Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista de la Sociedad Entomológica Argentina
versión impresa ISSN 0373-5680versión On-line ISSN 1851-7471
Rev. Soc. Entomol. Argent. vol.75 no.1-2 La Plata jun. 2016
TRABAJO CIENTÍFICO
Taxonomy and cladistics of the group of genera related to Cyrtomon Schoenherr (Coleoptera: Curculionidae: Naupactini)
Taxonomía y cladística del grupo de géneros relacionados con Cyrtomon Schoenherr (Coleoptera: Curculionidae: Naupactini)
Lanteri, Analía A. & M. Guadalupe Del Rio
Universidad Nacional de La Plata, CONICET, División Entomología, Museo de La Plata, Paseo del Bosque s/n, B1900FWA La Plata, Buenos Aires, Argentina. E-mail: alanteri@fcnym.unlp.edu.ar
Recibido: 3-II-2016
Aceptado: 25-V-2016
RESUMEN. En esta contribución se actualiza la información taxonómica y geográfica sobre cinco géneros de gorgojos (Curculionidae: Entiminae: Naupactini) distribuidos en Argentina, Brasil, Bolivia, Paraguay y Uruguay. Como resultado de un análisis cladístico basado en 48 caracteres morfológicos y 18 taxones terminales (dos del grupo externo y 16 del grupo en estudio) se obtuvo un árbol de máxima parsimonia con dos subgrupos principales, uno formado por Mendozella Hustache como taxón hermano de Cyrtomon Schoenherr, y otro por Lamprocyphopsis Lanteri, Priocyphopsis Lanteri y Priocyphus Hustache. De acuerdo con este resultado Cyrtomon hirsutus (Hustache) es la especie hermana de Mendozella curvispinis (Hustache), en consecuencia se estableció la nueva combinación Mendozella hirsuta (Hustache), y se establece que el género Mendozella incluye actualmente dos especies, ambas distribuidas en Mendoza, Argentina. Cyrtomon se recuperó como grupo monofilético con seis species, C. gibber (Pallas), C. pistor (Boheman), C. luridus (Boheman), C. inhalatus (Germar), C. ovalipennis (Hustache) y C. glaucus (Bovie). Priocyphus es monofilético e incluye cinco species, P. bosqi Hustache, P. hustachei Kuschel, P. inops Kuschel, P. kuscheli Lanteri y la especie nueva Priocyphus cordobensis; Lamprocyphopsis incluye dos especies, L. viridinitens (Kuschel) y L. paraguayensis Lanteri; y Priocyphopsis es monotípico, con la especie tipo P. humeridens (Hustache) como grupo hermano de Priocyphus. Se brindan diagnosis de todos los géneros, una clave para la identificación de todas las especies y fotografías de hábitos, en la mayoría de los casos de sus ejemplares tipo. Se ilustran los caracteres diagnósticos de la cabeza y rostro, tibias anteriores y genitalia con dibujos lineales. Se describen por primera vez los genitales de la hembra de Mendozella hirsuta y los genitales masculinos de Priocyphus kuscheli.
PALABRAS CLAVE: Priocyphus; Priocyphopsis; Lamprocyphopsis; Mendozella; América del Sur; Distribución geográfica
ABSTRACT. In this contribution we update taxonomic and geographical information on five genera of weevils (Curculionidae: Entiminae: Naupactini) distributed in Argentina, Brazil, Bolivia, Paraguay and Uruguay. As a result of a cladistic analysis based on 48 morphological characters and 18 terminal species (two outgroups and 16 species of the ingroup) we recover a single most parsimonious tree with two main subgroups, one including Mendozella Hustache as a sister group of Cyrtomon Schoenherr, and the other including Lamprocyphopsis Lanteri, Priocyphopsis Lanteri and Priocyphus Hustache. In this tree Cyrtomon hirsutus (Hustache) is the sister species of Mendozella curvispinis (Hustache), consequently we establish the new combination Mendozella hirsuta (Hustache), and herein consider that the genus Mendozella includes two species, both from Mendoza province, Argentina. We confirm the monophyly of Cyrtomon, which includes six species, C. gibber (Pallas), C. pistor (Boheman), C. luridus (Boheman), C. inhalatus (Germar), C. ovalipennis (Hustache) and C. glaucus (Bovie). Priocyphus is monophyletic and includes five species, P. bosqi Hustache, P. hustachei Kuschel, P. inops Kuschel, P. kuscheli Lanteri and the new species Priocyphus cordobensis herein described; Lamprocyphopsis ncludes two species, L. viridinitens (Kuschel) and L. paraguayensis Lanteri; and Priocyphopsis is monotypic, with the only species P. humeridens (Hustache) recovered as sister group of Priocyphus. We provide diagnosis of all the genera, a key and habitus photographs of all the species, most of them corresponding to type specimens. Diagnostic characters of head and rostrum, front tibiae and genitalia are illustrated with line drawings. The female genitalia of Mendozella hirsuta and the male genitalia of Priocyphus kuscheli are described for the first time.
KEY WORDS: Priocyphus; Priocyphopsis; Lamprocyphopsis; Mendozella; South America; Geographic distribution
INTRODUCTION
The genera Cyrtomon Schoenherr, 1823, Priocyphus Hustache, 1939, Mendozella Hustache, 1939, Priocyphopsis Lanteri, 1990 and Lamprocyphopsis Lanteri, 1990 (Curculionidae: Entiminae: Naupactini) include 16 species distributed in Argentina, Bolivia, southeastern and southern Brazil, Paraguay and Uruguay. According to Lanteri & Morrone (1991) this group of genera is monophyletic and has diversified in the biographical provinces of Atlantic and Parana forests, Cerrado, Chaco, Yungas, Espinal and Monte sensu Cabrera and Willink (1973). Cyrtomon is broadly distributed in Brazil (Atlantic and Parana forests and Cerrado), Bolivia (Yungas), Argentina (Chaco, Espinal and gallery forests of Paraná, Uruguay and La Plata rivers), Paraguay (Chaco) and Uruguay (gallery forest of Uruguay and La Plata rivers) (del Río et al., 2015). The remaining genera show smaller geographic distributions: Priocyphus ranges in Argentina, Paraguay and Uruguay, in Chaco, Espinal and Pampa biogeographic provinces; Lamprocyphopsis also ranges in Argentina and Paraguay, being distributed in the Chacoan biogeographic province; and Mendozella is endemic to Argentina, Monte biogeographic province, same as Priocyphopsis.
All the genera previously mentioned have been revised by Lanteri (1989, 1990a, 1990b) and detailed studies on body vestiture and mouthparts have been published with co-authors (Coscarón et al., 1991; Díaz et al., 1990a, b), nonetheless, the taxonomic position of some critical species is still doubtful. According to Lanteri (1990a) Cyrtomon Schoenherr, 1823 (senior synonym of Cyphus Germar, 1824 and Neocyphus Bedel, 1883) included the type species C. gibber (Pallas, 1781) and another five species, C. luridus (Boheman, 1840), C. pistor (Boheman, 1833), C. inhalatus (Germar, 1824), C. glaucus (Bovie, 1907) and C. ovalipennis (Hustache, 1938) (Lanteri, 1990a). However, Kuschel (1950) and Wibmer & O'Brien (1986) considered that the latter two species belong to Priocyphus, and that C. luridus, C. pistor and C. inhalatus are subspecies of C. gibber. These authors did not consider characters of the genitalia to make those decisions.
Priocyphus sensu Lanteri (1990b) included the type species P. bosqi Hustache, 1939 and other three species, P. hustachei Kuschel, 1950, P. inops Kuschel, 1950 and P. kuscheli Lanteri, 1990b. In the same paper Lanteri (1990b) described the new genus Priocyphopsis based on the type species Priocyphus humeridens (Hustache, 1926) and Lamprocyphopsis based on the type species Priocyphus viridinitens Kuschel, 1950. Moreover, she provisionally assigned Priocyphus hirsutus Hustache, 1939 to Priocyphopsis and described L. paraguayensis Lanteri, 1990b for Lamprocyphopsis. Priocyphopsis hirsutus was later transferred to Cyrtomon based on the result of a cladistic analysis (Lanteri & Morrone, 1991). Mendozella was considered as a monotypic genus based on the species M. curvispinis (Hustache, 1926) endemic to Argentina, Mendoza province (Lanteri, 1989).
The possibility to study more material from several entomological collections, including types of most species, gave us the chance to analyze new characters, to describe a new species and to provide new taxonomic and geographical information. The main objectives of this paper are to perform a new cladistic analysis for the group of genera closely related to Cyrtomon including all their species, to test the generic and species status of all these taxa, to take the taxonomic actions needed in agreement with the results obtained, to analyze the character evolution, to give a key and habitus photographs for the identification of all the species, and to provide new geographic records and data on type material.
MATERIALS AND METHODS
This study is based on the examination of specimens deposited in the following entomological collections:
Charles W. O'Brien Collection, Green Valley, Arizona, USA (CWOB). Dr. Charles O'Brien.
Departamento de Zoologia da Universidade Federal do Paraná, Curitiba, Brazil (DZUP). Dr. Germano RosadoNeto.
Fundación e Instituto Miguel Lillo, San Miguel de Tucumán, Argentina (FIML). Dr. Carolina Berta.
Museo Argentina de Ciencias Naturales, Bernardino Rivadavia, Buenos Aires, Argentina (MACN). Dr. Arturo Roig Alsina.
Museo de La Plata, La Plata, Argentina (MLP). Dr. Analía Lanteri.
Museu de Zoología da Universidade de São Paulo, Brazil (MZSP). Dr. Sergio Vanin.
Museu Nacional de Rio de Janeiro, Rio de Janeiro, Brazil (MNRJ). Dr. Marcela Monné.
Muséum National d' Histoire Naturelle, Paris, France (MNHN). Dr. Hélène Perrin.
Naturhistoriska Riksmuseet, Stockholm, Sweden (NHRS). Dr. Johannes Bergsten.
New Zealand Arthropod Collection, Auckland, New Zealand (NZAC). Dr. Richard Leschen.
United States National Museum, Washington DC., USA (USNM). Dr. David Furth.
We provide a diagnosis and description for a new species of Priocyphus, herein called P. cordobensis, comparative notes and habitus photographs for all species, and linedrawings of structures that provide diagnostic characters for the cladistic analysis. Dissections of genitalia were made according to standard entomological techniques. Their characters were drawn using a camera lucida adapted to a stereoscopic microscope Nikon SMZ800.
Measurements were taken with an ocular micrometer. Abbreviations used in the description are as follows: W F, width of frons between anterior margin of eyes; WR, width of rostrum across apex; LR, length of rostrum from anterior margin of eye to apex; WP+, maximum width of pronotum; WP-, minimum width of pronotum; LP, maximum length of pronotum; WE, maximum width of elytra (excluding humeri); and LE, maximum length of elytra. The terminology used for the morphological characters follows Marvaldi et al. (2014).
The material cited in previous revisions (Lanteri, 1989, 1990a, b) is not mentioned in the current paper. Illustrations of genitalia of each species and maps of distribution are also omitted because they are included in previous papers.
Cladistic analysis
The terminal taxa of the group under study are 16 species previously assigned to the genera Cyrtomon (7 spp.), Lamprocyphopsis (2 spp.), Mendozella (1 sp.), Priocyphopsis (1 sp.) and Priocyphus (4 known spp.) (Lanteri & Morrone, 1991). To these taxa we added the new species Priocyphus cordobensis. The outgroups selected are the type species of the closely related genera Briarius [Fischer de Waldheim] [type B. augustus (Illiger)] (Lanteri & del Río, 2003) and Stenocyphus Marshall [type S. bituberosus (Gyllenhall, 1833)] (del Río & Lanteri, 2013).
