Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de la Sociedad Entomológica Argentina
Print version ISSN 0373-5680On-line version ISSN 1851-7471
Rev. Soc. Entomol. Argent. vol.77 no.1 La Plata Mar. 2018
Trabajo científico - Article
Does nutrition influence sexual dimorphism in Triatoma infestans (Hemiptera: Reduviidae) of natural habitats?
Influye la alimentación sobre el dimorfismo sexual en Triatoma infestans (Hemiptera, Reduviidae) de hábitats naturales?
HERNÁNDEZ, María L.1, AMELOTTI, Ivana1,2, CATALÁ, Silvia1, & GORLA, David E.3
1 CRILAR-CONICET, Centro Regional de Investigaciones Científicas y Transferencia Tecnológica de La Rioja, UNLAR, SEGEMAR, UNCa, CONICET (5301) Anillaco, La Rioja, Argentina. E-mail: mlhernandez@crilar-conicet.gob.ar
2 Universidad Nacional de La Rioja (UNLaR), La Rioja, Argentina.
3 Instituto de Altos Estudios Espaciales "Mario Gulich" CONAE (Comisión Nacional de Actividades Espaciales), Córdoba, Argentina.
Received 21 - IX - 2017 | Accepted 30 - XI - 2017 | Published 30 - III - 2018
https://doi.org/10.25085/rsea.770101
ABSTRACT. Triatoma infestans is the main vector of the parasite that causes the Chagas disease in South America. It is known that T. infestans has different reproductive and development patterns depending on whether they feed on birds or mammals. Using the head of adult insects as an estimator of the specimen development, we attempt to determine if there are any differences in the sexual size dimorphism associated with the availability of the food resource in T. infestansof natural habitats in the Llanos Riojanos region (Argentina). The nutritional status resulted higher in chicken coops and, in both habitats, it was higher for females in relation to males. The centroid size was larger in females than in males from chicken coops, but not in the specimens from goat corrals. Centroid sizes revealed smaller medians in goat insects in comparison to those coming from chicken coops. Sexual size dimorphism occurs associated with differences in the nutritional status only for triatomines from chicken coops in natural habitats. The heads shape was not infuenced by the nutritional status. The sexual morphophysiological differences found in T. infestanshelp us understand aspects of the behavior of the species in diverse environments and its implications in the vectorial transmission o f Trypanosoma cruzi.
KEYWORDS. Head morphometry. Nutritional status. Sexual dimorphism. Triatominae.
RESUMEN. Triatoma infestanses el principal vector del parásito que causa la enfermedad de Chagas en Sudamérica. Se sabe que T. infestanstiene diferentes patrones reproductivos y de desarrollo dependiendo de si se alimentan de aves o mamíferos. Utilizando la cabeza de insectos adultos como estimador del desarrollo, se intenta determinar si existen diferencias en el dimorfismo de tamaño sexual asociado con la disponibilidad del recurso alimenticio e n T. infestans de hábitats naturales en la región de Llanos Riojanos (Argentina). El estado nutricional fue mayor en gallineros y, en ambos hábitats, fue mayor para las hembras en relación con los machos. El tamaño centroide fue mayor en las hembras que en los machos de gallineros, pero no en los especímenes de corrales de cabras. Los tamaños centroide revelaron medianas más pequeñas en insectos de cabra en comparación con los procedentes de gallineros. El dimorfismo sexual del tamaño se asocia con diferencias en el estado nutricional solo para triatominos de gallineros de habitats naturales. La conformación de las cabezas no se vio infuenciada por el estado nutricional. Las diferencias morfofisiológicas encontradas en T. infestans ayudan a comprender aspectos del comportamiento de la especie en diversos ambientes y sus implicaciones en la transmisión vectorial d eTrypanosoma cruzi.
PALABRAS CLAVE. Dimorfismo sexual. Estado nutricional. Morfometría de cabezas. Triatominae.
