Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista de la Sociedad Entomológica Argentina
versión impresa ISSN 0373-5680versión On-line ISSN 1851-7471
Rev. Soc. Entomol. Argent. vol.76 no.3-4 La Plata dic. 2017
Trabajo científico - Article
A preliminary study of aquatic Coleoptera in temporary ponds and the ecological variables influencing their richness and diversity
Estudio preliminar de coleópteros acuáticos en estanques temporales y variables ecológicas que influyen en su riqueza y diversidad
GÓMEZ LUTZ, María C. & KEHR, Arturo I.
Centro de Ecología Aplicada del Litoral (CECOAL (CONICET-UNNE)), Ruta 5, km 2.5 (3400) Corrientes, Argentina. Email: macogolu@gmail.com
Received 29 - IV - 2017 | Accepted 19 - IX - 2017 | Published 27 - XII - 2017 https://doi.org/10.25085/rsea.763402
ABSTRACT. Water beetles possess a wide variety of ecological attributes that allow them to occupy a wide range of aquatic habitats. Temporary ponds are environments that experience recurrent drought periods that may differ in duration; each pond is further characterized by a particular fauna, and by the size of the population that can be supported. The richness (S) and diversity (H') of water beetles collected in temporary ponds in Corrientes province were analyzed. Rarefaction was used to avoid estimation bias and to use comparative methodology. Pearson correlations were calculated using water temperature, depth, surface (m²), diversity (Shannon & Wiener) and richness. A total of 660 individuals, including 42 species, 22 genera and 7 families were recorded. The greatest percentage of species belonged to the family Hydrophilidae (45%) followed by Dytiscidae (32%) and Noteridae (15%), while the remaining families represent less than 10% of the total species. The Kruskal-Wallis tests did not find significant differences in diversity and richness between the temporary ponds analyzed. The most correlated variables in different ponds were diversity and hydroperiod, surface and depth.
KEYWORDS. Aquatic beetles. Community structure. Hydroperiod. Temporary pond.
RESUMEN. Los escarabajos de agua poseen una gran variedad de atributos ecológicos que les permiten ocupar una amplia gama de hábitats acuáticos. Los charcos temporarios son ambientes que experimentan periodos de sequía recurrentes y que pueden diferir en su duración; cada charco se caracteriza por la fauna particular que lo habita y por el tamaño de la población que puede soportar. Se analizaron la riqueza (S) y la diversidad (H') de los escarabajos acuáticos recolectados en charcos temporarios en la provincia de Corrientes. La rarefacción se utilizó para evitar el sesgo en la estimación y utilizar una metodología comparativa. Se calculó la correlacion de Pearson utilizando la temperatura del agua, la profundidad, la superficie (m²), la diversidad (Shannon & Wiener) y la riqueza. Se registraron un total de 660 individuos, incluyendo 42 especies, 22 géneros y 7 familias. El mayor porcentaje de especies perteneció a la familia Hydrophilidae (45%), seguido por Dytiscidae (32%) y Noteridae (15%), mientras que las restantes familias representaron menos del 10% del total de las especies. Las pruebas de Kruskal-Wallis no registraron diferencias significativas en la diversidad y riqueza entre los diferentes charcos temporarios analizados.Las variables más correlacionadas en los diferentes charcos fueron la diversidad con el hidroperiodo, la superficie y la profundidad.
PALABRAS CLAVE. Charcos temporarios. Coleópteros acuáticos. Estructura de la comunidad. Hidroperíodo.
INTRODUCTION
MATERIAL AND METHODS
Temporary environments are spread throughout the world and are characterized by diverse physical and chemical properties, regardless of their type and origin (Williams, 1996). Rain pools are small temporary ponds of variable duration formed in depressions where the rain is accumulated (Williams, 2006). Temporary ponds experience recurrent drought periods that may differ in duration, and are further characterized by the particular fauna that inhabits them and by the size of the populations that they can sustain (Williams, 1997). In these environments water permanence and water temperature are the most important abiotic factors affecting fauna (Eyre et al., 1992; Nilsson & Svensson, 1994; Eyre, 2006). Temporary environments impose strict conditions on the fauna that inhabits them and require the development of different morphological, physiological, and behavioral characteristics and adaptations to survive periods of drought, migration and changes in the life history (Wiggins et al., 1980; Wellborn et al., 1996; Williams, 1996). According to Nilsson & Svensson (1994) the adaptation of the fauna to this particular type of environment is conditioned by the ability of these organisms to complete their development cycles before the pool dries up.
In these environments, dispersion and colonization strategies are important for the succession process and life cycles of most aquatic insects (Sheldon, 1984); these processes infuence the population dynamics, abundance and structure of the communities (Dieckmann et al., 1999; Bilton et al., 2001; Colbert et al., 2001). Most aquatic insects are able to fy and disperse to other sites in order to reproduce and feed, both as part of their life cycle and as an adaptation for survival (Johnson, 1969).