We selected 48 morphological characters (see Table I), 32 belong to the external morphology and 16 to the terminalia (11 of female and five of male). Five characters are continuous and correspond to media of some ratios between measurements. This way we avoid ad hoc methods to establish ranges (Goloboff et al., 2008). Characters with intraspecific variation were treated as polymorphic, as indicated in TNT (e.g. [0 1]). Character states of species for which male or female genitalia could not be examined (because of insufficient material or because males or females are unknown for these species) were scored with "?" and treated as missing data (Maddison, 1993). All discrete multistate characters were treated as unordered.
The data matrix (Table II) was analyzed using the implicit enumeration algorithm of TNT (Goloboff et al., 2008). For character optimizations we used the UNAMBIGUOUS default option of TNT, which reconstructs character state changes only for those characters that have a single most parsimonious reconstruction on the preferred tree. The homoplasy in the tree was estimated using consistency and retention indices (Kluge & Farris, 1969; Farris, 1989). Branch supports were estimated using Symmetric resampling (Goloboff et al., 2003) with 1000 replicates; values over 50% are indicated below the branches of the cladogram (Fig. 1).The most parsimonious tree was rooted with Briarius augustus.
Fig. 1. Most parsimonious cladogram of 16 species of Cyrtomon, Mendozella, Lamprocyphopsis, Priocyphopsis and Priocyphus, and two outgroups. Numbers of nodes represent character changes given in Table III. Support values (Symmetric resampling) are below branches.
RESULTS
As a result of the cladistic analysis we obtained a single most parsimonious tree of 120.16 steps long, with CI= 0.61 and RI= 0.70, in which the genera under study are grouped in two main clades, one including Lamprocyphopsis, Priocyphopsis and Priocyphus, and the other, Mendozella and Cyrtomon. The group of genera under study (node 25) is well supported (SR 81%) by synapomorphies of the external morphology and genitalia: pronotum with broad median sulcus (16.2), front tibiae with large denticles (29.2), distal comb of hind tibiae shorter than dorsal comb (31.1); spermathecal duct sclerotized (40.1), penis with apical cuticular striation and a characteristic piece in the internal sac (47.1 and 48.1). Other well supported clades are those formed by the four species traditionally assigned to Cyrtomon, called C. gibber species group by Lanteri (1990a) (node 21, SR 76%); the genus Priocyphus, with the new species P. cordobensis within it (75%); and Mendozella, herein including two species, M. curvispinis and M. hirsuta n. comb. (SR 95%). The latter species is herein transferred from Cyrtomon to Mendozella based on the results of the current cladistic analysis. Moreover, within Cyrtomon, there is a well supported clade C.gibber + C. pistor (SR 61%) and within Priocyphus, the clade P. inops + P. kuscheli (SR 100%). The support values are lower for the genus Lamprocyphopsis, probably because all the characters of the females are unknown for L. paraguayensis. The synapomorphies and autapomorphies that support the clades and terminal species are listed in Table III.
TAXONOMY
Cyrtomon Schoenherr (Figs. 2-7)
Cyrtomon Schoenherr, 1823: col. 1140; Wibmer & O'Brien, 1986: 47; Lanteri, 1990b: 387-402. Lanteri & Morrone, 1991: 286; Alonso Zarazaga & Lyal, 1999: 164; Morrone, 1999: 152.
Cyphus Germar, 1824: 427; Blackwelder, 1947: 792.
Neocyphus Bedel, 1883: 23; Dalla Torre et al., 1936: 9; Hustache, 1947: 20-21.

Figs. 2-7. Habitus, dorsal views: 2, Cyrtomon gibber, female, MZSP; 3, Cyrtomon pistor, holotype female, NHRS; 4, Cyrtomon luridus, female, MLP; 5, Cyrtomon inhalatus, female, MLP; 6, Cyrtomon glaucus, holotype female, USNM; 7, Cyrtomon ovalipennis, syntype female, MACN. Scales: 2mm.

Figs. 8-11. Habitus, dorsal views: 8, Mendozella curvispinis, female, MLP; 9, Mendozella hirsuta, holotype, female, MNHN; 10, Lamprocyphopsis viridinitens, holotype male, MACN; 11, Lamprocyphopsis paraguayensis, holotype male, NZAC. Scales: 2mm.
Diagnosis: Length 11-24 mm. Vestiture of different colors (whitish, green, pinkish, grey or brown), composed of round scales usually overlapped (except C. glaucus ) and short recumbent setae. Rostrum not conical, with squamose carinae (Figs. 18, 23). Eyes convex. Supraocular border thickened or not thickened. Funicular article 2 less than twice as long as article 1. Prementum subrhomboidal, with 4-6 long setae on external face. Elytral base bisinuate, humeri well developed. Metathoracic wings well developed (in species with right angled humeri) or reduced (in species with posteriorly directed humeri). Front coxae contiguous, closer to anterior than posterior margin of pronotum. Mucro present on front and middle tibiae. Front tibiae with small to large denticles on inner side (Figs. 28-29); middle tibiae with or without denticles; hind tibiae usually lacking denticles (except males of C. ovalipennis). Corbel of hind tibiae broad, squamose; distal comb usually much shorter than dorsal comb (except C. glaucus). Ventrite 2 longer than 3+4. Sternite VIII of female subrhomboidal (Fig. 32). Coxites of ovipositor not posteriorly projected; styli apical, visible from ventral view; baculi straight (Fig. 38). Nodulus of spermatheca tubular, long; spermathecal duct very long (more than 15x as long as spermatheca) and curled (Fig. 45). Apex of penis usually arrowshaped and asymmetrical (Fig. 56); apodemes about as long as median lobe.
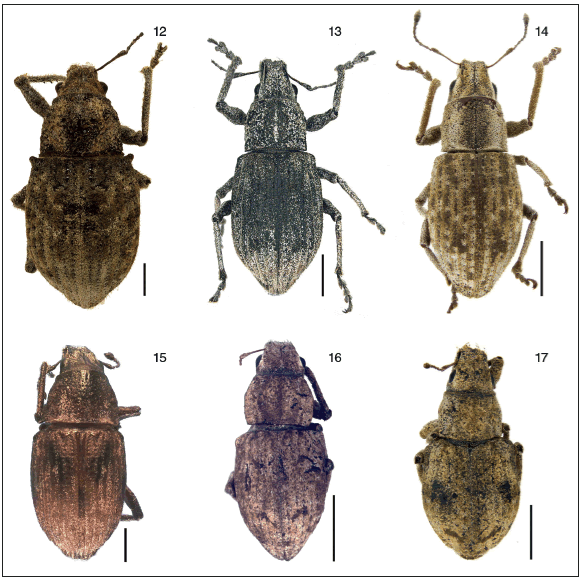
Figs. 12-17. Habitus, dorsal views: 12, Priocyphopsis humeridens, syntype female, MACN; 13, Priocyphus bosqi, paratype female, MLP; 14, Priocyphus hustachei, holotype female, NZAC; 15, Priocyphus cordobensis, holotype female, MLP; 16, Priocyphus kuscheli, holotype female, NZAC; 17, Priocyphus inops, paratype female, MLP. Scales: 2mm.
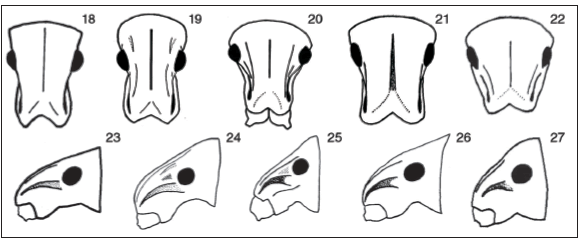
Figs. 18-27. Heads, frontal views and lateral views, females: 18, 23, Cyrtomon gibber; 19, 24, Lamprocyphopsis viridinitens; 20, 25, Priocyphopsis humeridens; 21, 26, Priocyphus bosqi; 22, 27, Priocyphus kuscheli.

Figs. 28-31. Front tibiae, lateral views: 28, Cyrtomon gibber; 29, Cyrtomon ovalipennis; 30, Mendozella curvispinis; 31, Priocyphus kuscheli.

Figs. 32-37. Sternite VIII of females: 32, Cyrtomon gibber; 33, Cyrtomon glaucus; 34, Mendozella curvispinis; 35, Lamprocyphopsis viridinitens; 36,Priocyphopsis humeridens; 37, Priocyphus bosqi.
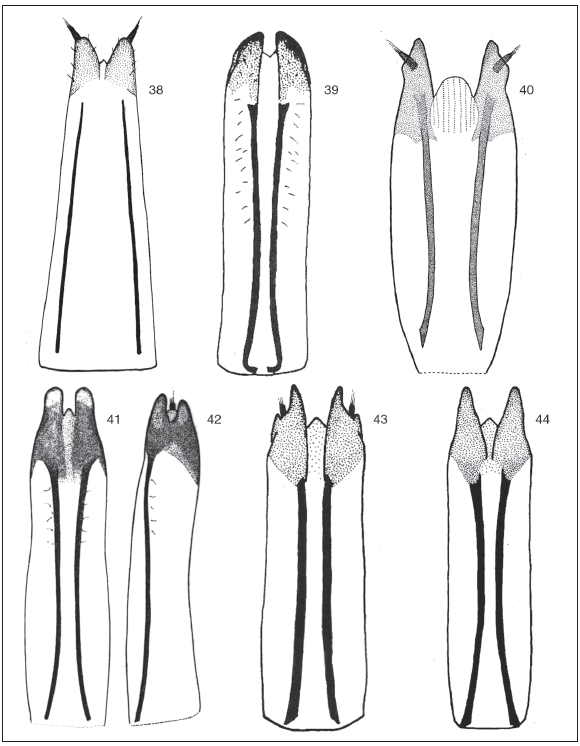
Figs. 38-44. Ovipositors: 38, Cyrtomon gibber; 39, Mendozella curvispinis; 40, Lamprocyphopsis viridinitens; 41, Priocyphus bosqi, ventral view; 42, P. bosqi, lateral view; 43, Priocyphus inops; 44, Priocyphus kuscheli.

Figs. 45-51. Spermathecae and spermathecal ducts: 45, Cyrtomon gibber; 46, Mendozella hirsuta; 47, Lamprocyphopsis viridinitens; 48, Priocyphus hustachei; 49, Priocyphus cordobensis; 50, Priocyphus inops; 51, Priocyphus kuscheli.
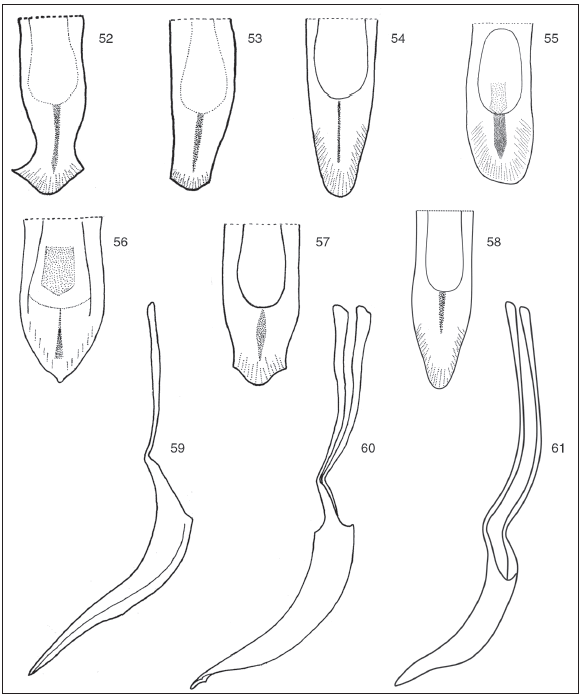
Figs. 52-61. Penis. Apices 52-58: 52, Cyrtomon gibber; 53, Cyrtomon ovalipennis; 54, Lamprocyphopsis viridinitens; 55, Lamprocyphopsis paraguayensis; 56, Priocyphopsis humeridens; 57, Priocyphus bosqi; 58, Priocyphus kuscheli. Lateral views 59-61: 59, Lamprocyphopsis viridinitens; 60, Priocyphus bosqi; 61, Priocyphus kuscheli.