INTRODUCTION
One of the most significant differences in the morphology of some animals is the body size of males and females. This difference is known as sexual size dimorphism and it is often affected by ecological, physiological, developmental, and environmental factors, among others (Fairbairn et al., 2007). The large amount of scientific information and literature related to the body size of animals refects the relevance of this feature in biology (LaBarbera,1986, 1989; Calder, 1996; Smith & Lyons, 2013). It is known that body size is strongly infuenced by both biotic and abiotic environmental factors (Gaston, 1991; Chown & Gaston, 2010, 2013; Price et al., 2011), although it is not always easy to understand the adaptive significance of dimorphism. Triatoma infestans is the main vector of the parasite that causes the Chagas disease in South America and its vectorial capacity is closely associated to the feeding behavior of this insect (Wisnivesky-Colli et al., 1982). Triatoma infestans is an almost exclusively domestic species, although it is also commonly found in peridomestic habitats such as chicken coops, goat or pig corrals, or storage rooms, which could act as a source of T. infestans that can disperse towards human dwellings after control interventions such as residual spraying (Gürtler et al., 1993; Cecere et al., 1997). Laboratory experiments revealed that T. infestans have different reproductive and development patterns depending on whether they feed on birds or mammals (Guarneri et al., 2000; Nattero et al., 2011). In natural conditions, this species maintains significant populations in chicken coops and goat corrals, particularly in the Arid and Semi-arid Chaco region, as it is associated with mammals of the Andean region (Noireau et al., 2005). In the Llanos Riojanos region (Argentina), successive collections showed that chicken coop insects reached higher nutritional status than those collected in goat corrals (Abrahan et al., 2011; Hernández, 2012), and, in turn, females were the largest blood consumers, promoting egg laying (Catalá, 1989, 1994; Catalá et al., 1992). The probability of achieving good nutritional status depends on the interaction with their host, since, being large insects, they require considerable blood intake. Nymphs gain between eight and nine times their own weight, while adults do so by two to four times (Schofeld, 1994). The irritability of the host during feeding is a crucial factor, since it hampers the procurement of resources. Successive interruptions
in feeding condition the nutritional status by a limitation in the accessibility to food (Rabinovich, 1985). The nutritional status is a factor that can affect reproduction, moulting, dispersion, vectorial capacity and may show differences between sexes. This sexual difference in nutritional status may occur due to the feeding priority of one sex, which guarantees population growth, thus making the most of the available resources. In some cases, the resource is shared equally among members of the population, and in other cases it is distributed unequally, giving rise to hierarchical or non-hierarchical competition mechanisms for the exploitation of a resource as in the case of food (Townsend et al., 2008). The head of the triatomines is an organ that does not undergo any modifications once the insect reaches its adult stage and its phenotype refects the ontogenetic development in a particular environment. In addition, since adult body size refects the growth of the insect in its nymphal stages, it is possible to use adult head sizes as estimators of the insect's development. The head of triatomines has proven to be a useful organ for solving identification problems of reinfestants (Dujardin et al., 1997; Borges et al., 2005; Hernández et al., 2013) for the differentiation of species (Gurgel-Gonçalves et al., 2011), and where variables associated with fight potential can be recognized (Hernández et al., 2015). The existence of a sexual dimorphism in Triatomines is a known fact, with females being, on average, larger than males (Lent & Wygodzinsky, 1979), although what happens in natural habitats and how it relates to their nutritional status has not yet been studied. We attempt to determine if there are any differences in the sexual size dimorphism associated to the availability of the food resource for T. infestans in natural habitats (chicken coops and goat corrals). The aim is to verify whether differences in the nutritional status correlate with a sexual head size dimorphism and analyze its implications regarding the utilization of food.