Water beetles are an important part of the aquatic invertebrate community (Fernández & López Ruf, 1999); they present a variety of ecological attributes that allow them to occupy a wide range of habitats within the wetlands (Fairchild et al., 2003). In temporary ponds, physiological and behavioral mechanisms to survive dry periods have been documented in aquatic coleopterans (Fernando, 1958; Fernando & Galbraith, 1973; Wiggins et al., 1980; Wellborn et al., 1996, Williams, 2006).
The aims of this study were to analyze some attributes of aquatic Coleoptera communities in temporary environments: describing the abundance and diversity of aquatic Coleoptera in temporary ponds and their changes over time, in relation to the modifications in the different temporary environments.
Study sites
The study was conducted in northeastern Argentina, in Corrientes province, which has a network of wetlands composed of marshes, dams and lagoons; occupying 16.000 km2 of the total area. This area of wetlands corresponds to the Central East Region. Water bodies are partially or completely covered by different species of aquatic plants (Bonetto et al., 1978). Some of the most common areEgeria najasPlanch.,Cabomba caroliniana Gray, Salvinia biloba Raddi, Limnobium laevigatum (Humb. and Bonpl. ex Willd.) Heine, Pistia stratiotesL., Eichhornia azurea (Sw.) Kunth, Hydrocotyle ranunculoidesL. f. and Nymphoides indica(L.) Kuntze, (Poi & Galassi, 2013). The climate is humid subtropical without a dry season (Escobar et al., 1990; Bruniard, 1999). Rainfall is abundant, frequent and irregular; occurring mainly during the spring-summer and decreasing from east to west (between 1.700 - 1.900 m m peryear and between 1.300 - 1.500 mm peryear, respectively) (Acosta et al., 2009).
All temporary ponds included in this study were formed by rainfall in depressed areas of land and are characterised by drying out regularly. Samples were collected in those temporary ponds (Fig. 1). The shape, size, and periodicity in water level varied in each pond, although they are generally shallow (less than 40 cm) and of short duration.

Fig. 1. Pictures of some of the temporary ponds sampled in Corrientes Province. Pond: a) No. 5; b) No. 7; c) No. 8; d) No. 2.
In total, nine temporary ponds were sampled. They were numbered 1 to 9 in the order in which samples were taken. The dynamics, duration, and estimated variables for each pond are detailed in Table I.
Table I. Temporary pond sampled in Corrientes province.
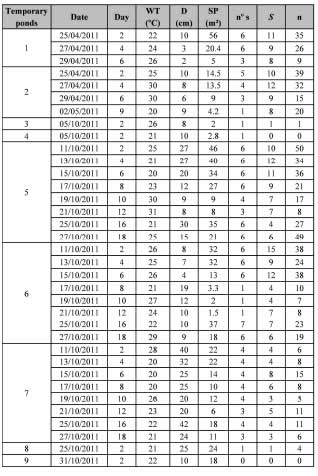
Date: date of sample; Day: day after rain; WT: water temperature; D: depth; SP: surface of the pond; nº s: number of samples taken; S: species richness and n: number of individuals.
Experimental design
Samplings were performed at a frequency of 48 hours (beginning 24 hours after the frst rain) due to their limited duration. Samples were collected during April, May and October 2010 (where accumulation of water was registered), which correspond to the fall and spring seasons. Beetles were captured with an aquatic hand net (mesh size 300 μm, diameter 30 cm). The number of samples in each pond varied according to the hydroperiod dynamics of each of the aquatic environments. The extraction of the samples was carried out in a cup perpendicular to the main axis of the temporary ponds, i.e.occupying the entire width of the same. To ensure sample independence, the distance between sampling units was 3 m. Samples were usually collected between 10 AM and 1 PM and were fxed in situ in 5% formaldehyde. Subsequently, samples were transferred to the laboratory for identification, and then stored in 70% ethanol. Puddles were sampled for a period of up to 20 days in cases where rainfall persisted.
The systematics follows Lawrence & Newton (1995); Hansen (1999) for Hydrophiloidea classification, Short & Fikáček (2011, 2013) for Hydrophilidae, and Nilsson (2001) and subsequent updates for Dytiscidae. Taxonomic identifications were done to the lowest possible taxonomic level, using available keys and literature (Young, 1974, 1990; Trémouilles & Bachmann, 1980; Trémouilles, 1984, 1989, 1995, 1996; Trémouilles et al., 1995, 2005; Miller, 2000, 2005; Oliva et al., 2002; Vondel & Spangler, 2008; Archangelsky et al., 2009; Libonatti et al., 2011). The studied material was deposited in the collection of the Centro de Ecología Aplicada del Litoral (CECOAL, CONICET-UNNE), Corrientes. Only adults were considered in the analyses.