Type species: Curculio gibber Pallas, 1781, by original designation.
Cyrtomon gibber (Pallas) (Fig. 2)
Curculio gibber Pallas, 1781: 39.
Cyphus gibber: Germar, 1824: 428; Marshall, 1922: 183; Blackwelder, 1947: 792.
Cyrtomon gibber: Schoenherr, 1833: col. 1140.
Cyphus argillaceus Germar, 1824: 429 [Syn. by Schoenherr, 1833: 621]
Cyphus chevrolati Boheman, 1840: 142 [Syn. by Kuschel, 1958: 793]. Type material: holotype female from Brazil, at NHRS, Stockholm.
Neocyphus gibber: Bedel, 1883: 23; Dalla Torre et al., 1936: 9.
Cyphus gibber gibber: Kuschel, 1958: 793.
Cyrtomon gibber gibber: Wibmer & O´Brien, 1986: 47.
Cyrtomon gibber: Lanteri, 1990a: 392; Morrone, 1999: 152.
Other material examined: BRAZIL. Bahía: Cándido Sales, XI-1971, Seabra & Roppa (1m MNRJ); Monte Pascoal, 13-XI-1967, A Aguirre (2m MNRJ); Encruzilhada, XI-1971, Seabra & Roppa (1f MNRJ). Espirito Santo: Colatina, XI-1969, A Silva (1f 1m MNRJ); Conceção da Barra (1f MZSP); Corrego Itá, XI-1956, W Zikán (2f 3m MNRJ); Jabaeté, V-1938, A Maller (1f MNRJ); Linhares, X-1969, B Silva; X-1972, Roppa & Alvarenga (10f 5m MNRJ); Rio Bonito (2f MZSP); Rio Lamego, I-1953 (1m MNRJ); Tijuco Preto, X-1937 (1m MNRJ). Minas Gerais: Lagoa Santa, XII-1960 (1m MNRJ); Machacalis, XII-1954 B Silva (1f MNRJ); Mar de Hespanha, X-1987, D Barres (1m MNRJ); Pedra Azul, 700m, X-1977, Roppa (1f MNRJ). Rio de Janeiro: Alto da Boa Vista, Tijuca, III-1950, C Seabra (1f MNRJ); Angra dos Reis, X-1934 (1m MNRJ); Deodoro, 25-I-1936 (1f MNRJ); Guaratiba, 28-II-1940, M Alvarenga (1f MNRJ); Nova Friburgo, XII-1933 (1f MNRJ); São Bento, XI-1966, Telles (1m MNRJ); Santa Teresa, 10-VII-1899 (1f MNRJ); Teresópolis, XI-1940, Freitas (2f MNRJ); 92 km de R de Janeiro, Mendés (4f MNHN). São Paulo: Barueri, 27-I-1955, Lenko (1f MZSP); 47 km Estrada Rio-São Paulo, X-1949, D Mendes (1f 2m MNRJ); Santo Amaro, IV-1961, Reichardt (1f MZSP).
Geographic distribution: Brazil (Bahía, Espirito Santo, Minas Gerais, Rio de Janeiro and São Paulo). It is distributed along the Atlantic forest, being partially sympatric with C. pistor in its northernmost distribution (Bahía to Rio de Janeiro), and with C. luridus in its southernmost distribution (São Paulo). Bahía is a new state record.
Host plants: Solanaceae (Silva et al., 1968).
Remarks: Cyrtomon gibber distinguishes from all remaining species of the C. gibber species group for the whitish color of the vestiture and the usually larger body size. The elytra are strongly humped, with a pair of pronounced impressions on sides, same as in C. pistor, but the rostrum and elytra are distinctly longer than in the latter species.
Cyrtomon pistor (Boheman) (Fig. 3)
Cyphus pistor Boheman, 1833: 821. Type material: holotype female from Brazil, at NHRS, Stockholm.
Neocyphus pistor: Dalla Torre et al., 1936: 9; Hustache, 1947: 20-21.
Cyphus pistor: Blackwelder, 1947: 792.
Cyphus gibber pistor: Kuschel, 1958: 793.
Cyrtomon gibber pistor: Wibmer & O'Brien, 1986: 48.
Cyrtomon pistor: Lanteri, 1990a: 394; Morrone, 1999: 152.
Other material examined: BRAZIL. Bahía: Maracas, XI-1968, FM Oliveira (2f MNRJ). Rio de Janeiro: Tijuca (2f MNHN).
Geographic distribution: Brazil (Bahía and Rio de Janeiro). It is restricted to the northernmost extreme of the Atlantic forest, where it is partially sympatric with C. gibber. Rio de Janeiro is a new state record.
Remarks: Cyrtomon pistor distinguishes from C. gibber because of the brown color of the vestiture, the absence of thickened supraocular border and the shorter rostrum and elytra.
Cyrtomon luridus (Boheman) (Fig. 4)
Cyphus luridus Boheman 1840: 144. Type material: from Brazil, Rio Grande do Sul, at NHRS, Stockholm.
Neocyphus luridus: Dalla Torre et al., 1936: 9; Hustache, 1947: 20-21.
Cyphus luridus: Blackwelder, 1947: 792.
Cyphus gibber luridus: Kuschel, 1958: 794.
Cyrtomon gibber luridus: Wibmer & O'Brien, 1986: 48.
Cyrtomon luridus: Lanteri, 1990a: 396; Morrone, 1999: 152.
Other material examined: ARGENTINA. Misiones: Puerto Londero, Dto. San Javier, 30-X-1946, Viana (4f MLP); San José, I-1953, R Fuembuena (1f MLP); 25 de Mayo, 14-XI-1946, Viana (2f MLP). BRAZIL. Paraná: Arapongas, XII-1951, A Maller (2f 2m MNRJ); Curitiba (1f MNHN); Matelandia, X-1961 (1f DZUP); Pato Branco, 6-XII-1983 (1f DZUP); Piraguará. 4-X-1968, Moure-Marinoni (1f 1m DZIP); Rio Negro, 25-IX-1924 M Witte (2f 2m MNRJ); Rolāndia, XII-1982, Proksch (1f 2m MZSP). Rio Grande do Sul: Cruz Alta (3f MNHN); Santa María, 8-IX-1972 (1f DZUP). Santa Catarina: Blumenau, VI-1919 (1f MZSP); Corupá, XI-193, A Maller (1f MNRJ); Joinville (1m MNRJ); Mafra, XI-1937, A Maller (2f MNRJ); Mafra (2f MNHN); Pinhal, XII-1951, A Maller (1m MNRJ); São Bento, II-1952, A Maller (3f MNRJ). São Paulo: Araras, 26-II-1982, M Campos (1f DZUP); Anhangai, 29-XI-1926 (2f MZSP); São Manuel, I-1982, G Andrade (1m MNRJ); Vale du Rio Pardo (10ex MNN). PARAGUAY. Alto Paraná: Ciudad del Este (1f CWOB). Central: Villa Elisa, XII-1939, Denier (2f MLP). Itapúa: Hohenau (2f 2m MNHN). San Pedro: San Pedro de Ycuamandiyú (1f MLP).
Geographic distribution: Northeastern Argentina (Misiones), Brazil (Paraná, Río Grande do Sul, Santa Catarina and São Paulo) and Paraguay (Alto Paraná, Central, Itapúa and San Pedro). It ranges in the Paraná forest, being partially sympatric with C. gibber in São Paulo, and with C. inhalatus in northeastern Argentina (Misiones) and eastern Paraguay.
Host plants: Solanum viarum Dunal (Solanaceae) in Misiones, Argentina (Lanteri et al., 2002); Solanum mauritianum Scopoli, Cestrum intermedium Sendt. and Duboisia sp. (Solanaceae) in Paraná, Brazil (Tironi et al., 2005). Duboisia sp. is a medicinal plant introduced in Brazil from Australia. The larvae of C. luridus feed on the roots of this plant causing 100% damage in Arapongas, Paraná state (Tironi et al., 2005). It is also associated with Eucalyptus spp. (Myrtaceae) in São Paulo (Silva et al., 1968).
Remarks: Cyrtomon luridus distinguishes from C. inhalatus because the vestiture is pinkish or grey always lighter on head, venter and legs; the sides of the pronotum are more straight, the elytra more rugose, with supernumerary striae always distinct, and the spermathecal duct is wider (Lanteri, 1990a). This species differentiates from C. gibber because the elytral disc is less steeped towards posterior third and lacks pronounced impressions; the uneven intervals are slightly more convex than even intervals; and the spermathecal duct is wider and less curled (with only one loop).
Microctonus sp. (Hymenoptera: Braconidae) is a parasitoid of C. luridus that has been investigated for its biological control (Tironi et al., 2004).
Cyrtomon inhalatus (Germar) (Fig. 5)
Cyphus inhalatus Germar, 1824: 430. Type material: holotype female from Brazil at Univ. Halle, Germany.
Neocyphus inhalatus: Dalla Torre et al., 1936: 9; Hustache, 1947: 19-20.
Cyphus inhalatus: Blackwelder, 1947: 792.
Neocyphus inhalatus var intermedius: Hustache, 1947. Type material: holotype female from Argentina, Santiago del Estero, Río Salado, MNHN, Paris.
Cyphus gibber inhalatus: Kuschel, 1958: 794.
Cyrtomon gibber inhalatus: Wibmer & O'Brien, 1986: 48.
Cyrtomon inhalatus: Lanteri, 1990a: 397; Morrone, 1999: 152.
Other material examined: ARGENTINA. Buenos Aires: Luján (2f MNRJ); Punta Lara, 10-IV-1938, Maldonado (1f MLP). Catamarca: city, XI-1959, A Martinez (1f MZSP). Córdoba: Achiras (MNHN); Río de los Sauces, 28-II1942, Maldonado (1f MLP); Valle Hermoso, XII-1942, Viana (2f MLP), idem, II-1943, Viana (10f MLP); Villa Carlos Paz (MLP). Corrientes: Corrientes (MLP). Entre Ríos: Victoria, 32° 36.923'S, 60° 10.324'W, 3-III-2013, Lanteri col. (2f 1m MLP); Paraná, 13-XI-1967, M del P Arce (1f MLP). Jujuy: Ledesma, 24-II-1945, Birabén (1f MLP). Misiones: Iguazú, Cuerda & Pérez (1f MLP). Salta: PN Finca El Rey, VIII-1958, A Martínez (1f MZSP); Tartagal, 26-II-1945, Birabén (1f MLP). Santa Fe: Achiras (1m MNHN). Santiago del Estero: Río Salado (8f 3m MNHN); Turena, Dto. Robles, 22-XI-1939, Maldonado (1f MLP). Tucumán: Burruyacu, Villa Padre Monti, 7-II-1948, Golbach (1f 1m FIML); El Morenillo, III-1936 (1f FIML); Raco, 30-III-1969, Ronderos-Schnack (1f MLP); Tapia, III-1972, Zimmermann (1f FIML). BOLIVIA. La Paz: Coroico, XII-1948, A Martinez (1m MZSP). Santa Cruz: Lagunillas, XI-1917 (2f MNHN); Montero (1f CWOB). Tarija: Yacuiba, XII-1917 (1f MNHN). BRAZIL. Goiás: Jatai, XI-1972, FM Oliveira (1f 1m MLPC). Minas Gerais: Campina Verde, X_1960, Alvarenga (1f MZSP); Monte Alegre, 23-X-1962, K Lenko (1f 1m MZSP); Rio Verde, 400m (DZUP); Uberlandia, XI-1962 Exp. Dep. Zool. (10f MZSP). Mato Grosso: Três Lagoas, margen Izq. Rio Sucurim, Faz Canãa, XI-1966, F Lane (1f MZSP); Empalme ruta 163 (1f MLP). São Paulo: Mogi Guaçú, 17-19-XI-1967, Reichhardt (2f 1m MZSP). URUGUAY. Montevideo: Montevideo (1f CWOB).