MATERIAL AND METHODS
Study area
The work was conducted in the Independencia Department, located West of Los Llanos (La Rioja, Argentina). This department is located at the South end of the Gran Chaco region (Arid Chaco). In the Llanos Riojanos region, the rearing of goats and chickens is common for the subsistence of small producers. This activity is associated with the construction of precarious corrals and chicken coops which become the main refuge for peridomestic populations of T. infestans (Cecere et al., 1997; Gürtler et al., 2004; Porcasi et al., 2006). The sampling was conducted in three localities of the said department: La Torre, La Aguadita, and Patquía Viejo. In this study, we chose chicken coops and goat corrals, which are the main peridomestic structures in the study area.
Insects
For morphometric analysis, 68 females (34 from goat corrals and 34 from chicken coops) and 82 males (32 from goat corrals and 50 from chicken coops) from all three localities were used. These habitats were actively searched by hand during 30 minutes, using tetramethrin 0.2% to dislodge the insects (Spacial 0.2, Ministerio de Salud de la Nación/ National Health Ministry). The samplings were conducted in January, April, and October 2007 and 2009, in each of the peridomestic structures. The weight and length of the insects were recorded in the laboratory immediately afiter the collection. The insects' heads were detached and kept in 70% ethanol until the time of the morphometric analysis.
Geometric morphometrics of the heads
The heads were separated at the collar and mounted on a pin attached to a metal support. Photographs were taken with a Kodak C613 (6.2 MP) digital camera, and using a stereo microscope (10X). Ten type II landmarks were selected on the ventral surface of the adult T. infestans heads (Fig. 1). The average of the landmarks on both sides of the head was used (fve landmarks), which allowed to reduce intraindividual variation and minimize digitization errors. For head size comparisons between groups, the isometric size estimator or centroid size (CS), derived from the coordinates, was used. This is defned as the square root of the sum of the squared distances between the centre of the landmark confguration and each individual landmark (Bookstein, 1991). The statistical significance of the CS differences was assessed using a non-parametric test based on permutations (5000 runs).

Fig. 1. Landmarks measured as coordinates of heads of Triatoma infestans. The number indicates the order of landmark capture
The shape variables were obtained through a generalized Procrustes analysis and the subsequent projection of the residuals into the Euclidean space (Rohlf, 1999).
Principal and partial warps were used as conformation variables. Principal warps describe the global variation such as elongation and compression, while partial warps correspond to the local variation (Zelditch et al., 2004). These two components describe the differences in the conformation, as well as the deviations from the average landmark confguration. For the landmark digitization and morphometric data analysis, the CLIC99 module developed by J.P. Dujardin was used (www.mpl.ird.fr/morphometrics). The statistical significance of the head size and conformation analysis was assessed using a non-parametric test based on permutations (5000 runs) included in the CLIC module. The equality of variances analysis was conducted using an F-Test with InfoStat software (Di Rienzo et al., 2016).
Nutritional status
The nutritional status of the adult T. infestans was estimated through the proportion between body weight (P) and total length (L), (P/L index), as suggested by Schofeld (1980). Each insect was weighed on an analytical balance accurate to 0.01 mg, and measured from the clypeus to the end of the abdomen using a caliper. The nutritional status was considered low for those insects with values of P/L<8 mg/mm, as suggested by Lehane and Schofeld (1982). The comparison of the P/L index in males and females from both ecotopes was accomplished using STATISTICA (StatSoft Inc, 2005).
Nutritional status by sex and habitat
The nutritional status proved to be significantly different between habitats, comparing each sex separately, with higher means in T. infestans from chicken coops than those from goat corrals (p<0.05). For each habitat, females showed higher nutritional status than males (p<0.05) (Fig. 2).
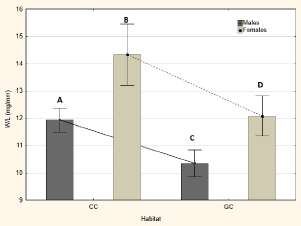
Fig. 2. Nutritional status by sex and habitat. The markers and the lines represent the mean and standard error. CC: Chicken Coops, GC: Goat Corral. W/L: Weight/Length in mg/ mm. Different letters indicate, statistically, significant differences between groups.