Methods used to assess the diversity and species richness
Only temporary environments from which a minimum of 3 samples could be taken were considered in calculations (i.e. puddles that remained at least fve days after the frst rain). The richness (S) and diversity (H') of the species in each community studied were analyzed with EcoSim Software (Gotelli & Enstminger, 2011). Diversity was estimated using Shannon Diversity Index (Shannon & Weaver, 1949).
The richness and diversity of each temporary pond was compared using the rarefaction method (1,000 permutations obtained from real data, with the average and standard deviation for diversity and species richness in each community) (Simberloff, 1972), as implemented in EcoSim software. This method allowed us to estimate, by calculating probabilities, the diversity and richness of two or more communities with different sample sizes. The data were unifed by the number of individuals: 10, 20, 30, 40, 50 and 60 by sample. Using the data obtained in this analysis, comparisons between the richness and diversity of the different temporary environments were carried out by means of Kruskal-Wallis tests with XLSTAT 2009 software (Hamann et al., 2006; Duré et al., 2008).
Relationship between the different variables
Pearson correlations were calculated with EcoSim software in different temporary ponds. The correlated variables were: water temperature, depth, size (m2), diversity and species richness.
RESULTS
Diversity and species richness
More than 10 temporary ponds have been identifed for being sampled after the rainfall, however 48 hours after their formation they disappeared, perhaps the water was absorbed due to the characteristics of the soil. In different temporary environments sampled, hydroperiod varied from two days (pond No. 3) to more than 18 days (ponds No. 5, 6, and 7). Water temperature ranged from 20 °C (ponds No. 2, 5, and 7) to 31 °C (pond No. 5), with an average of 24 °C. The depth of the ponds ranged from 2 cm (pond No. 1) to 42 cm (pond No. 7), and an average of 16 cm.
A total of 660 individuals of aquatic Coleoptera, including 42 species, 22 genera and 7 families were recorded. Water beetles were the only species found in 7 of the 9 sampled ponds. The taxonomic list and the presence of each species by pond can be found in Table II.
Table II. List of aquatic Coleoptera species found in temporary ponds of Corrientes province, Argentina.
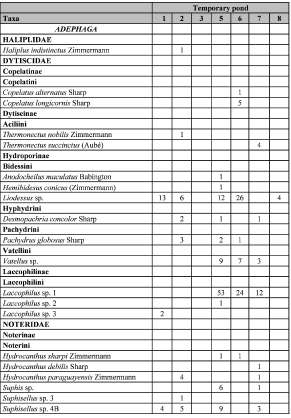
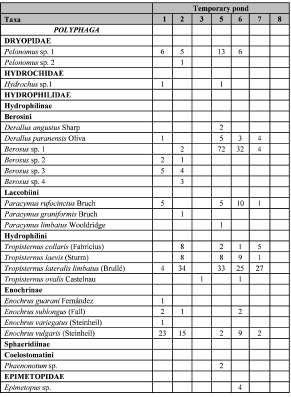
The greatest percentage of species belonged to the family Hydrophilidae (45%), followed by Dytiscidae (32%) and Noteridae (15%), while the remaining families represent less than 10% of the diversity. The most frequent and abundant species in different ponds were Liodessus sp., Laccophilus sp. 1, Berosus sp. 1 and T ropisternus lateralis. Other species were recorded in a single sample site, as was the case of Haliplus indistinctus, Copelatus alternatus, C. longicornis, Thermonectus nobilis, T. succinctus, Anodocheilus maculatus, Hemibidesus conicus, Laccophilus sp. 2, Laccophilus sp. 3, Pelonomus sp. 2, Derallus angustus, Paracymus graniformis, P. limbatus, Enochrus guarani, Phaenonotum sp. and Epimetopus sp.
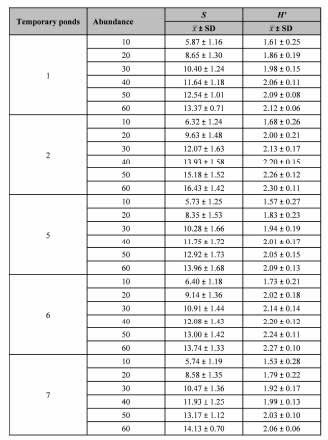
Table III shows the results of the rarefaction method used to analyze diversity and richness of species. No significant differences were found between ponds with regard to these two variables (diversity: Kruskal-Wallis Tests KW = 7.64, df 4; p > 0.05; richness: Kruskal-Wallis Test KW= 1.78, df 4; p > 0.05).
Table III. Summary of main results of the method of rarefaction. The mean and the standard deviation ( ± SD) of the diversity (H') and species richness (S) for different temporary ponds were estimated.