Geographic distribution: Argentina (Buenos Aires, Catamarca, Córdoba, Corrientes, Chaco, Entre Ríos, Jujuy, La Pampa, La Rioja, Mendoza, Misiones, Salta, San Luis, Santa Fe, Santiago del Estero and Tucumán), Bolivia (La Paz, Santa Cruz and Tarija), Brazil (Goiás, Minas Gerais, Mato Grosso and São Paulo), Paraguay and Uruguay (Montevideo). This species is broadly distributed in the biogeographical provinces of Cerrado, Chaco, Yungas and Pampa. It is partially sympatric with C. luridus in São Paulo. Misiones, Corrientes and Entre Ríos (Argentina), La Paz and Tarija (Bolivia) and Goiás, Minas Gerais, Mato Grosso and São Paulo (Brazil) are new state records.
Cyrtomon inhalatus was cited for Paraguay (Wibmer & O'Brien, 1986) but we could not confirm its presence in this country, because we studied material for the eastern side of Paraguay, that corresponds to the Paraná forest, where the most common species is C. luridus. Cyrtomon inhalatus probably occurs on the western side of this country.
Host plants: Species associated with wild and cultivated Solanaceae, Cestrum parqui L'Hér., Solanum glaucophyllum Desf., Solanum tuberosum L. and Nicotiana tabacum L. (Lanteri et al., 2002). It was also found on cotton Gosypium hirsutum L. (Malvaceae) and Medicago sativa L. (Fabaceae) in Argentina (Lanteri, 1990a). Solanum glaucophyllum is a toxic plant that shows similar geographic distribution than C. inhalatus plus C. luridus (Argentina, Bolivia, Brazil, Paraguay and Uruguay).
Remarks: Cyrtomon inhalatus distinguishes from C. luridus because the color of the vestiture is light green, the sides of the pronotum are more curved, the elytra longer and more rugose, and the spermathecal duct is narrower and slightly shorter (about 10x as long as spermatheca). The supernumerary striae and punctures of regular striae are almost indistinct in some specimens with moderately long elytral setae. In the variety intermedius described by Hustache (1947) based on material from Santiago del Estero, Argentina, the supernumerary striae are distinct. The specimens sampled from the Cerrado biogeographic province in Brazil (Goias, Mato Grosso and Minas Gerais) are larger (more than 20 mm long) than those of other areas (about 15 mm long).
Cyrtomon glaucus (Bovie) (Fig. 6)
Cyphus glaucus Bovie, 1907: 326. Type species: lectotype female from Argentina, Buenos Aires, La Plata, at USNM, Washington D.C.
Neocyphus glaucus: Dalla Torre et al., 1936: 9; Hustache, 1947: 20-21.
Cyphus glaucus: Blackwelder, 1947: 792.
Priocyphus glaucus: Kuschel, 1950: 546; Wibmer & O'Brien, 1986: 48.
Cyrtomon glaucus: Lanteri, 1990a: 398; Morrone, 1999: 152.
Other material examined: ARGENTINA. Buenos Aires: La Plata (1f 1m MNHN). URUGUAY. San José: San José (1f MLP).
Geographic distribution: Species endemic to Argentina (Buenos Aires) and Uruguay (San José), and restricted to the margins of La Plata river, where it inhabits in the southernmost distribution of the Paraná forest. This forest extends from Brazil along the gallery forests of the Paraná and Uruguay rivers, down the mouth of La Plata River. In this area it is partially sympatric with C. inhalatus.
Cyrtomon glaucus is a rare species probably in risk of extinction due to human impact on this area. Uruguay is a new country record.
Remarks: Cyrtomon glaucus mainly distinguishes from C. inhalatus because the vestiture is iridescent green, darker than in the latter species, and composed of very small scales with smooth surface (in the remaining species of Cyrtomon scales have microscopic ribs) (see Coscarón et al., 1991). The pronotum is subcylindrical with smooth surface, the rostral groove is very broad, the elytral striae are similarly flat and with small punctures, and the distal comb of the hind tibiae is longer than the dorsal comb. The ovipositor and the apodeme of the female sternite VIII are much longer than those of the remaining species of the genus, and of the whole group under study (Fig. 33).
Cyrtomon ovalipennis (Hustache) (Fig. 7)
Neocyphus ovalipennis Hustache, 1938: 95; Hustache, 1947: 20-21. Type material: 6 females and 4 males syntypes, from Rio Salado, Santiago del Estero, Argentina, at MNHN, Paris.
Cyphus ovalipennis: Blackwelder, 1947: 792.
Priocyphus ovalipennis: Kuschel, 1950: 546; Wibmer & O'Brien, 1986: 48.
Cyrtomon ovalipennis: Lanteri, 1990a: 398; Morrone, 1999: 152.
Other material examined: ARGENTINA. Córdoba: no loc, 16-II-1939 (1f MNRJ). Chaco: Las Breñas, 6-IX-1941, PA Berry (6f USNM). La Rioja: I- 1904 (1m MACN, labeled as cotype); Salta: Metán, II-1957 (1f USNM). Tucumán: Las Cejas, 6-IV-1917 (1f FIML); no loc. (1f 1m MACN).
Geographic distribution: Endemic to Argentina (Córdoba, Chaco, La Rioja, Salta, Santiago del Estero and Tucumán), Chacoan bio-geographic province. In this area is sympatric with C. ovalipennis. Córdoba and Chaco are new state records.
Host plants: The main hosts are trees and shrubs of the genus Prosopis (Fabaceae). It was occasionally found on Gossypium hirsutum L. (Malvaceae) (Lanteri et al., 2002).
Remarks: Cyrtomon ovalipennis differentiates from any other species of Cyrtomon by the subcylindrical and punctuate pronotum and the presence of large denticles on the inner side of front and middle tibiae (female) or the three pairs of tibiae (male). Based on the characters of the pronotum and legs is similar to Lamprocyphopsis paraguayensis, but the vestiture is brown, same as in C. pistor. The apex of the penis is rounded with vanished lateral points (Fig. 53).
Mendozella Hustache (Figs. 8-9) Mendozella Hustache, 1939: 42; Blackwelder, 1947: 795; Wibmer & O'Brien, 1986: 52; Lanteri, 1989: 142-143; Lanteri & Morrone, 1991: 286; Alonso Zarazaga & Lyal, 1999: 164; Morrone, 1999: 156.
Diagnosis: Length 11-14 mm. Vestiture brown, moderately dense, composed of overlapped smooth scales and long suberect setae. Rostrum not conical. Eyes convex. Supraocular border thickened. Funicular article 2 less than twice as long as article 1. Prementum subcircular, lacking setae on external face. Pronotum subcylindrical, with rought integument, broad sulcus and distinct longitudinal impressions on sides. Elytral base slightly bisinuate, humeri well developed, tuberculate or not tuberculate; striae 3-4 and 5-6 interrupted on anterior third; uneven intervals convex, 7° interval riblike. Metathoracic wings reduced. Front coxae contiguous, closer to anterior than posterior margin of pronotum. Mucro and denticles large (Fig. 30), present in the three pairs of tibiae or in front and middle tibiae. Corbel of hind tibiae broad, squamose; distal comb shorter than dorsal comb. Ventrite 2 as long as ventrites 3+4. Sternite VIII subrhomboidal without differentiated apical half (Fig. 34). Coxites of ovipositor slightly sclerotized and with styli terminal (same as in Cyrtomon) or coxites posteriorly projected and concealing styli (same as in the remaining genera of the group under study) (Figs. 38, 39). Nodulus of spermatheca conical or subcylindrical long; spermathecal duct long (about 10x as long as spermatheca) and curled (with a single loop) (Fig. 46). Apex of penis arrow-shaped, asymmetrical (Fig. 52); apodemes about as long as median lobe.
Type species: Trichocyphus humeridens Hustache, 1926, by original designation.
Mendozella curvispinis (Hustache) (Fig. 8)
Trichocyphus curvispinis Hustache, 1926: 161. Type material: holotype female from Paramillo, Mendoza, Argentina, II- 1907, at MNHN, Paris. Length 12mm.
Mendozella curvispinis: Hustache, 1939: 42; Morrone, 1999: 156.
Other material examined: ARGENTINA. Mendoza: Picheuta, 27-I-1970 (17 ex CWOB).
Geographic distribution: Endemic to central-western Argentina (Mendoza). Monte bio-geographic province.
Host plants: Larrea divaricata Cav. (Zygophyllaceae) (Lanteri et al., 2002), plant species typical of the Monte province.
Remarks: The presence of long, erect setae covering the integument, and the interrupted 3-4 and 5-6 elytral striae in their basal third are synapomorphies of Mendozella, not seen in any other genera of the group under study. The latter character was not observed in previous studies (Lanteri, 1989; Lanteri & Morrone, 1991).
Mendozella hirsuta (Hustache) n. comb. (Fig. 9)
Priocyphus hirsutus Hustache, 1939: 42. Type material: holotype female from Carrizal, Mendoza, Argentina, 25-I-1907, at MNHN, Paris. Length 12mm.
Priocyphopsis hirsutus: Lanteri, 1990b.
Cyrtomon hirsutus: Lanteri & Morrone, 1991: 286; Morrone, 1999: 152.
Female genitalia: Sternite VIII subrhomboidal, without differentiated apical half (Fig. 34); apodeme almost twice as long as plate, slightly slender than in M. curvispinis. Ovipositor about half length of abdomen, without strongly sclerotized coxites that conceal styli; styli apicolateraly directed; baculi slightly curved outward near both extremes, lacking short setae on external sides (Fig. 39). Spermatheca subcylindrical, 0.5 mm long; nodulus tubular, slightly exceeding level of ramus; cornu slightly shorter than spermathecal body; spermathecal duct slightly narrower than nodulus, long (about 12x as long as spermatheca) and curled (Fig. 46).
Geographic distribution: Endemic to central-western Argentina (Mendoza), Monte bio-geographic province.
Remarks: Mendozella hirsuta differentiates from M. curvispinis because it is slightly smaller (about 11 mm long), the pronotum is more slender, the elytral humeri lack tooth-like tubercle, and the hind tibiae lack mucro and denticles on their inner side. The female genitalia are herein described for the first time. The female sternite VIII is equal to that of M. curvispinis (Fig. 34) but the ovipositor is similar to those of species of Cyrtomon, because it does not have sclerotized coxites that conceal the styli.
Lamprocyphopsis Lanteri (Fig. 10-11)
Lamprocyphopsis Lanteri, 1990b: 416; Lanteri & Morrone, 1991: 286; Alonso Zarazaga & Lyal, 1999: 164; Morrone, 1999: 154.
Diagnosis: Length 15-17 mm. Vestiture iridescent green, with pale pinkish pattern stripes along sides of pronotum, anterior third of elytra, venter of head and legs; overlapped scales with microscopic ribs; setae short, recumbent. Rostrum not conical, with strong carinae (Figs. 19, 24). Eyes convex. Supraocular border thickened. Funicular article 2 less than twice longer than article 1. Prementum subhexagonal, lacking setae on external face. Elytral base bisinuate; humeri well developed, posteriorly curved. Metathoracic wings reduced. Front coxae contiguous, closer to anterior than posterior margin of pronotum. Front and middle tibiae with line of strong denticles on inner side (female). Corbel of hind tibiae broad, squamose; distal comb slightly shorter than dorsal comb. Ventrite 2 about as long as 3+4. Female sternite VIII cordiform, apodeme very short (about 1.5x as long as plate). Coxites of ovipositor projected and partially concealing styli; membrane separating coxites very expanded (Fig. 40). Spermatheca broad, with very short nodulus; spermathecal duct thin, short (about 2.5X as long as spermatheca), not curled (Fig. 47). Apex of penis truncate or rounded, symmetrical or asymmetrical (Figs. 57, 59); apodemes shorter than median lobe (Fig. 58).
Type species: Priocyphus viridinitens Kuschel, 1950, by original designation.