Comparison of head centroid size by sex and habitat.
The centroid size of the heads resulted significantly higher (p0.05) in females than in males for chicken coop T. infestans, but not in insects from the goat corrals.
The centroid sizes of the heads displayed smaller medians in goat corral insects, compared to those collected in chicken coops. As for variances, chicken coop males and females show similar values (p=0.72; F=1.11), while in goat corrals, variability in females resulted higher than that observed in males (p=0.03; F=1.85) (Table I, Fig. 3).
Table I. Median values and variances of the centroid size variable for the different groups. n: number of T. infestans in each group. Fem: Females, Mal: Males.
| Habitat | Sex | n | Medians | Absolute differences between CS | Variances |
| Goat corral | Fem | 34 | 353.45 | 6.72 | 176.07 |
| Mal | 32 | 346.73 | 95.18 | ||
| Chicken coop | Fem | 34 | 372.94 | 13.9 | 108.65 |
| Mal | 50 | 359.04 | 97.82 |

Fig. 3. Centroid size (CS) of heads of Triatoma infestans. The boxes show the median and the 25 and 75% quartiles. Different letters indicate, statistically, significant differences between groups. CC: Chicken Coops, GC: Goat Corral, Mal: Males, Fem: Females
Comparison of head shape between sexes and habitats
The analyzed groups showed sexual differentiation in their head shape (males were significantly different from females in insects from both chicken coops and goat corrals, p0.05, using the Bonferroni correction), but there were no differences between the two habitats for each sex.
The Mahalanobis distances were larger for habitat differences than for sexual differentiation (Table II). The allometric effect was 0% and 9% for the frst two canonical factors, evidencing that the conformation of sexual dimorphism is not a consequence of sexual size dimorphism. The first factor explains 71% of the total
Table II. Mahalanobis distances between groups extracted from discriminant multivariate analysis. The Mahalanobis distances were larger for habitat differences than for sexual differentiation Fem: Females, Mal: Males, CC: Chicken coops, GC: Goat corrals.
| FemGC | MalGC | FemCC | MalCC | |
| FemGC | ||||
| Mal GC | 1.89 | - | ||
| Fem CC | 2.63 | 3.94 | ||
| Mal CC | 1.83 | 2.2 | 2.15 |
DISCUSSION
variation, while the second factor explains the remaining 29%.
The metric disparity did not reveal, statistically, significant differences between males and females of both habitats (Table III). These results show that both sexes show no significant variability in the head shape. Head shape is therefore not infuenced by the nutritional status.
Table III. Metric disparity of male and female T. infestans collected in chicken coops and goat corrals. The different letters indicate the statistically significant differences (p0.05). Fem: Females, Mal: Males, CC: Chicken coops, GC: Goat corrals.