Relationship between variables
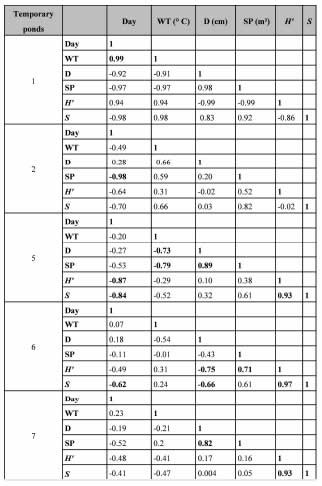
Analyzes were performed in fve ponds. Results of all of the correlations are available in Table IV. In the puddles that lasted less than 10 days (for example No. 1 and No. 2) few significant correlations were recorded. However, in other ponds (example No. 5 and No. 7) a greater number of significant correlations was recorded. Species richness and diversity were highly correlated to depth and hydroperiod (Table IV).
Table IV. Pearson correlation of temporary ponds No. 1, 2, 5, 6 and 7. Day: day after rain; WT: water temperature; D: depth; SP: surface of the pond; H': diversity and S: species richness. Bold values are different from 0 with a significance level alpha = 0.05
DISCUSSION
Some researchers found a greater species richness in permanent ponds in comparison to that registered in temporary ponds (Williams, 1996; Boix et al., 2001; Fontanarrosa et al., 2004). In this study, the richness of aquatic Coleoptera is lower than that reported for permanent ponds in Corrientes province (107 species; Gómez Lutz et al., 2012). However, the Coloptera taxa collected in temporary ponds were similar to those previously reported in permanent ponds (Gómez Lutz et al., 2012, 2015; Torres et al., 2012; Libonatti et al., 2013).
Of the 42 taxa recorded in this study, 18 (45%) were Hydrophilidae, dominated mainly by Derallus paranensis and Tropisternus lateralis limbatus in terms of abundance. This dominance of diversity and abundance of the Hydrophilidae among the Coleoptera is a common phenomenon in permanent and temporary ponds (Torres et al., 2007; Gómez Lutz et al., 2012; Macchia et al., 2015). Hydrophilidae and Dytiscidae were the most diverse water beetle families, together accounting 77% of the total species. Ribera et al. (2003) considered both families typical of temporary environments. These two families have also been reported in other studies as the best represented Coleoptera both in Argentina (Fischer et al., 2000; Fontanarrosa et al., 2004, 2009) and other regions of the world (Williams, 1983; Lake et al., 1989; Bazzanti et al., 1996; Boix et al., 2001; Boix & Sala, 2002; Pérez-Bilbao et al., 2015). According to Ribera & Vogler (2000) the presence of Hydrophilidae and Dytiscidae in temporary ponds is due to their exceptional capacity to disperse.
The total number of Coloptera taxa reported here is high when compared with earlier studies by Fischer et al. (2000) and Fontanarrosa et al. (2004), which reported 18 (including 13 genera and 3 families) and 36 taxa (26 genera and 7 families), respectively, from different environments of Buenos Aires city. In a different study of rainwater puddles in the same city, Fontanarrosa et al. (2009) recognized 36 morphospecies of water beetles belonging to seven families.
Of all the taxa collected, only four (Liodessus sp., Laccophilus sp. 1, Berosus sp. 1 and T. lateralis) were the most abundant and frequent in all the temporary ponds, perhaps being the main opportunistic species in this area. Helped by their biological capacity, these species might use the temporary ponds as transitory places in order to disperse to more stable environments. These four species have been previously registered in permanent environments of Corrientes city (Gómez Lutz et al., 2012) and temporary ponds of Buenos Aires city (Fischer et al., 2000; Fontanarrosa et al., 2004).On the other hand, most of the species in this study were recorded either in low abundance or at only one of the sites. Some of them (C. alternatus, C. longicornis, T. nobilis, T. succinctus, A. maculatus, H. conicus, Laccophilussp. 2, Laccophilussp. 3, Pelonomussp. 2, D. angustusand E. guarani) were previously registered for different environments in the province of Corrientes (Torrres et al., 2012; Gómez Lutz et al., 2012, 2015). They could be interpreted as opportunistic species, however less common than Liodessus sp., Laccophilus sp. 1, Berosus sp. 1 and T. lateralis.
In addition, two of the taxa collected in this work have not been previously recorded from Corrientes Province. Phaenonotum belongs to the subfamily Sphaeridiinae (Hydrophilidae). In Argentina, three species have been cited: P. argentinense Bruch (Tucumán, Buenos Aires), P. meriones (Orchymont) (Argentina: Chaco; Brasil: Mato Grosso), and P. regimbarti Bruch (Formosa, Chaco, Buenos Aires) (Oliva et al., 2002). Acording to Archangelsky et al. (2009) Phaenonotum needs a taxonomic revision. The specimens collected in our samples correspond to an unidentifed species and this is the frst mention of the genus in Corrientes (Table II). The Epimetopidae include 18 species in the genus Epimetopus, fve of which occur in Argentina (Oliva et al., 2002). In this study we collected one species that could not be identifed. This is the frst record of the Family and genus from Corrientes Province.
