Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de la Sociedad Entomológica Argentina
Print version ISSN 0373-5680On-line version ISSN 1851-7471
Rev. Soc. Entomol. Argent. vol.78 no.2 La Plata June 2019
http://dx.doi.org/https://doi.org/10.25085/rsea.780201
https://doi.org/10.25085/rsea.780201
Artículo-Article
Host influence on the nutritional and reproductive status of Triatoma infestans (Klug) (Hemiptera: Reduviidae) peridomiciliary populations
Influencia del hospedador en el estado nutricional y reproductivo de poblaciones peridomiciliarias de Triatoma infestans (Klug) (Hemiptera: Reduviidae)
SORIA, Carola1,*, CARDOZO, Miriam1, CANAVOSO, Lilián E.2, CROCCO, Liliana B.1, NATTERO, Julieta3, ORTIZ, Valeria A.P.1, LEYRIA, Jimena2 & RODRÍGUEZ, Claudia S.1
1 Cátedra de Introducción a la Biología, Instituto de Investigaciones Biológicas y Tecnológicas (IIByT-CONICET/UNC), Facultad de Ciencias Exactas Físicas y Naturales, Universidad Nacional de Córdoba. Córdoba, Argentina. * E- mail: soriacarola@gmail.com
2 Departamento de Bioquímica Clínica-CIBICI-CONICET, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba. Córdoba, Argentina.
3 Laboratorio de Eco-Epidemiología, Departamento de Ecología, Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires. C.A.B.A., Argentina.
Received 06 - X - 2018 | Accepted 25 - III - 2019 | Published 27 - VI - 2019
RESUMEN. Triatoma infestans es el principal vector de la enfermedad de Chagas en el Cono Sur de Sudamérica. Esta especie está bien adaptada a vivir en el domicilio y estructuras utilizadas para albergar animales domésticos (hábitats peridomésticos). En este trabajo evaluamos la relación entre la fuente de sangre consumida por los adultos de T. infestans recolectados de diferentes hábitats peridomésticos de dos localidades del departamento de Cruz del Eje (Córdoba, Argentina) y su estado nutricional y reproductivo. En cada individuo, la relación entre el peso y la longitud corporal total se utilizó como un indicador del estado nutricional (EN). La presencia de espermatozoides en espermatecas y el número de ovocitos corionados en ovarios y oviductos se consideraron indicadores del estado reproductivo (ER) de las hembras. La fuente de alimentación en el promesenterón de insectos machos y hembras se identifcó utilizando antisueros anti-gallina, anti-cabra, anti-humano y anti-perro. Los gallineros fueron las principales estructuras peridomésticas presentes en el área de estudio, así como los sitios con el mayor porcentaje de T. infestans. Los insectos recolectados en las diferentes estructuras peridomésticas mostraron un EN entre 8 y 15 mg / mm. De las hembras evaluadas, el 35,7% presentó ovocitos corionados. Los análisis del perfl alimentario revelaron que las gallinas fueron la principal fuente de sangre. Independiente de la fuente de sangre los triatominos presentaron EN entre 8 y 15 mg / mm. No se encontraron ejemplares alimentados exclusivamente con sangre humana; sin embargo, del 31,48% de los insectos que se alimentaron de fuentes de sangre mixtas, el 59% incluía sangre humana. Todas las muestras de T. infestans que incluían sangre humana en la fuente de sangre se recolectaron en gallineros y depósitos ubicados en un área de 12 m alrededor de los domicilios. La sangre humana presente en las fuentes de alimentación mixta sugiere que T. infestans se mueve de los domicilios a los peridomicilios y viceversa.
PALABRAS CLAVE. Estado nutricional. Estado reproductivo de las hembras. Hábitats peridomésticos. Perfl alimentario. Vector de la enfermedad de Chagas.
ABSTRACT. Triatoma infestans is the main vector of Chagas disease in the southern cone of South America. This species is well adapted to living in rural houses and structures used for housing domestic animals (peridomestic habitats). In this study, we evaluated the relationship between the source of blood consumed by adults of T. infestans collected from different peridomestic habitats from two localities from Cruz del Eje department (Córdoba, Argentina) and their nutritional and reproductive status. In each individual, the ratio between body weight and total body length was used as an indicator of nutritional status (NS). The presence of sperm in spermathecae and the number of chorionated oocytes in ovaries and oviducts were considered indicators of reproductive status (RS) of females. The feeding source in the promesenteron of male and female insects was identifed using anti-chicken, anti-goat, anti-human and anti-dog antisera. Chicken coops were the main peridomestic structure present in the study area as well as the peridomestic sites with the highest percentage of T. infestans. Insects collected from the different peridomestic structures showed a NS between 8 and 15 mg/mm. Of the evaluated females, 35.7% presented chorionated oocytes. Food profle analyses revealed that chicken was the main blood source. Independently of the blood source, the triatomines presented a NS between 8 and 15 mg/mm. No specimens feeding exclusively on human blood were found; nevertheless, of 31.48% of insects feeding on mixed blood sources, 59% included human blood. All T. infestansspecimens that included human blood in the mixed blood source were collected from chicken coops and storerooms located in a 12-m area around domiciles. Human blood present in mixed blood meal of adult insects suggests that T. infestansmoves from domiciles to peridomicilies and vice versa.
KEYWORDS. Chagas disease vector. Female reproductive status. Host-feeding source. Nutritional status. Peridomestic habitats.
INTRODUCTION
Triatoma infestans(Klug), one of the 151 recognized species of triatomines (Hemiptera: Reduviidae: Triatominae), is the main vector of Trypanosoma cruzi, the etiological agent of Chagas disease, in the southern cone of South America (Justi & Galvão, 2017). This species is successfully adapted to thrive in human dwellings and other human-made or modifed structures used by domestic animals (peridomiciles), such as chicken coops, goat and pig corrals, and storerooms (Coura et al., 2014; Gürtler et al., 2014).