| Habitat/sex | Metric disparity |
| GC Fem | 0.000183 a |
| GC Mal | 0.000233 |
| CC Fem | 0.000231 |
| CC Mal | 0.000296 |
| Total | 0.000250 b |
Body size is probably the most important quantitative variable that can be recorded in animals since it has a strong infuence on ecological and adaptive aspects (Peters, 1983; Schmidt-Nielsen, 1984). Sexual dimorphism constitutes a manifestation of sexual selection and is one of the most conspicuous differences in the morphology of males and females, as described by Darwin (1871). Phenotypic responses depend on both the environmental features, particularly those that act during the development of the individual, and on the genetic properties of species (Daly, 1985; Williams, 2001). Our results show that sexual size dimorphism occurs associated with differences in the nutritional status only for triatomines from chicken coops in natural habitats. Results also show that T. infestans do not behave equally in both habitats, since sexual size dimorphism could only be observed in chicken coop insects. It is possible that in chicken coops, given a lower irritability of the host, females get the maximum amount of blood their body size allows. Although no specific studies were conducted on the digestive contents to assure the unique source of blood of the individuals of each habitat, the number of hosts in each of the analyzed habitats and the distance between them allow to consider that the primary food source came from the animals present in the habitat where the insects were collected. The lack of differentiation in the head shape of the two habitats could be interpreted as an exchange of triatomines between both habitats, although four years of studies in the Department showed a low rate of inter-habitat exchange (Hernández, 2012). existing data of populations in the same area. Chicken For this reason, the idea of the exchange of individuals coop insects achieve higher nutritional status than those between habitats cannot be ratifed based on the that feed on goat blood, and this fact infuences their size, as refected by the centroid sizes of their heads. Studies conducted in eight localities of the Llanos Riojanos region revealed the same results in terms of nutritional status (Hernández, 2012). These results are similar to those found for other species under laboratory conditions (Crocco & Catalá, 1997; Guarneri et al., 2000; Sant’Anna et al., 2010; Nattero et al., 2011).
The observed intrapopulation morphometric variations can be due to phenotypic variations in response to environmental features, particularly those that act during the development of the individual, as well as the genetic properties of the species (Daly, 1985; Williams, 2001). There are several hypotheses that could explain the morphometric differences between females and males in T. infestans. According to the fecundity hypothesis, the greater size of the female could be aimed at building up energy reserves prone to achieve high ovipositions (Higgins, 2000). Sexual selection factors could also be occurring since, usually, in insects, larger females present higher fecundity, as well as dominance in confrontations over resources, and male preference towards this type of females (Clutton-Brock, 2017). In laboratory-bred triatomines, a general downsizing of the insects has been observed over the generations (Szumlewicz, 1976; Zeledón, 1983). In T. infestans, the lack of sexual dimorphism is usually associated to a domiciliation process. It has been suggested that, as a result of incomplete feeding in domiciles, the periods between generations are longer, and this results in a smaller body size (Zeledón et al., 1970; Zeledón & Ravinovich, 1981). What could be happening in goat corrals is an incomplete feeding in both sexes, probably due to a lower availability of hosts. In our study area, the fock of goats is moved to grazing lands for several days and the moments with greatest host availability, and therefore available blood, occur during goatling season (twice a year). Under these conditions, neither sex competitively displaces the other. A non-hierarchical competition would take place, where each individual (regardless of its sex) perceives the limitation in the food supply in the same way. In addition, in the case of triatomines that feed on goats, the thickness of the goatskin may difficult the access to blood capillaries. Based on observations of chicken coop triatomines, there would be sexual differences in the assignment of the available blood. In chicken coops, where there seems to be no restriction for T. infestanst o access blood, each sex obtains as much as it can ingest according to its body size. In goat corrals, where there would be a restriction to access the food resource, both sexes obtain a similar amount of blood, without evident competitive displacement between them. Conversely, it is possible that the difference is not given solely by the amount of blood available in both habitats, but it may also be infuenced by the quality of the blood or the differential utilization of the blood of both hosts by T. infestans. In addition to the aforementioned, the possible irritability of the host cannot be ruled out, since it would make T. infestansachieve higher nutritional status when feeding on birds, which are less irritable than mammals.
The variability of the centroid size of the head in both habitats allows the discussion on intraspecific competition. It has been observed that the females found in goat corrals show more variation in the size of their heads in comparison to those of chicken coops. This could indicate competition within the female group when the resource is scarce. The size variability in females of goat corral would be explained by a differential access to food in less resourceful habitats. As a consequence of this competition among females, larger and smaller head centroid sizes are observed in this habitat.
It has been evidenced that in these two habitats there are different head conformations in both sexes, although this difference would not be a passive consequence of the difference in size.