Lamprocyphopsis viridinitens (Kuschel) (Fig. 10)
Priocyphus viridinitens Kuschel, 1950: 546; Wibmer & O'Brien, 1986: 48. Type material: holotype male, Argentina, Santiago del Estero, Wagner col., at MACN, Buenos Aires.
Lamprocyphopsis viridinitens: Lanteri, 1990b: 420; Morrone, 1999: 154.
Geographic distribution: Endemic of north-central Argentina (Córdoba, Salta and Santiago del Estero). It occurs in the Chacoan biogeographic province.
Host plants: It is mainly associated with Prosopis sp. (Fabaceae).
Remarks: Lamprocyphopsis viridinitens mainly distinguished from L. paraguayensis because of the subconical pronotum, the smaller elytral punctures and the shape of the penis, narrowed towards apex and rounded at the tip (Fig. 57).
Lamprocyphopsis paraguayensis Lanteri (Fig. 11)
Lamprocyphopsis paraguayensis Lanteri, 1990b: 420; Morrone, 1999: 154. Type material: holotype males from Paraguay, San Luis, Roimoser leg., Kuschel collection at NZAC, Auckland.
Geographic distribution: Endemic to Paraguay, Cordillera de San Luis, extended throughout Amambay and Concepción departments, towards the coasts of Paraguay River. It ranges in the Chacoan biogeographic province.
Remarks: Lamprocyphopsis paraguayensis is similar to L. viridinitens and C. ovalipennis in the subcylindrical and punctuate pronotum, and the presence of large, curve denticles on the inner side of front and middle tibiae. It mainly differentiates because of the shape of the pronotum (subcylindrical instead of subconical) and the apex of the penis wide, truncate and asymmetrical (Fig. 59).
Priocyphopsis Lanteri (Fig. 12) Priocyphopsis Lanteri, 1990b: 415-416; Alonso-Zarazaga & Lyal, 1999: 165; Morrone, 1999: 165.
Diagnosis: Length 10-13.5 mm. Vestiture grey, moderately dense, composed of over-lapped scales and short recumbent setae. Rostrum strongly conical and with very sharp carinae (Figs. 20, 25). Eyes convex. Supraocular border not thickened. Funicular article 2 slightly longer than article 1. Prementum subrhomboidal, lacking setae on external face. Elytral base slightly bisinuate; humeri reduced, having anteriourly directed tooth-like tubercle; interval 7 rib-like. Metathoracic wings reduced. Front coxae contiguous, closer to anterior than posterior margin of pronotum. Front tibiae with line of strong sharp denticles on inner side. Corbel of hind tibiae broad, squamose; distal comb longer than dorsal comb (tibial apex expanded). Ventrite 2 about as long as 3+4. Female sternite VIII suboval (Fig. 36). Coxites of ovipositor projected and concealing styli (Fig. 39). Spermatheca subcylindrical, having very short nodulus; spermathecal duct medium length (about 6x as long as spermatheca), not curled. Apex of penis acute (Fig. 56); apodemes about as long as median lobe.
Type species: Priocyphopsis humeridens (Hustache, 1926), by original designation.
Priocyphopsis humeridens (Hustache) (Fig. 12)
Trichocyphus humeridens Hustache, 1926: 162. Type material: two syntypes females from Argentina, Tucumán, Bañado, V-1921, W Weiser, at MNHN, Paris and MACN, Buenos Aires. Priocyphus humeridens: Hustache, 1939: 43.
Priocyphopsis humeridens: Lanteri, 1990b: 416; Morrone, 1999: 165.
Other material examined: ARGENTINA. Neuquén: no loc. (1m MLP).
Geographic distribution: Endemic to north and central-western Argentina (Catamarca, Tucumán and Neuquén). Monte biogeographic provinces in its limits with Chaco (northern extreme) and Espinal (southern extreme). Neuquén is a new state record.
Remarks: Priocyphopsis humeridens mainly differentiates from Priocyphus bosqi and other species of this genus, because it has convex eyes, tuberculate humeri, few large denticles on the inner side of front tibiae, penis acute at apex, oval sternite VIII and very short nodulus of the spermatheca. It is similar to Priocyphus cordobensis in the shape of the pronotum and in the rib-like interval 7; and it is similar to Mendozella curvispinis in the characters of the ovipositor.
Priocyphus Hustache (Figs. 13-17)
Priocyphus Hustache, 1939: 43; Wibmer &O'Brien, 1986: 48; Lanteri, 1990b: 403-422; Lanteri & Morrone, 1991: 286; Alonso Zarazaga & Lyal, 1999: 165; Morrone, 1999: 165.
Prionocyphus: Blackwelder, 1947: 795 (lapsus). Priocyphis: Lanteri & Morrone, 1991: 278 (lapsus).
Diagnosis: Length 6.5-11 mm. Vestiture grey or brown, moderately dense, composed of over-lapped scales having microscopic ribs and short recumbent setae. Rostrum slightly conical to strongly conical (Figs. 21, 22) Eyes flat. Supraocular border not thickened. Funicular article 2 slightly longer than article 1, or both articles subequal in length. Prementum subrhomboidal, with 6-12 long setae on external face. Elytral base straight; humeri reduced, not tuberculate. Metathoracic wings reduced. Front coxae contiguous, closer to anterior than posterior margin of pronotum. Front tibiae with line of small to medium-sized denticles on inner side. Corbel of hind tibiae broad, squamose; distal comb slightly shorter than dorsal comb. Ventrite 2 about as long as 3+4. Female sternite VIII subrhomboidal (Fig. 37). Coxites of ovipositor projected, partially or completely concealing styli (Figs. 41-44). Spermatheca with long or very long tubular nodulus (reaching level of gland entrance or exceeding spermathecal length); spermathecal duct short to medium length (1.5- 5x as long as spermatheca), not curled (Figs. 48-51). Apex of penis arrow-shaped or rounded, symmetrical; apodemes about as long as median lobe (Figs. 57-60).
Type species: Priocyphus bosqi Hustache, 1939, by original designation.
Priocyphus bosqi Hustache (Fig. 13)
Priocyphus bosqi Hustache, 1939: 43. Type material: holotype female from Argentina, La Pampa, General Pico, IV-1934, Bosq leg. at MNHN, Paris; 4 paratypes (3f 1m) at MNHN, with same data as holotype; 14 paratypes (8f 6m) at MLP, La Plata, Argentina, with same data as holotype.
Priocyphus bosqi: Lanteri, 1990b: 408; Morrone, 1999: 165.
Other material examined: ARGENTINA. La Pampa: General Pico, III-1943, on alfalfa (3f 2m FIML). San Luis: no loc. (1f MLP).
Geographic distribution: Endemic to sierras of central Argentina (Buenos Aires, Córdoba, La Pampa and San Luis). It inhabits the Espinal bio-geographic province, but it has invaded some areas of Pampa. San Luis is a new state record.
Host plants: Priocyphus bosqi is part of the alfalfa weevil complex in Argentina (Lanteri, 1994).
Besides Medicago sativa L. (Fabaceae), this weevil was recently found associated with Glycine max (L.) Merr. (Fabaceae) in Córdoba province, and with Gossypium hirsutum L. (Malvaceae) (Lanteri et al., 2002).
Remarks: Priocyphus bosqi distinguishes from P. hustachei because the elytral striae are more convex, the sides of the pronotum are more curved, the disc of the pronotum has a broader sulcus and a pair of slight lateral impressions, and the nodulus of the spermathecae is shorter. The first instar larva of P. bosqi was described by Marvaldi & Loiácono (1994).
Priocyphus hustachei Kuschel (Fig. 14)
Priocyphus hustachei Kuschel, 1950: 548. Type material: holotype from Argentina, Córdoba, Anisacate, II-1943, Argentina, at Kuschel's collection, NZAC, Auckland; two paratypes females, same data as holotype, at Kuschel's collection, NZAC, Auckland.
Priocyphus hustachei: Lanteri, 1990b: 410; Morrone, 1999: 165.
Other material examined: ARGENTINA. Catamarca: no loc. (1h MLP).
Geographic distribution: Endemic to central Argentina (Córdoba and Catamarca). Chacoan biogeographic province. Catamarca is a new state record.
Remarks: Priocyphus hustachei distinguishes from P. bosqi because its eyes are more oval and flat, it has more slender pronotum and sulcus, lacking longitudinal impressions. The nodulus of the spermatheca is longer than in P. bosqi.
Priocyphus cordobensis new species (Fig. 15)
Holotype: female from Argentina, Córdoba, Oliva, 1-IV-1974, sweeping, deposited at MLP.
Differential diagnosis: The new species distinguishes from P. bosqi and P. hustachei by the strongly conical pronotum, having convex disc and lineal sulcus only extended along apical third; elytra are broader; spermatecal duct is slightly longer and nodulus of spermatheca is recurved near duct junction.
Description holotype female: Length 14 mm. Integument brown, with darker areas on anterior half of elytra, over intervals 3-4 and 7-8. Vestiture grey, composed of round, overlapped scales and short recumbent setae. Head. Rostrum about 1.20x as long as wide at apex; epistome large, covered with small, sparse scales; rostral groove wide; rostral carinae very strong, divergent towards frons, denuded; frons about 1.50x as wide as rostrum at apex; eyes oval and flat. Prementum subrhomboidal, impressed, with 6 long setae on external face. Antennae sparsely covered with setae; scape not exceeding hind margin of eyes; funicular article 2 slightly longer than article 1; funicular articles 3-6 slighlty longer than wide at apex; funicular article 7 conical, about as long as wide at apex; club oval, about 2.25x as long as wide. Prothorax sub-conical, W+/W-: 1.64; disc convex and slightly punctuated; median groove restricted to apical third; base slightly undulate; postero-lateral angles slightly projected and impressed. Scutellum glabrous. Elytra oval; base almost straight; humeri reduced, without tubercles; all striae well developed, with medium- sized punctures; intervals slightly convex. Metathoracic wings reduced. Legs. Front femora slightly wider than hind femora; front tibiae having large mucro and 8 medium-sized denticles on inner side; middle tibiae with small mucro and minute denticles; hind tibiae lacking mucro and denticles; corbels broad, squamose; distal comb slightly shorter than dorsal comb; tarsite 2 elongate. Abdomen. Ventrite 2 about as long as ventrites 3+4.
Female genitalia. Sternite VIII subrhomboidal, with differentiated apical half, basal V-shaped sclerotization and tuft of short setae at apex; apodeme about twice as long as plate (Fig. 37). Ovipositor about half length of abdomen; coxites distally projected, with truncate apex; styli concealed by coxites from ventral view (Figs. 41-42). Spermatheca subcylindrical (0.78 mm long), nodulus tubular, very long (almost as long as spermatheca), slightly recurved near duct junction; ramus well developed; cornu slightly shorter than spermathecal body. Spermathecal duct sclerotized, slightly narrower than nodulus and about 3x as long as spermatheca (Fig. 49).
Geographic distribution: Endemic to central Argentina (Córdoba). Espinal biogeographic province.
Remarks: The new species is similar to P . bosqi and P. hustachei. The conical pronotum resembles that of Priocyphopsis humeridens.
Priocyphus inops Kuschel (Fig. 17) Priocyphus inops Kuschel, 1950: 549. Type material: holotype female from Argentina, Formosa, Clorinda, 10- I-1940, Denier leg., Kuschel collection at NZAC; allotype male from Argentina, Corrientes, I-1921, De Carlo leg., Kuschel col. at NZAC; paratype female, same data as holotype. Priocyphus inops: Lanteri 1990b: 412; Morrone, 1999: 165.
Other material examined: ARGENTINA. Corrientes: 3km Corrientes city, 17-1989, C & L O'Brien & Wibmer (1f CWOB); Laguna Brava, 7 km E Corrientes, Hwy 5, 17-I-1989, C & L O'Brien & Wibmer (1m CWOB). Formosa: Laguna Oca, 2-I-1940, Denier leg. (1f MLP). URUGUAY. Montevideo, 14-XII-1944, Parker (1f USNM).