Pioneer species have adaptations that permit their appearance soon after inundation; the differences in the frequency of occurrence of some taxa suggest that these groups might be using the temporary ponds in a diferent way, in relation to their bionomic requirements, or the ability to inhabit all types of aquatic environments. For example, some species of Berosus remain embedded (both larvae and adults) in the sediment, which allows them to finish the metamorphosis (Thiéry, 1979; Barbero et al., 1982).
In this study temporary ponds were sampled during fall and spring, which were the periods of greatest rain fall in the study area and resulted in the formation of the ponds. No significant differences were found between the temporary ponds across both seasons. It is likely that the abundance and richness of aquatic Coleoptera in these seasons are associated with heavy precipitation that favored the formation of temporary ponds and individuals dispersal fights. In a study of structural patterns in a community of aquatic insects in Argentina, Fontanarrosa et al. (2009) documented peaks of richness and diversity during spring and fall, which they attributed to prolonged fooding and weather conditions. Additionally, Fernando (1958) and Southwood (1962) attributed these peaks of richness and diversity to fight dispersion, mainly in these seasons. Lundkvist et al. (2002) showed two peaks of abundance in studies of Dytiscidae dispersion over different months, one in spring / summer (attributed to reproductive dispersion) and another in autumn (opportunistic dispersion).
Schneider & Frost (1996), Wellborn et al. (1996), Spencer et al. (1999) and Boix et al. (2001) postulated the presence of various factors that infuence the structure of the communities living in temporary environments, emphasizing the importance of hydroperiods. An association between species richness and water permanence has already been reported in the literature (Stout, 1964; Driver, 1977; Ebert & Balko, 1987; Nilsson & Svensson, 1994; Bazzanti et al., 1996;
Schneider & Frost, 1996; Schneider, 1999; Spencer et al., 1999; Fontanarrosa et al., 2009), this association was also found in several temporary ponds during this study. At longer hydroperiods, more species will be able to complete their development and maintain viable populations. According to Spencer et al. (1999) the longer permanence of ponds also implies a longer time available for colonization. In this contribution, the temporary ponds that remained for a maximum of 10 days showed very few significant correlations between the analyzed variables, perhaps due to the rapid drying.
This is the frst of a series of contributions which intend to study and evaluate the dynamics of macroinvertebrates in ephemeral environments. Based on our results, we conclude that the beetle communities inhabiting temporary ponds in Corrientes Province are diverse, and include several species, such as Liodessus sp., T. lateralis and Enochrus vulgaris, that frequently inhabit these environments due to their biological adaptations. Also, less frequent and abundant species, such as Phaenonotum sp. and Epimetopus sp., were registered for the frst time in Corrientes.
Finally, the hydroperiod, surface, and depth are the factors that mainly determine the composition of these environments. We are aware that there are numerous open questions and unresolved issues that need to be addressed in future investigations. However, these data contribute to the knowledge about aquatic beetles, as well as the ecology of the species (diversity, abundance, pioneer species, etc) that inhabit these ephemeral environments, which is currently very limited.
ACKNOWLEDGEMENTS
Dra. Liliana Fernández is acknowledged for comments about the manuscript and collaboration in Coleoptera identification
LITERATURE CITED
Acosta, F ., Giménez, L., Richieri, C., & Calvi, M. (2009) Zonas Agroeconómicas Homogéneas de Corrientes. Descripción Ambiental, Socioeconómica y Productiva. Ediciones INTA. Estudios Socioeconómicos de la Sustentabilidad de los Sistemas de Producción y Recursos Naturales,8. [ Links ]
Archangelsky, M., Manzo, V., Michat, M., & Torres, P.L.M. (2009) Coleoptera. Macroinvertebrados bentónicos sudamericanos. Sistemáticaybiología.(ed. Domínguez, E. & Fernández, H.R.), pp. 411-468. Fundación Miguel Lillo, Tucumán, Argentina. [ Links ]
Barbero, M., Giudicelli, J., Loisel, R., Quezel, P., & Terzian, E. (1982) Etude des biocénoses des mares et des ruisseaux temporaires à Ephémérophytes dominants en région méditerranéenne. Bulletin d'Ecologie, 13, 387-400. [ Links ]
Bazzanti, M., Baldoni, S., & Seminara, M. (1996) Invertebrate macrofauna of a temporary pond in central Italy: composition, community parameters and temporal succession. Archivfür Hydrobiologie, 137(1), 77-94. [ Links ]
Bilton, D.T., Freeland, J.R., & Okamura, B. (2001) Dispersal in freshwater invertebrates. Annual Review of Ecology and Systematics, 32, 59-181. [ Links ]
Boix, D., & Sala, J. (2002) Riqueza y rareza de los insectos acuáticos de la laguna temporal de Espolla (Pla de lEstany, Cataluña). Boletín de la Asociación española de Entomología, 26, 45-57. [ Links ]
Boix, D., Sala, J., & Moreno-Amich, R. (2001) The faunal composition of Espolla pond (NE Iberian Peninsula): the neglected biodiversity of temporary waters. Wetlands, 21(4), 577-592.