Further studies might prove useful to understand what happens with these features in other geographic areas or even examine sexual size dimorphism of T. infestans considering other uncommon ecotopes for the vector. Morphophysiological sexual differences in T. infestans may help understand behavioral aspects of the species in different environments such as dispersive capacity, female fertility infuenced by size, population density, blood intake volume and its implication in the vectorial transmission of Trypanosoma cruzi.
ACKNOWLEDGEMENTS
This work was funded by CONICET and ANPCyT (PICT 2012-2883). We thank Natalia Folguera for the technical support and Luciana Abrahan for her feld and laboratory work.
BIBLIOGRAFÍA CITADA
- Abrahan, L.B., Gorla D.E., & Catalá S.S. (2011) Dispersal of T riatoma infestans and other Triatominae species in the arid Chaco of Argentina - Flying, walking or passive carriage? The importance of walking females. Memorias do Instituto Oswaldo Cruz, 106, 232-239. [ Links ]
- Bookstein, F.L. (1991) Thin-plate splines and the atlas problem for biomedical images. In: Biennial International Conference on Information Processing in Medical Imaging, Berlin. pp. 326-342. [ Links ]
- Borges, E.C., Dujardin, J.P., Schofeld, C.J., Romanha A.J., & Diotaiuti, L. (2005) Dynamics between sylvatic, peridomestic and domestic populations of Triatoma brasiliensis (Hemiptera: Reduviidae) in Ceará State, Northeastern Brazil. Acta Tropica, 93, 119-126. [ Links ]
- Calder, W.A. (1996) Size, function, and life history. Dover, New York. [ Links ]
- Catalá, S. (1989) Relaciones entre consumo de sangre y ovogénesis en Triatoma infestans Klug 1834 (Hemiptera, Reduviidae). Chagas, 5, 3-10. [ Links ]
- Catalá, S. (1994) Bloodmeal size and nutritional status of Triatoma infestansunder natural climatic conditions. Medical and Veterinary Entomology, 8, 104-106. [ Links ]
- Catalá, S., Giojalas, L., & Crocco, L. (1992) Temperature effect upon blood consumption inTriatoma infestans. Memorias do Instituto Oswaldo Cruz, 87, 473-476. [ Links ]
- Cecere, M.C., Gürtler, R.E., Canale D., Chuit, R., & Cohen, J.E. (1997) The role of the peridomiciliary area in the elimination of Triatoma infestans from rural Argentine communities. Revista Panamericana de Salud Pública, 1, 273-279. [ Links ]
- Chown, S.L., & Gaston, K.J. (2010) Body size variation in insects: a macroecological perspective. Biological Reviews, 85, 139-169. [ Links ]
- Chown, S.L., & Gaston K.J. (2013) Macroecological patterns in insect body size. Animal body size: linking pattern and process across space, time, and taxonomic group (ed. Smith, F .A., & Lyons, S.K.), pp. 13-61. The University of Chicago Press, Chicago. [ Links ]
- Clutton-Brock, T. (2017) Reproductive competition and sexual selection. Philosophical T ransactions of the Royal Society B, 372, 20160310.
- Crocco, L., & Catalá, S. (1997) Host preferences of Triatoma sordida. Annals of T ropical Medicine and Parasitology, 91, 927-930.
- Daly, H.V. (1985) Insect morphometrics. Annual Review of Entomology, 30, 415-438.
- Darwin, C. (1871) The Descent of man and selection in relation to sex. John Murray, London.
- Di Rienzo J.A., Casanoves, F ., Balzarini, M.G., Gonzalez, L., Tablada, M., & Robledo, C.W. (2016) Grupo InfoStat, ficA, Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar
- Dujardin, J.P. (2013) MoMe-CLIC: Morphometrics in Medical Entomology, Collection of Landmarks for Identification and Characterization. Disponible en: http://momeclic.com
- Dujardin, J.P., Bermudez, H., & Schofeld, C.J. (1997) The use of morphometrics in entomological surveillance of sylvatic foci of T riatoma infestans in Bolivia. Acta T ropica, 66, 145-153.