Geographic distribution: Argentina (Corrientes and Formosa) and Uruguay. Chacoan and Pampean biogeographic province. Uruguay is a new country record.
Remarks: Priocyphus inops differentiates from the closely related species Priocyphus kuscheli because it shows smaller body size, subcylindrical pronotum and more slender elytra. The coxites of the ovipositor are posteriorly projected and acute, but they do not completely conceal the styli from ventral view (Fig. 43). The nodulus of the spermatheca is recurved towards the base of the cornu (Fig. 50). unfortunately, we could not study the male genitalia of this species.
Priocyphus kuscheli Lanteri (Fig. 16)
Priocyphus kuscheli Lanteri, 1990b: 412; Morrone, 1999: 165. Type material: holotype female from Paraguay, Asunción, 23-XII-1946, Williner col., at NZAC, Auckland.
Description of male: More slender than female. Penis progressively narrowed towards apex; apex rounded and symmetrical; apodemes of penis (= temones) longer than median lobe; pieces of internal sac typical of the genera under study (Figs. 58-61).
Other material examined: PARAGUAY. Cordillera: San Bernardino, 18-I-1939 (1f 1m MLP).
Geographic distribution: Paraguay (Central and Cordillera). Chacoan biogeographic province. Cordillera is a new state record.
Remarks: Priocyphus kuscheli differentiates from P. inops because it has conical pronotum, wider elytra, coxites of the ovipositor concealing the styli from ventral view (Fig. 44) and nodulus of the spermatheca recurved and directed towards the apex of cornu (Fig. 51). The male genitalia, herein described for the first time, is similar to that of Lamprocyphopsis viridinitens (Fig. 57), but the apodemes are longer than the median lobe, instead of shorter.
Key to species of the group of genera related to Cyrtomon
1. Body length usually not exceeding 11 mm. Vestiture dull colored, grey or brown. Eyes flat. Elytral base straight. Prementum with 6-12 long setae on external face ….…………….......... 2
1'. Body length usually 12 to 24 mm. Vestiture of different colors, grey, brown, pale green, whitish or iridescent green. Eyes convex. Elytral base bisinuate. Prementum with 4-6 long setae on external face or lacking setae ……………...……. 6
2. Species less than 8 mm long. Vestiture light brown, with pair of oblique dark brown stripes on distal third of elytra, from interval 5 to interval 2. Rostrum conical, short (L/W-: 1 to 1.10x) (Figs. 22, 27); rostral carinae indistinct. Funicular article 2 about as long as article 1. All tibiae lacking denticles on inner side, front tibiae with long stiff setae (Fig. 31). Coxites of ovipositor strongly projected and apically acute (Figs. 43-44). Spermathecal duct thin, less than 2x as long as spermatheca; nodulus of spermatheca recurved (Figs. 50-51) …............. …. P. inops species group … 3
2'. Species more than 8 mm long. Vestiture grey, usually lacking dark brown maculae on distal third of elytra. Rostrum slightly conical, more than 1.25x as long as wide at apex; rostral carinae strong, denuded (Figs. 21, 26). Funicular article 2 longer than article 1. Front tibiae with denticles on inner side. Coxites of ovipositor projected and apically truncate (Figs. 41-42).
Spermathecal duct 2-5x as long as spermatheca; nodulus not recurved, or slightly recurved near duct junction (Figs. 48-49) …............... 4
3. Pronotum conical. Styli of ovipositor completely concealed by coxites in ventral view (Fig. 44). Nodulus of spermatheca directed towards apex of cornu (Fig. 51) ..….. P. kuscheli (Fig. 16)
3'. Pronotum subcylindrical. Styli of ovipositor partially concealed by coxites in ventral view (Fig. 43). Nodulus of spermatheca directed towards base of cornu (Fig. 50) …......... P. inops (Fig. 17)
4. Pronotum strongly conical (W+/W- more than 1.50); disc convex, with narrow groove on anterior third. Nodulus of spermatheca slightly curved near duct juction (Fig. 49) ..……....……… Priocyphus cordobensis n. sp. (Fig. 15)
4'. Pronotum slightly conical (W+/W- 1.35-140); disc flat or impressed, with groove extended along whole length. Nodulus of spermatheca not recurved near duct junction …................................................... …P. bosqi species group ... 5
5. Eyes oval. Pronotum rough, with curved flanks, wide central sulcus and longitudinal impressions along sides. Nodulus of spermatheca long, but shorter than spermathecal body ... P. bosqi (Fig. 13)
5'. Eyes strongly oval. Pronotum almost smooth, with almost straight flanks, slender central sulcus and lacking lateral impressions. Nodulus of spermatheca very long, exceeding length of spermathecal body (Fig. 48) …..... P. hustachei (Fig. 14)
6. Dorsum, venter and legs covered with long suberect setae. Pronotum subcylindrical (W+/W- less than 1.50), with broad median sulcus and deep impressions along sides. Elytral striae 3-4 and 5-6 interrupted on anterior third; punctures of striae large …………............. Mendozella ... 7
6'. Dorsum and venter lacking long suberect setae. Prothorax either conical (W+/W- more than 1.50) or subcylindrical, but then lacking longitudinal impressions. Elytral striae 3-4 and 5-6 not interrupted in anterior third; punctures of striae large or small ………………. 8
7. Elytral humeri having large tooth-like tubercle. Three pairs of tibiae with mucro and large denticles on inner side (Fig. 30). Coxites of ovipositor projected in short nail-shaped pieces that conceal styli from ventral view (Fig. 39). Nodulus of spermatheca conical, not reaching gland entrance …………. Mendozella curvispinis (Fig. 8)
7'. Elytral humeri lacking tooth-like tubercle. Mucro and line of denticles present in front and middle tibiae. Coxites of ovipositor not projected in short nail-shaped pieces that conceal styli (Fig. 38). Nodulus of spermatheca tubular, slightly exceeding gland entrance (Fig. 46) ……..……. Mendozella hirsuta n. comb. (Fig. 9)
8.. Elytral humeri reduced, with distinct anteriorly directed toothlike tubercle. Vestiture grey. Front tibiae with 4-5 large and sharp denticles on inner side. Distal comb of hind tibiae longer than dorsal comb. Sternite VIII of female oval (Fig. 36). Apex of penis acute (Fig. 56) .........……Priocyphopsis humeridens (Fig. 12)
8'. Elytral humeri well developed, lacking anteriorly directed toothlike tubercle. Vestiture palegreen, iridescent green, whitish or brown. Front tibiae with 6-8 small denticles, or front and middle tibiae with 7-11 large and curved denticles. Distal comb usually shorter than dorsal comb. Sternite VIII of female subrhomboidal or cordiform. Apex of penis not acute at apex …..............… 9
9. Pronotum usually subcylindrical, not impressed along center and sides, punctuated or smooth. Elytra without supernumerary striae; humeri posteriorly curved and projected. Metathoracic wings reduced. Front tibiae with line of 9-10 large denticles on inner side. Disc of pronotum with distinct punctuation or smooth. Prementum with or without long setae on external face. Apex of penis arrow-shaped, truncate or rounded …............................................. 10
9'. Pronotum conical, impressed along center and sides, integument rough, with irregularly shaped fovea. Elytra with supernumerary striae; humeri about right angled. Metathoracic wings well-developed. Front tibiae with line of 7-8 small denticles. Prementum with 4 to 6 long seta on external face. Apex of penis arrow-shaped ……………......... …C. gibber species group ... 13
10. Vestiture pale green, irididescent; with 2 pairs of pinkish strips along sides of pronotum, one prolonged along 8-10 elytral interval, anterior third of interval 1°, area close to humeri, venter of head and legs also pinkish. Supraocular border thickened. Prementum lacking setae. Sternite VIII subcordiform (Fig. 35); ovipositor very short (about 1/3 length of abdomen); coxites strongly sclerotized, projected and partially concealing styli; baculi slightly curved in both extremes (Fig. 40). Spermatheca broad, with very short nodulus (Fig. 47). Apodemes of penis shorter than median lobe (Fig. 59) ……….. Lamprocyphopsis ... 11
10'. Vestiture uniformly iridescent green or brown. Supraocular border not thickened. Prementum with 4-6 long setae on external face. Sternite VIII subrhomboidal (Figs. 32-33); ovipositor long to very long (half length or equal to length of abdomen), coxites slightly sclerotized, not projected; styli apical not concealed by coxites; baculi straight to slightly posteriorly convergent (Fig. 38). Spermatheca slender, with tubular, long nodulus (Fig. 45). Apodemes of penis about as long as median lobe (Fig. 54) ………. 12
11. Pronotum conical (maximum width near base). Elytral striae medium -sized; uneven interval more convex than even interval. Penis narrowed towards apex, symmetrical (Fig. 54) …….…. Lamprocyphopsis viridinitens (Fig. 10)
11'. Pronotum subcylindrical (maximum width near middle). Elytral striae broad; even and uneven intervals similarly convex. Penis not narrowed towards truncate, apex truncate, asymmetrical (Fig. 55) …. Lamprocyphopsis paraguayanus (Fig. 11)
12. Vestiture iridescent green; scales very small, contiguous, with smooth surface. Rostral groove very wide. Pronotum almost smooth. Front tibiae with line of medium sized denticles; middle tibiae with very small denticles (Fig. 28). Punctures of elytral striae small, somewhat indistinct. Distal comb longer than dorsal comb. Apodeme of sternite VIII about 3x as long as plate (Fig. 33). Ovipositor about as long as abdomen. Apex of penis arrow-shaped (with distinct subapical points) (Fig. 52) ……Cyrtomon glaucus (Fig. 6 )
12'. Vestiture brown; scales overlapped, distinctly larger than in C. glaucus, surface with microscopic ribs. Rostral groove wide. Pronotum punctuate. Front and middle tibiae with line of large denticles (females) or all tibiae with denticles (males) (Fig. 29). Punctures of elytral striae large. Apodeme of sternite VIII about 2x as long as plate (Fig. 32). Ovipositor about half length of abdomen. Apex of penis lacking distinct lateral points (Fig. 53) …. Cyrtomon ovalipennis (Fig. 7)
13. Elytra humped, with pair of oblique impressions on each side of maximum high (near apical third); uneven interval strongly convex regarding even interval. Preocular impression strong. Central and lateral impressions of pronotum very pronounced. Spermathecal duct with three loops (Fig. 45) ................................................... 14
13' Elytra convex but not humped, with pair of very slight impressions on each side of maximum high; uneven interval slightly more convex than even intervals. Preocular impression vanished. Central and lateral longitudinal impressions of pronotum moderately pronounced. Spermathecal duct with one or two loops (Fig. 46) …….. 15
14. Vestiture light brown, pair of oblique impressions on each side of maximum high, dark brown. Rostrum less than 1.20x as long as wide at apex. Elytra less than 1.40x as long as wide about middle ………….. C. pistor (Fig. 3)
14'. Vestiture whitish. Rostrum about 1.50x or more, as long as wide at apex. Elytra more than 1.50x as long as wide about middle ............................................................. C. gibber (Fig. 2)
15. Vestiture pale green. Sides of pronotum curved. Elytral intervals slightly rugose, covered with moderately long setae. Spermathecal duct narrower than nodulus ……. C. inhalatus (Fig. 5)
15'. Vestiture usually pinkish or grey, head, legs and venter usually lighter. Sides of pronotum slightly curved. Elytral intervals strongly rugose, covered with short setae. Spermathecal duct wider than nodulus …………..……C. luridus (Fig. 4)
DISCUSSION
The cladogram herein obtained (Fig. 1) shows some differences with that one published by Lanteri & Morrone (1991), in which the phylogenetic sequence was Lamprocyphopsis, Priocyphopsis humeridens, Mendozella curvispinis, Priocyphopsis hirsutus, Cyrtomon and Priocyphus. As a consequence of that result, Lanteri & Morrone (1991) transferred P. hirsutus to Cyrtomon. This species was originally classified in Priocyphus and provisionally assigned to Priocyphopsis by Lanteri (1990a) because in that opportunity the type material of P. hirsutus was not available. The possibility to study this material in detail, including the characters of the female genitalia, reveals that this species is closely related to M. curvispinis (95% of support). Both species are endemic to Mendoza, Argentina, Monte biogeographic province, and have long setae covering their bodies (6.1), pronotum with broad median sulcus (16.2), striae 3-4 and 5-6 interrupted on anterior third (23.1), uneven intervals of elytra convex (22.3) and sternite VIII subrhomboidal (33.1). Mendozella curvispinis has more autapomorphies than M. hirsuta, e.g., tuberculate humeri (21.2), mucro and denticles in all tibiae (28.2, 29.2) and coxites of the ovipositor posteriorly projected and concealing the styli (36.1, 38.1). Moreover, we recovered the clade Mendozella + Cyrtomon (Fig. 1, node 24), mainly justified by the pronotum with maximum width near middle (15.1), the spermathecal duct long (39.1), nodulus long (42.1), and penis arrow-shaped and asymmetrical (46.1).