Bonetto, A.A., Corrales, M.A., Varela, M.E., Rivero, M.M., Bonetto, C.A., Vallejos, R.A., & Zalokar, J. (1978) Estudios limnológicos en la cuenca del Riachuelo II. Lagunas Totoras y González. Ecosur, 5(9), 17-55.
Bruniard, E. (1999) Los Regímenes hídricos de las Formaciones Vegetales. Aporte para un Modelo Fitoclimático Mundial. Ed. EUDENE. Resistencia, Argentina.
Colbert, J., Danchin, E., Dhondt, A.A., & Nichols, D.J. (2001) Dispersal. Oxford University Press, Oxford.
Dieckmann, U., Ohara, B., & Weisser, W. (1999) The evolutionary ecology of dispersal. Trends in Ecology and Evolution, 14(3), 88-90.
Driver, E. A. (1977) Chironomid communities in small prairie ponds: some characteristics and controls. Freshwater Biology, 7, 121-133.
Duré, M.I., Kehr, A.I., Schaefer, E.F., & Marangoni, F. (2008) Diversity of amphibians in rice felds from northeastern Argentina. Interciencia, 33, 523-527.
Ebert, T.A., & Balko, M.L. (1987) Temporary pools as islands in space and in time: The biota of vernal pools in San Diego, Southern California, USA. Archivfür Hydrobiologie, 110(1), 101-123.
Escobar, E.H., Ligier, H.D., & Matteio, H.R. (1990) Suelos de la Provincia de Corrientes. Atlas de suelos de la República Argentina,tomo 1, pp. 517-590. SAGyP - INTA-CIRN
Eyre, M.D. (2006) A strategic interpretation of beetle (Coleoptera) assemblages, biotopes, habitats and distribution, and the conservation implications. Journal of Insect Conservation, 10, 151-160.
Eyre, M.D., Carr, R., McBlane, R.P., & Foster, G.N. (1992) The effects of varying site-water duration on the distribution of water beetle assemblages, adults and larvae (Coleoptera: Haliplidae, Dytiscidae, Hydrophilidae). Archivfür Hydrobiologie, 124, 281-291.
Fairchild, G.W., Cruz, J., & Faulds, A.M. (2003) Microhabitat and landscape infuences on aquatic beetle assemblages in a cluster of temporary and permanent ponds. Journal of the North American Benthological Society, 22, 224-240.
Fernández, L.A., & López Ruf, M.L. (1999) Coleoptera y Heteroptera acuáticos y semiacuáticos de la isla Martín García (Provincia de Buenos Aires). Physis (B), 57(132-133), 1-4.
Fernando, C.H. (1958) The colonization of small freshwater habitats by aquatic insects. 1. General discussion, methods and colonization in the aquatic Coleoptera. The Ceylon Journal Science, 1, 117-154.
Fernando, C.H., & Galbraith, D. (1973) Seasonallity and dynamics of aquatic insects colonizing small habitats. Verhandlungen des Internationalen Verein Limnologie, 18, 1564-1575.
Fischer, S., Marinone, M.C., Fontanarrosa, M.S., Nieves, M., & Schweigmann, N. (2000) Urban rain pools: seasonal dynamics and entomofauna in a park of Buenos Aires. Hydrobiologia, 441, 45-53.
Fontanarrosa, M.S., Torres, P.L.M., & Michat, M.C. (2004) Comunidades de insectos acuáticos de charcos temporarios y lagunas en la ciudad de Buenos Aires (Argentina). Revista de la Sociedad Entomológica Argentina, 63, 55-65.
Fontanarrosa, M.S., Collantes, M.B., & Bachmann, A.O. (2009) Seasonal patterns of the insect community structure in urban rain pools of temperate Argentina. Journal of Insect Science, 9(10), 1-18.
Gómez Lutz, M.C., Fernández, L.A., & Kehr, A.I. (2012) Coleópteros acuáticos de lagunas situadas en el noroeste de la provincia de Corrientes, Argentina. Revista de la Sociedad Entomológica Argentina, 71, 73-85.
Gómez Lutz, M.C., Kehr, A.I., & Fernández, L.A. (2015) Abundance, diversity and community characterization of aquatic Coleoptera in a rice feld of Northeastern Argentina. Revista de Biología Tropical, 63(3), 629-638.
Gotelli, N., & Enstminger, G. (2011) EcoSim: null models software for ecology, version 7.72, Acquired Intelligence Inc. and Kesey-Bear, http://homepages.together.net/wgentsmin/ ecosim.htm
Hamann, M.I., Kehr, A.I., & González, C.E. (2006) Species affinity and infracommunity ordination of helminths of Leptodactylus chaquensis (Anura: Leptodactylidae) in two contrasting environments from Northeastern Argentina. Journal of Parasitology, 92, 1171-1179.