- Fairbairn, D.J. (2007) Introduction: the enigma of sexual size dimorphism. Sex, size and gender roles: Evolutionary studies of sexual size dimorphism (ed. Fairbairn, D.J., Blanckenhorn, W.U., & Szekely, T.), pp. 1-12. Oxford University Press, Oxford.
- Gaston, K.J. (1991) Body size and probability of description: the beetle fauna of Britain. Ecological Entomology, 16, 505-508.
- Guarneri, A.A., Pereira, M.H., & Diotaiuti, L. (2000) Infuence of the Blood Meal Source on the Development of Triatoma infestans, Triatoma brasiliensis, Triatoma sordida, and Triatoma pseudomaculata (Heteroptera, Reduviidae). Journal of Medical Entomology, 37, 373-379.
- Gurgel-Gonçalves, R., Ferreira, J.B.C., Rosa, A.F., Bar, M.E., & Galvão, C. (2011) Geometric morphometrics and ecological niche modelling for delimitation of near-sibling triatomine species. Medical and Veterinary Entomology, 25, 84-93.
- Gürtler R.E., Schweigmann, N.J., Cecere, M.C., Chuit, R., & Wisnivesky-Colli, C. (1993) Comparison of two sampling methods for domestic populations of Triatoma infestans in north-west Argentina. Journal of Medical Entomology, 7, 238-242.
- Gürtler, R.E., Canale D.M., Spillmann, C., Stariolo, R., Salomón, O.D., Blanco, S., & Segura E.L. (2004) Effectiveness of residual spraying of peridomestic ecotopes with deltamethrin and permethrin on Triatoma infestans in rural western Argentina: a district-wide randomized trial. Bulletin of the World Health Organization, 82, 196-205.
- Hernández, M.L. (2012) Efecto del tratamiento con insecticidas sobre la estructuración de las poblaciones de Triatoma infestans (Hemiptera, Reduviidae) en distintos hábitats de los Llanos de La Rioja. Tesis. Universidad Nacional de Córdoba, Córdoba, Argentina, 152 pp.
- Hernández, M.L., Dujardin, J.P., Gorla, D.E., & Catalá, S.S. (2013) Potential sources of Triatoma infestans reinfesting peridomiciles identifed by morphological characterization in Los Llanos, La Rioja, Argentina. Memorias do Instituto Oswaldo Cruz, 108, 91-97.
- Hernández, M.L., Dujardin, J.P., Gorla, D.E., & Catalá, S.S. (2015) Can body traits, other than wings, refect the fight ability of Triatominae bugs? Revista da Sociedade Brasileira de Medicina Tropical, 48, 682-691.
- Higgins, L. (2000) Female gigantism in a New Guinea population of the spider Nephila maculata. Oikos, 99, 377-385.
- Labarbera, M. (1986) The evolution and ecology of body size. Patterns and processes in the history of life(ed. Raup, D.M., & Jablonski, D.), pp. 69-98. Springer, Berlin.
- Labarbera, M. (1989) Analyzing body size as a factor in ecology and evolution. Annual Review of Ecology and Systematics, 20, 92-117.
- Lehane, M.J., & Schofeld C.J. (1982) Flight initiation in Triatoma infestans (Klug) (Hemiptera, Reduviidae). Bulletin of Entomological Research, 72, 497-510.
- Lent, H., & Wygodzinsky, P. (1979) Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas' disease. Bulletin of the American Museum of Natural History, 163, 1-520.
- Nattero J., Leonhard G., Rodríguez C.S., & Crocco, L. (2011) Infuence of the quality and quantity of blood ingested on reproductive parameters and life-span in T riatoma infestans (Klug). Acta Tropica,9119, 183-187.