Another difference between results of the two phylogenetic analyses is that in the present tree (Fig. 1) Priocyphopsis humeridens is recovered as the sister group of Priocyphus, the latter being a well supported group (SR 75%) of five species, including the new one herein described. Some of the synapomorphies of this genus (Fig. 1, node 32) are the flat eyes (11.1), the presence of 6-12 long setae on the external side of the prementum (14.1) and the elytral base straight (19.2). Within Priocyphus, P. inops and P. kuscheli, from Argentina, Paraguay and Uruguay (Chacoan and Pampean biogeographic provinces) share several synapomorphies, e.g. rostral carinae indistinct (7.0), rostral sulcus narrow (8.0), funicular articles 1 and 2 subequal (13.2), all tibiae lacking denticles (29.0), coxites of the ovipositor strongly posteriorly projected in acute pieces (36.2), spermathecal duct very short (39.2) and nodulus of the spermatheca recurved (43.1). These species form a so called P. inops species group (Lanteri, 1990b) and they mainly differentiate in the shape of the pronotum and the orientation of nodulus of the spermatheca.
The other three species of Priocyphus (P. bosqi, P. hustachei and P. cordobensis) are more similar to each other and mainly differentiate in characters of the integument and morphometrics. They inhabit in the Espinal, a biogeographic province that forms an arch limiting with Chaco in the north, Monte in the west and Pampa in the east, following the Central Sierras of Argentina, located in Córdoba, San Luis, La Pampa and Buenos Aires province (Tandilia and Ventania) (Cabrera & Willink, 1973). Priocyphus bosqi mainly occurs in the southern half of the Espinal (San Luis, La Pampa and Buenos Aires), P. hustachei in the northwest (Córdoba and Catamarca) and P. cordobensis in the center (Córdoba province). This geographic pattern, in which some closely related species are partially sympatric in central Argentina, mainly Córdoba province, is also seen in other genera of Naupactini, e.g. Pantomorus auripes species group (Lanteri et al., 1991).
Priocyphopsis distinguishes from Priocyphus because it has humeral tubercles (21.2) (same as M. curvispinis, although smaller and with different orientation), riblike interval 7 (22.2), distal comb of hind tibiae longer than dorsal comb (31.0), oval sternite VIII (33.2) and acute apex of penis (45.0). Priocyphopsis humeridens inhabits in the Monte, same as Mendozella, but it is not recorded for Mendoza and its range extends near the limits with the biogeographic provinces of Espinal and Chaco, where Priocyphus has diversified.
Lamprocyphopsis, described based on a type species previously assigned to Priocyphus (P. viridinitens) is herein recovered as monophyletic (Fig. 1, node 26), mainly based on the following synapomorphies: humeri posteriorly projected (21.1) and apodemes of penis shorter than median lobe (44.0). Despite some external similarity with some species of Cyrtomon (e.g. C. inhalatus and C. glaucus) it is recovered in the clade including Priocyphopsis and Priocyphus.
The taxonomic position of C. ovalipennis and C. glaucus has changed according to different authors who classified them either in Cyrtomon (or its synonyms Cyphus and Neocyphus) (Blackwelder, 1947; Hustache, 1947) or in Priocyphus (Kuschel, 1950; Wibmer & O'Brien, 1986). Kuschel (1950) transferred C. ovalipennis and C. glaucus to Priocyphus when he described P. viridinitens, because the three species are similar in several external features, e.g. shape and sculpture of the pronotum, shape of the elytral humeri, presence of large denticles in two pairs of tibiae. The cladistic analysis of Lanteri & Morrone (1991) and the current analysis confirm that C. ovalipennis and C. glaucus are closer to the remaining species of Cyrtomon than to those of Priocyphus, especially for the presence of long setae on the prementum (14.1) and distal comb of hind tibiae longer than dorsal comb (31.2).
The remaining species of Cyrtomon form a well supported group (Fig. 1, node 21) described as C. gibber species group by Lanteri (1990a). Among the main synapomorphies it is worth mentioning the pronotum with median impression (16.0), the uneven intervals convex (22.3) and supernumerary striae in the elytra (23.2). The species included, although easy to recognize, are mainly separated by characters of the integument and morphometrics (see Table III), probably due to their recent evolution. The degree of separation among them is correlated with their geographic distribution. Cyrtomon gibber and C. pistor inhabit the southernmost and the northernmost distributions of the Atlantic forest, respectively, and they form a monophyletic group well separated from C. luridus, ranging in the Parana forest, and C. inhalatus, ranging in the Cerrado, Chaco, Yungas, and Pampa. This pattern explains why Kuschel proposed to treat these last two species as subspecies of C. gibber. Considering that the category of subspecies is not commonly used in Curculionidae, we prefer to keep them as closely related species of the same complex or species group. In Lanteri & Morrone (1991) the C. gibber species group was treated as a single terminal unit, same as the P. bosqi species group (P. bosqi plus P. hustachei) and the P. inops species group (P. inops plus P. kuscheli), and this kind of discussion at species level was not done.
ACKNOWLEDGEMENTS
We thank all the curators for loaning material and helping us during our visits to their collections; to Bruno Pianzola and Paulina Hernández (MLP, Argentina) and to Birgit Rhode (NZAC) for their help with the photographs of the specimens; to Sonia Suárez for her technical support; to the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT) and the Universidad Nacional de La Plata, Argentina for their financial support through grants PIP 0355, BID-PICT 2012/2524, and 11/N763, respectively.
LITERATURE CITED
1. ALONSO- ZARAZAGA, M. A. & LYAL, C. H. C. 1999. A world catalogue of families and genera of Curculionoidea (Insecta: Coleoptera). Entomopraxis, D.C.P., Barcelona, Spain. [ Links ]
2. BEDEL, L. 1882-1888. Faune des Coléoptères du basin de la Seine. Annales de la Societe Entomologique de France 6 (hors série), Rynchophora, 1-442. [ Links ]
3. BLACKWELDER, R. E. 1947. Checklist of the Coleopterous insects of Mexico, Central America, the West Indies, and South America. Part 5. Bulletin - United States National Museum 185 (1-5): 765-925. [ Links ]
4. BOVIE, A. 1907. Notes sur les Curculionides. Annales de la Société Entomologique de Belgique 51: 67-71 (Première partie), 326-328 (Deuxième partie). [ Links ]
5. CABRERA, A. L. & A. WILLINK. (1973) Biogeografía de América Latina. Serie de Biología, Monografía 13. Secretaría General de la Organización de los Estados Americanos, Washington D.C. [ Links ]
6. COSCARÓN, M. del C., N. B. DÍAZ, A. A. LANTERI & M. S. LOIÁCONO. 1991. Importancia taxonómica del revestimiento tegumentario en la tribu Naupactini I. Género Cyrtomon y taxa afines (Coleoptera: Curculionidae). Neotrópica 37 (97): 31-54. [ Links ]
7. DALLA TORRE, K.W. VON, M. VAN EMDEN & F. VAN EMDEN. 1936. Coleopterorum Catalogus. Pars 147, Curculionidae: Bracyderinae I, pp 11-132, vol. 27. [ Links ]
8. DEL RÍO, M. G. & A. A. LANTERI. 2013. Taxonomic revision of the genus Stenocyphus Marshall (Coleoptera, Curculionidae) from Brazil. Zookeys 357: 29-43. [ Links ]
9. DEL RíO, M. G., J. J. MORRONE & A. A. LANTERI. 2015. Evolutionary biogeography of South American weevils of the tribe naupactini (oleoptera: Curculionidae). Journal of Biogeography 42: 1293-1304. [ Links ]
10. DÍAZ, N. B., M. S. LOIÁCONO, M. del C. COSCARÓN & A. A. LANTERI. 1990a. Importancia taxonómica de las piezas bucales en la tribu Naupactini. I. Género Cyrtomon Schoenherr y taxa afines (Coleoptera: Curculionidae). Revista Brasileira de Entomologia 34 (4): 861-876. [ Links ]
11. DÍAZ, N. B., M. S. LOIÁCONO, A. A. LANTERI & M. DEL C. COSCARÓN. 1990b. Importancia taxonómica de las piezas bucales en la tribu Naupactini. II. Las especies del género Cyrtomon (Coleoptera: Curculionidae). Neotrópica 36 (96): 93-99. [ Links ]
12. FARRIS, J. S. 1989. The retention index and the rescaled consistency index. Cladistics 5: 417-419. [ Links ]
13. GERMAR, E. F. 1824. Insectorum species novae aut minus cognitae, descriptionibus illustratae. Halde, Hendel & Sons, vol 1, Coleoptera, XXIV+624 pp, illus (Curc. Pp 185-461 & pls. I-II). [ Links ]
14. GOLOBOFF, P. A., J. S. FARRIS, M. KALERSJO, B. OXEL MAN, M.J. RAMIREZ & C. SZUMIK. 2003. Improvement to resampling measures of groups support. Cladistics 19: 324-332. [ Links ]
15. GOLOBOFF, P. A., J. S. FARRIS & K.C. NIKON. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24: 774-786. [ Links ]
16. HUSTACHE, A. 1926. Contribution á l'étude des Curculionides de la République Argentine (première note). Anales del Museo Nacional de Historia Natural "Bernardino Rivadavia" 34: 155-261. [ Links ]
17. HUSTACHE, A. 1938. Nouveaux Naupactini de l'Amerique méridionale (Col. Curculionidae). Bulletin de la Societe Entomologique de France 43: 93-96. [ Links ]
18. HUSTACHE, A. 1939. Curculionides nouveaux de l'Argentine et autres régions Sud-Américaines. Anales de la Sociedad Científica Argentina 128: 38-64. [ Links ]
19. HUSTACHE, A. 1947. Naupactini de l'Argentine et des regions limitrophes (Col. Curculion.). Revista de la Sociedad Entomológica Argentina 13 (1-5): 3-146. [ Links ]
20. KLUGE, A. G. & J. S. FARRIS. 1969. Quantitative phyletics and the evolution of anurans. Systematic Zoology 18: 1-32. [ Links ]
21. KUSCHEL, G. 1950. Die Gattung Priocyphus Hustache, 1939. Revista de Entomologia, Rio de Janeiro 21 (3): 545-550. [ Links ]
22. KUSCHEL, G. 1958. Neotropische Rüsselkäfer aus dem Museum G. Frey (Col. Curculionidae). Entomologische Arbeiten aus dem Museum G. Frey 9 (3): 750-798. [ Links ]
23. LANTERI, A. A. 1989. Estudio sistemático de los géneros Trichocyphus Heller y Mendozella Hustache (Coleoptera: Curculionidae). Boletín de la Sociedad de Biología de Concepción 60: 139-147. [ Links ]
24. LANTERI, A. A. 1990a. Revisión sistemática del género Cyrtomon Schönherr (Coleoptera: Curculionidae). Revista Brasileira de Entomologia 34 (2): 387-402. [ Links ]
25. LANTERI, A. A. 1990b. Revisión sistemática del género Priocyphus Hustache 1939 y creación de los géneros Priocyphopsis y Lamprocyphopsis (Coleoptera: Curculionidae). Revista Brasileira de Entomologia 34 (2): 403-422. [ Links ]
26. LANTERI, A. A. (dir.). 1994. Bases para el control integrado de los gorgojos de la alfalfa. De la Campana Ediciones, La Plata. [ Links ]
27. LANTERI, A. A. & M. G. DEL RÍO. 2003. Revision of the genus Briarius [Fischer de Waldheim] (Coleoptera: Curculionidae). Insect Systematics and Evolution 34 (3): 281-294. [ Links ]
28. LANTERI, A. A. & J. J. MORRONE. 1991. Cladistic analysis of Priocyphus Hustache and related genera (Coleoptera: Curculionidae). Procceding Entomological Society of Washington 93 (2): 278-287. [ Links ]
29. LANTERI, A.A., M.S. LOIACONO, M. del C. COSCARÓN & N.B. DÍAZ. 1991 (90). Estudio sistemático del grupo de especies afines a Pantomorus auripes Hustache (Coleoptera: Curculionidae). Revista de la Sociedad Entomológica Argentina 49 (1-4): 3-16. [ Links ]
30. LANTERI, A. A., A. E. MARVALDI & S. M. SUÁREZ. 2002. Gorgojos de la Argentina y sus plantas huéspedes. Tomo I: Apionidae y Curculionidae. Publicación Especial Nº 1, de la Sociedad Entomológica Argentina. [ Links ]
31. MADDISON, W. P. 1993. Missing data versus missing characters in phylogenetic analysis. Systematic Biology 42: 576-581. [ Links ]
32. MARSHALL, G. A. K. 1922. On new genera and species of Neotropical Curculionidae. Transactions of the Entomological Society of London (1-2): 181-224. [ Links ]
33. MARVALDI, A. E. & M. S. LOIÁCONO. 1994. First instar larvae in the tribe Naupactini (Coleoptera, Curculionidae). Revista Brasileira de Entomologia 38(2): 453-466. [ Links ]
34. MARVALDI, A. E., A. A. LANTERI, M. G. DEL RíO & R. G. OBERPRIELER. 2014. Chapter 3.7.5. Entiminae Schoenherr 1823. En: Leschen R. A. B. & R. G. Beutel (eds.) Handbook of Zoology, Arthropoda: Insecta: Coleoptera Volume 3: Morphology and Systematics (Phytophaga), de Gruyter Press, Berlín, pp. 503-523. [ Links ]
35. MORRONE, J. J. 1999. The species of Entiminae (Coleoptera: Curculionidae) ranged in America south of the United States. Anales del Instituto de Biología, UNAM 70: 99-168. [ Links ]
36. PALLAS, P. S. 1781. Icones insectorum praescum Rossiae Sibiraeque peculiarium quae collegit et descriptionibus illustravit. Walther, Erlangae. [ Links ]
37. SCHOENHERR, C. J. 1823. Curculionides Isis Oken, 1823, heft X, columns 1123-1146; heft V, columns 581-588. Title usually cited as "Tabula synoptic familiae curculionidum" [ Links ].