Hansen, M. (1999) Hydrophiloidea (s. str.) (Coleoptera). World Catalogue of Insects, vol. 2. Apollo Books, Stenstrup, Denmark.
Johnson, C.G. (1969) Migration and Dispersal of Insects by Flight. Methuen, London.
Lake, P.S., Bayly, I.A.E., & Moton, D.W. (1989) The phenology of a temporary pond in western Victoria, Australia, with special reference to invertebrate succession. Archivfür Hydrobiologie, 115(2), 171-202.
Lawrence, J.F., & Newton, A.F. (1995) Families and subfamilies of Coleoptera (with selected genera, notes, references and data on family-group names). Biology, Phylogeny, and classification of Coleoptera: Papers celebrating the 80th Birthday of Roy A. Crowson. (ed. Pakaluk, J., & Slipinski, S.A.) pp. 779-1092. Muzeumi Instytut Zoologii PAN, Warszawa
Libonatti, M.L., Michat, M.C., & Torres, P.L.M. (2011) Key to the subfamilies, tribes and genera of adult Dytiscidae of Argentina (Coleoptera: Adephaga). Revista de la Sociedad Entomológica Argentina, 70(3-4), 317-336.
Libonatti, M.L., Michat, M.C., & Torres, P.L.M. (2013) Aquatic Coleoptera from two protected areas of the Humid Chaco eco-region (Chaco Province, Argentina). Revista de la Sociedad Entomológica Argentina, 72(3-4), 155-168.
Lundkvist, E., Landin, J., & Karlsson, F. (2002) Dispersing diving beetles (Dytiscidae) in agricultural and urban landscapes in south-eastern Sweden. Annales Zoologici Fennici, 39, 109-123.
Macchia, G.A., Libonatti, M.L., Michat, M.C., & Torres, P.L.M. (2015) Aquatic Coleoptera from El Cristal Natural Reserve (Santa Fe Province, Argentina). Revista de la Sociedad Entomológica Argentina, 74(3-4), 111-116.
Miller, K.B. (2000) Revision of the Neotropical genus Hemibidessus Zimmermann (Coleoptera: Dytiscidae: Hydroporinae: Bidessini). Aquatic Insects, 23(4), 253-275.
Miller, K.B. (2005) Revision of the New World and south-east Asian Vatellini (Coleoptera: Dytiscidae: Hydroporinae) and phylogenetic analysis of the tribe. Zoological Journal of the Linnean Society, 144, 415-510.
Nilsson, A.N. (2001) Word catalogue of insects. Vol. 3. Apollo Books, Stenstrup, Denmark.
Nilsson, A.N., & Svensson, B.W. (1994) Dytiscidae predators and culicid prey in two boreal snowmelt pools differing in temperature and duration. Annales Zoologici Fennici, 31, 365-376.
Oliva, A.L., Fernández, L.A., & Bachmann, A.O. (2002) Sinopsis de los Hydrophiloidea acuáticos de la Argentina (Insecta, Coleoptera). Monografas del Museo Argentino de Ciencias Naturales, 2, 1-67.
Pérez-Bilbao, A., Benetti, C.J., & Garrido, J. (2015) Assessment of the effects of the dry period on the faunal composition of aquatic macroinvertebrate assemblages in two temporary ponds in NW Spain. Journal of Limnology,74(3), 467-476.
Poi, A., & Galassi, E. (2013) Sistemas 3b. Humedales de la planicie aluvial del río Paraná entre Confuencia y Reconquista. Inventario de los Humedales de Argentina: Sistemas de paisajes de humedales del corredor fuvial Paraná–Paraguay. (ed. Benzaquén et al.), pp. 161-168. Primera edición. Buenos Aires.
Ribera, I., & Vogler, A.P. (2000) Habitat type as a determinant of species range sizes: the example of lotic-lentic differences in aquatic Coleoptera. Biological Journal of the Linnean Society, 71, 33-52.
Ribera, I., Foster, G.N., & Vogler, A.P. (2003) Does habitat use explain large scale species richness patterns of aquatic beetles in Europe? Ecography, 26, 145-152.
Schneider, D.W. (1999) Snowmelt ponds in Wisconsin: Infuence of hydroperiod on invertebrate Community structure. Invertebrates in Freshwater Wetlands of North America: Ecology and Management(ed. Batzer, D.P., Rader, R.R., & Wissinger, S.A.), pp. 299-318. John Wiley and Sons, New York.
Schneider, D.W., & Frost, T.M. (1996) Habitat duration and community structure in temporary ponds. Journal of the North American Benthological Society, 15(1), 64-86.
Shannon, C.E., & Weaver, W. (1949)The mathematical theory of communication.University of Illinois Press. Urbana, IL.
Sheldon, A. (1984) Colonization dynamics of aquatic insects. The ecology of aquatic insects (ed. Reh, V.H., & Rosenberg, D.M.), pp. 401-429. Praeger, New York.
