021-2030. [ Links ]
22.SERVICIO METEOROLOGICO NACIONAL. 2000. Atlas Climático. Available at: http://www.smn.gov.ar/serviciosclimaticos/?mod=elclima&id=3. [Acceso: 10 Jun 2013]. [ Links ]
23. STEWART, J. L., A. J. DUNSDON, J. J. HELLIN, & C. E. HUGHES. 1992. Wood biomass estimation of bentral american dry zone trees. Oxford University Press, Oxford. [ Links ]
24. THORP, J. R. & R. LYNCH. 2000. The determination of weeds of national significance. National weeds strategy Executive Committee, Launceston. [ Links ]
25. VAN KLINKEN, R. D., L. FLACK & B. LUKITSCH. 2008. What limits predation rates by a specialist seed feeder? Journal of Applied Ecology 45(6): 1600-1611. [ Links ]
26. VAN KLINKEN, R. D., S. D. CAMPBELL, T. A. HEARD, J. MCKENZIE, & N. MARCH. 2009. The biology of Australian weeds: 54. Parkinsonia aculeata L. Plant Protection Quarterly 24(3): 100-117. [ Links ]
27. VAN KLINKEN, R. D. & T. A. HEARD. 2012. Parkinsonia aculeata. In: Julien, J., R. McFadyen & J. Cullen (eds.), Biological control of weeds in Australia, CSIRO Publishing, Melbourne, pp. 437-447. [ Links ]
28. WAGNER, W. L., D. R. HERBST & S. H. SOHMER. 1999. Manual of the flowering plants of Hawaii. University of Hawaii and Bishop Museum Press, Honolulu. [ Links ]
29. WALTER, H., E. HARNICKELL & D. MUELLER-DUMBOIS. 1975. Climate-diagram maps of the individual continents and the ecological climate regions of the earth. Springer-Verlag, New York. [ Links ] [ Links ]