38. SCHOENHERR, C. J. 1833. Genera et species curculionidum cum synopnymia hujus familiae, vol 1. Roret, Paris, Fleischer, Lipsiae. [ Links ]
39. SCHOENHERR, C. J. 1840. Genera et species curculionidum cum synopnymia hujus familiae, vol. 6. Roret, Paris, Fleischer, Lipsiae. [ Links ]
40. SILVA, A. G. D'A., C. R. GONÇALVEZ, D. MONTEIRO GALVÃO, A. J. L. GONÇALVEZ, J. GOMES, M. DO NACIMENTO SILVA & L. DE SIMONI. 1968. Quarto Catálogo dos insetos que vivem nas plantas do Brasil, seus parasitos e predadores. Departamento de Defesa e Inspecão Agropecuaria, Serviço de Defesa Sanitaria Vegetal, Laboratorio Central de Patología Vegetal, Río de Janeiro, Brasil. [ Links ]
41. TIRONI, P., A Von TREUNFELS & J. R. P. PARRA. 2004. Biology of Microctonus sp. (Hymenoptera: Braconidae), a parasitoid of Cyrtomon luridus Boh. (Coleoptera: Curculionidae). Scientia Agricola, Piracicaba 61 (5): 538-541. [ Links ]
42. TIRONI, P., A. Von TREUENFELS & J. R. P. PARRA. 2005. Population dynamics of Cyrtomon luridus Boheman (Coleoptera: Curculionidae) on Duboisia sp. (Solanaceae) in Brazil. Scientia Agricola, Piracicaba 62 (5): 473-477. [ Links ]
43. WIBMER, G. J. & C. W. O'BRIEN. 1986. Annotated checklist of the weevils (Curculionidae sensu lato) of South America (Coleoptera: Curculionidae). Memoirs of the American Entomological Institute 39: 563pp. [ Links ]
APPENDIX
Table I. List of morphological characters used in the cladistic analysis.
- Rostrum, length relative to width at apex (excluding scrobes in dorsal view).
- Head, width of frons (distance between anterior margin of eyes) relative to width of rostrum at apex (excluding scrobes in dorsal view).
- Pronotum, maximum width relative to width at apex.
- Elytra, length along midline relative to maximum width (excluding humeri when well developed).
- Elytra, length along middle relative to length of pronotum.
- Vestiture of elytra: setae, short recumbent (0); setae long, erect (1).
- Rostrum, pair of lateral carinae: carinae indistinct (0); carinae subparallel squamose (1); carinae denuded, divergent towards frons (2).
- Rostrum, median groove: narrow (0) wide (1), very wide (2).
- Head, supraocular borders: not thickened (0); thickened (1).
- Head, preocular impression: strong (0); slight (1); indistinct (2).
- Head, shape and convexity of eyes: eyes round, convex (0); eyes oval, flat (1).
- Antennae, length and width of scape: wide, not reaching hind margin of eye (0), slender, reaching to exceeding hind margin of eye (1).
- Antennae, length of funicular article 2 (antennomere 3) with respect to length of funicular article 1 (antennomere 2): funicular article 2 about twice as long as funicular article 1 (0); funicular article 2, 1.1 to 1.9x as long as article 1 (1); funicular article 2, about as long as article 1 (2).
- Mouthparts, setation of prementum: prementum with about 20 short fine setae on whole external surface (0); with 4 to 12 long setae on distal half of external surface (1); lacking setae (2).
- Pronotum, maximum width: near base (0); near middle length (1).
- Pronotum, median impression and longitudinal sulcus: disc of pronotum with median impression (0); with broad median sulcus (1); with fine to indistinct sulcus (2).
- Pronotum, pair of lateral, longitudinal impressions: lateral impressions present (0); lateral impressions absent (1).
- Pronotum, disc surface: disc of pronotum rough, with irregularly shaped fovea (0); disc of pronotum with distinct punctuation (1); slightly punctuate to smooth (2).
- Elytra, outline of base: base strongly bisinuate (0); slightly bisinuate (1); straight (2).
- Elytra, development of humeri: humeri well-developed (0); humeri reduced (1).
- Elytra, shape of humeri and tubercle: humeri at about right angle, lacking tubercle (0); humeri posteriorly directed, with small prominence (1); humeri antero laterally projected in a tubercle (2).
- Elytra, curvature of interstriae: interstriae flat (0); interstriae convex (1); interstriae 7° convex (0 rib-like) (2); all uneven intervals (except suture) convex, even interstriae flat (2)
- Elytra, presence of supernumerary or interrupted striae: elytra with 10 continuous striae (0); with striae 3-4 and 5-6 interrupted in anterior third (1); with supernumerary striae (2).
- Elytra, size of punctures of striae: punctures small (0); medium sized (1); large (2).
- Elytra, curvature in lateral view: not humped (0); humped (1).
- Elytra, oblique lateral impressions on each side of the beginning o declivity: absent (0); present (1).
- Metathoracic wings, development: wings well-developed (0); wings reduced to absent (1).
- Legs, presence of mucro at apex of all tibiae: mucro present only on front tibiae (0); present on front and middle tibiae (1); present on three pairs of tibiae (2).
- Legs, row of denticles on inner margin of tibiae (female): denticles minute to indistinct, only in front tibiae (0); denticles medium to large in front tibiae, minute in middle tibiae (1); large in front and middle tibiae (2); large in the three pairs of tibiae (3).
- Legs, corbel at metatibial apex: corbel broad, setose (2 to 2.5x as long as wide) (0); broad, squamose (1).
- Legs, length of distal comb relative to dorsal comb of hind tibiae: distal comb longer than dorsal comb (0); distal comb about as long as to slightly shorter than dorsal comb (1); distal shorter than dorsal comb (2).
- Legs, length of tarsite 2 of hind tibiae relative width at apex: tarsite 2 wider than long (0); tarsite 2 longer than wide (1).
- Female terminalia, shape of sternite VIII: subrhomboidal with distinct apical half (0); subrhomboidal without distinct apical half (1); suboval (2); subcordiform (3).
- Female terminalia, length of apodeme of sternite VIII: apodeme about 2x as long as plate (0); apodeme about 3 x as long as plate (1); less than 2x as long as plate.
- Female terminalia, length of ovipositor relative to abdomen: ovipositor less than half of abdominal length (0); ovipositor about half length of abdomen (1); ovipositor about as long as abdomen (2).
- Female terminalia, shape of distal gonocoxites (= coxites): gonocoxites not projected (0); gonocoxites projected in a short nail-shaped piece (1); gonocoxites strongly projected in a long nail- shaped piece (2).
- Female terminalia, membrane between gonocoxites: not expanded, gonocoxites close to each other (0); expanded, gonocoxites strongly separated (1).
- Female terminalia, styli visible or not from ventral side: styli not concealed by distal gonocoxites (0); styli partially or completely concealed by distal gonocoxites (1).
- Spermathecal duct, length: medium length (2.5-6 x as long as spermatheca, not curled (0); long (more than 10x as long as spermatheca) and curled (1); very short (less than 2x as long as spermatheca) (2).
- Spermathecal duct, sclerotization: duct membranous (0); duct sclerotized (1).
- Spermatheca, shape of corpus: corpus broad (0); corpus slender (about as wide as base of cornu) (1).
- Spermatheca, length of nodulus (= collum): nodulus very short (0); nodulus medium length (it does not exceed level of ramus) (1); nodulus long (it exceeds level of ramus) (2); nodulus very long (it exceeds length of spermatheca) (3).
- Spermatheca, orientation of nodulus: nodulus not recurved (0); nodulus completely recurved (1).
- Aedeagus, length of apodemes of penis (= temones): apodemes about as long as median lobe (0); apodemes shorter than median lobe (1); apodemes longer than median lobe (2).
- Aedeagus, shape of apex of median lobe: acute (0); truncate to rounded (1); arrow- shaped (2).
- Aedeagus, apex of median lobe: symmetrical (0); asymmetrical (1).
- Aedeagus, fine cuticular striation at apex of median lobe: lacking cuticular striation around apex (0); with cuticular striation around apex (1).
- Aedeagus, sclerites of the internal sac: sclerites absent or not Cyrtomon type (0); sclerites present as a pair of lateral struts on each side of a pyriform piece connected with ejaculatory duct (= Cyrtomon type) (1).
Table II. Data matrix of 48 morphological characters and 18 taxa (16 of the ingroup and two of the outgroup).
Table III. List of synapomorphies and autopomorphies of the cladistic analysis. Number of nodes corresponds to those given in Fig. 1 (cladogram).