- Noireau, F., Rojas Cortez, M.G., Monteiro F.A., Jansen, A.M., & Torrico, F. (2005) Can wild Triatoma infestansfoci in Bolivia jeopardize Chagas disease control efforts? Trends in Parasitology, 21, 7-10.
- Peters, R.H. (1983) The Ecological Implications of Body Size. Cambridge Univ. Press, New York.
- Porcasi, X., Catalá, S.S., Hrellac, H., Scavuzzo, M.C. & Gorla, D.E. (2006). Infestation of Rural Houses by Triatoma infestans(Hemiptera: Reduviidae) in Southern Area of Gran Chaco in Argentina. Journal of Medical Entomology, 43, 1060-1067.
- Price, P.W., Denno, R.F., Eubanks, M.D., Finke, D.L., & Kaplan, I. (2011) Insect ecology. behavior, populations and communities. Cambridge University Press, Cambridge.
- Rabinovich, J.E. (1985) Ecología poblacional de Triatominos. Factores biológicos y entomológicos de la Enfermedad de Chagas, (ed. OMS), tomo I 13, 121-147.
- Rohlf, F.J. (1999) Shape Statistics: Procrustes Superimpositions and Tangent Spaces. Journal of Classification, 16, 197-223.
- Sant'Anna, M.R.V., Nascimento, A., Alexander, B., Dilger, E, Cavalcante, R.R., Diaz-Albiter, H.M., Bates, P .A., & Dillon, R.J. (2010) Chicken blood provides a suitable meal for the sand fy Lutzomyia longipalpis and does not inhibit Leishmania development in the gut. Parasites & Vectors 2010, 33(1): 3.
- Schmidt-Nielsen, K. (1984) Scaling. Why is Animal Size so Important? Cambridge Univ. Press, New York.
- Schofeld, C.J. (1980) Nutritional status of domestic populations of Triatoma infestans. Transactions of the Royal Society of Tropical Medicine and Hygiene, 74, 770-778.
- Schofeld, C.J. (1994) Triatominae-Biologíay Control. Eurocomunica Publications, West Sussex, U.K.
- Smith, F.A., & Lyons, S.K. (2013) Animal body size: linking pattern and process across space, time and taxonomic group. University of Chicago Press, Chicago.
- Stat Sofit Inc (2005) STATISTICA. Disponible en: www.statsoft.com
- Szumlewicz, A.P. (1976) Laboratory colonies of Triatominae, biology and population dynamics. American T ripanosomiasis Research. PAHO Scientific Publication, 318, 63-82.
- Townsend, C.R., Begon, M., & Harper, J.L. (2008) Essentials of Ecology. Wiley-Blackwell, Oxford. UK.
- Williams, B.L. (2001) Patterns of morphological variation in Speyeria idalia (Lepidoptera: Nymphalidae) with implications for taxonomy and conservation. Annals of the Entomological Society of America, 94, 239-243.
- Wisnivesky-Colli C., Gürtler, R.E., Solarz, N., & Ruiz, A.M. (1982) Feeding patterns of Triatoma infestans (Hemiptera, Reduviidae) in relation to transmission of American Trypanosomiasis in Argentina. Journal of Medical Entomology, 19, 645-654.
- Zelditch, M. (2004) Geometric morphometrics for biologists: a primer. Academic Press, San Diego.
- Zeledón, R. (1983) Vectores de la enfermedad de Chagas y sus características ecofisiológicas. Interciencia, 66, 384-395.
- Zeledón, R., & Rabinovich, J.E. (1981) Chagas Disease: an Ecological Appraisal With Special Emphasis on its Insect Vectors. Annual Review of Entomology, 26, 101-133.
- Zeledón, R., Guardia, V.M., Zuñiga, A., & Swartzwelder, J.C. (1970) Biology and ethology of Triatoma dimidiata (Latreille, 1811) II. Life span of adults and fecundity and fertility of females. Journal of Medical Entomology, 77, 462-469.