Short, A.E.Z., & Fikáček, M. (2011) World catalogue of the Hydrophiloidea (Coleoptera): additions and corrections II (2006–2010). Acta Entomologica Musei Nationalis Pragae, 51(1), 83-122.
Short, A.E.Z., & Fikáček, M. (2013) Molecular phylogeny, evolution and classification of the Hydrophilidae (Coleoptera). Systematic Entomology, 38(4), 723-752.
Simberloff, D.S. (1972) Properties of the rarefaction diversity measurement. The American Naturalist, 106, 414-418.
Southwood, T.R.E. (1962) Migration of terrestrial arthropods in relation to habitat. Biological Reviews, 37, 171-214.
Spencer, M., Blaustein, L., Schwartz, S.S., & Cohen, J.E. (1999) Species richness and the proportion of predatory animal species in temporary freshwater pools: relationships with habitat size and permanence. Ecology Letters, 2(3), 157-166.
Stout, R.R. (1964) Studies on the temporary ponds in Canterbury, New Zeland. Verhandlungen des Internationalen Verein Limnologie, 15, 209-214.
Thiéry, A. (1979) Infuence de lassèchement estival sur le peuplement dInsectes aquatiques dun marais saumâtre en Crau (Bouches-du- Rhône). Annales de Limnologie, 15, 181-191.
Torres, P.L.M., Mazzucconi, S.A., & Michat, M.C. (2007) Los coleópteros y heterópteros acuáticos del Parque Nacional El Palmar (Provincia de Entre Ríos, Argentina): lista faunística, diversidad y distribución. Revista de la Sociedad Entomológica Argentina, 66(3-4), 127-153.
Torres, P.L.M., Michat, M.C., Libonatti, M.L., Fernández, L.A., Oliva, A., & Bachmann, A.O. (2012) Aquatic Coleoptera from Mburucuyá National Park (Corrientes Province, Argentina). Revista de la Sociedad Entomológica Argentina, 71(1-2), 57-71.
Trémouilles, E.R. (1984) El género Rhantus Dejean en la Argentina (Coleoptera, Dytiscidae). Physis, 42, 9-24.
Trémouilles, E.R. (1989) Contribución para el conocimiento del género Thermonectus Dejean en la Argentina y áreas limítrofes. Revista de la Sociedad Entomológica Argentina, 46(1-4), 95-115.
Trémouilles, E.R. (1995) Dytiscidae: Methlinae - Hydroporinae. Fauna de Agua Dulce de la República Argentina (dir. Castellanos, Z.A.), pp. 1-82. FECIC, Buenos Aires.
Trémouilles, E.R. (1996) Revisión del género Hydaticus Leach en América del Sur, con descripción de tres nuevas especies (Coleoptera, Dytiscidae). Physis, 52(122-123), 15-32.
Trémouilles, E.R., & Bachmann, A.O. (1980) La tribu Cybisterini en la Argentina (Coleoptera, Dytiscidae). Revista de la Sociedad Entomológica Argentina, 39(1-2), 101-125.
Trémouilles, E.R., Oliva, A., & Bachmann, A.O. (1995) Insecta, Coleoptera. Ecosistemas de Aguas Continentales, metodologías para su estudio. (ed. Lopretto, E.C., & Tell, G.), tomo III, pp. 1133-1197. Ediciones Sur, La Plata.
Trémouilles, E.R., Michat, M.C., & Torres, P.L.M. (2005) A synopsis of the South American Hydrovatus (Coleoptera: Dytiscidae: Hydroporinae), with notes on habitat and distribution, and a key to species. Revista de la Sociedad Entomológica Argentina, 64(1-2), 61-69.
Vondel, B.J.V., & Spangler, P.J. (2008) Revision of the Haliplidae of the Neotropical Region including Mexico (Coleoptera: Haliplidae). Koleopterol Rundsch, 78, 69-194.
Wellborn, G.A., Skelly, D.K., & Werner, E.E. (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecological Systematics, 27, 337-363.
Wiggins, G.B., Mackay, R.J., & Smith, M.I. (1980) Evolutionary and ecological strategies of animals in annual temporary pools. Archivfür Hydrobiologie, 58(97), 206.
Williams, D.D. (1983) The Natural History of a Neartic Temporary Pond in Ontariowith Remarks on Continental Variation in such Habitáis. Internationale Revite der gesamten Hydrobiologie, 68, 239-253.
Williams, D.D. (1996) Environmental constraints in temporary fresh waters and their consequences for the insect fauna. Journal of the North American Benthological Society, 15, 634-650.
Williams, D.D. (1997) Temporary ponds and their invertebrate communities. Aquatic Conservation, 7, 105-117.
Williams, D.D. (2006) The biology of temporary waters. Oxford Univ. Press. New York.