Although constant vector control efforts via pyrethroid insecticide applications have greatly reduced T. infestans distribution range, this species persists in several areas of the Gran Chaco ecoregion of Argentina, Bolivia and Paraguay (Schofeld et al., 2006). Particularly in the Argentine Chaco rural zones, the area surrounding human dwellings is highly important because their peridomestic structures are usually heavily infested with triatomines (e.g.: Cécere et al., 1997; López et al., 1999; Chartier & Crocco, 2007; Gorla et al., 2013; Ortiz et al., 2015). These areas may act as potential sources of re-infestation after insecticide application (Cécere et al., 1997, 2004). Several studies suggest that chicken coops and goat and pig corrals are the most important peridomestic structures associated with Triatominae in this region (e.g.: López et al., 1999; Ceballos et al., 2005; Gurevitz et al., 2011; Hernández et al., 2011).
Fitness-related measures, such as bug abundance, blood-feeding rates, engorgement status and reproductive status (RS) are good indicators of the state of the population within the peridomestic structures (Gürtler et al., 2014). The nutritional status (NS) of triatomines affects all vital rates and the propensity of these bugs to fy large distances (Schofeld, 1980; Lehane et al., 1992). Thus, a low NS has been found to be associated with the probability of initiation of fight in search of new sources of food (Mc Ewen & Lehane, 1993; Ceballos et al., 2005). Additionally, in domiciles, the presence of females with low NS and chorionated oocytes suggested that the dispersion of gravid females might be a potential process of domicile colonization (Payet et al., 2009; Abrahan et al., 2011). In turn, different peridomestic structures vary in habitat quality for triatomines. The populations of T. infestansoccurring in chicken coops have a better NS and RS than those present in goat corrals, pig corrals and other structures that are not related to the presence of chickens (Hernández et al., 2011; Gürtler et al., 2014). Hence, considering habitat suitability and stability, goat and pig corrals would have higher probability of being the source of domicile re-infestation after spraying (Cécere et al., 1997; Ceballos et al., 2005; Hernández et al., 2011; Gürtler et al., 2014).
On the other hand, knowing the host-feeding source of the populations of peridomiciliary triatomines allows us to identify mixed sources of food, which show the real dispersion of these insects between different peridomestic structures or between the domicile and the peridomicile (Wisnivesky-Colli et al., 1982, 1987; Salvatella et al., 1994). In addition, a host-feeding source provides complementary information of the NS to understand the potential dispersion movements (Zeledón, 1976).
The types of peridomestic structures that hold triatomine populations are relevant for T. infestans eradication, since they present different levels of risk for domicile re-infestation. Considering that infested peridomiciles might lead to domicile re-infestation or colonization, in this work we propose analyzing the infuence of habitats and sources of consumed blood on NS and RS in adults of T. infestans peridomiciliary populations in western Córdoba province (Cruz del Eje department), Argentina. The province of Córdoba, in the southern extreme of the Argentine Chaco region, is a historically endemic area for Chagas disease and shows a heterogeneous scenario of T. cruzitransmission (Moreno et al., 2010; 2012). In addition, the presence of peridomiciliary T. infestans populations, mostly in chicken coops, together with risk factors, such as the building materials used in peridomicile construction (Ortiz et al., 2015), defne a complex scenario of diffcult diagnosis in this area.
Triatomines are opportunistic in host choice and, therefore, the blood source tends to refect the local host abundance and availability (Gürtler et al., 1997). It is expected that the host choice of the peridomiciliary populations of T. infestans will be biased towards the resident host in each habitat, which would be evidenced in a low preference for mixed blood sources by triatomines, mainly in chicken coops.
MATERIAL AND METHODS
Study Area
The study was conducted in three rural sites of Cruz del Eje department (30° 44' S; 64° 48' W), Córdoba province, Argentina (Fig. 1). This department is part of a Chagas disease endemic area with intermediate risk of vector transmission, and with a re-infestation rate of 5% (National Chagas Program Report, 2017). This area is included within the Arid Chaco of Argentina, the southern extreme of Gran Chaco geographic region, characterized by a dry subtropical climate with warm summer (monthly average of the warmest month: 26 °C). Winters are mild, with average monthly temperature of the coldest month of about 12 °C and with frequent frosts. Rainfalls are concentrated in summer, with 70% of rains occurring in the four warmest months (Karlin, 2013).
Two feld trips were conducted in April and November 2013, with 30 houses being surveyed in El Brete and Guanaco Muerto villages in the frst trip, and 15 houses in Villa de Soto village in the second trip (Fig. 1). The houses were selected according to the plans of the National and Province Chagas Programs. Vector control personnel had sprayed the study area with pyrethroid insecticides approximately three years before our feldwork, and no further interventions were made.
Field Activities
The study was conducted in peridomestic structures that housed a host. In each house, the number of peridomestic structures and the distance between the structure and the house (in meters) were recorded.

Fig. 1. A. Location of Cruz del Eje department, Cordoba province, Argentina. B. Sampling sites (indicated with dots): Guanaco Muerto, El Brete and Villa de Soto.
Triatomines were collected using the man/hour method (Chuit et al., 1992). The collected triatomines were placed in plastic containers with house identifcation labels and the peridomestic structure type to which they belonged, and were maintained in iceboxes at 10 ºC for further transport to the laboratory. The periodomestic structures observed were chicken coops, goat and pig corrals, and storerooms. A single woodpile and a cage with birds were found in two different houses and were categorized as others".
Laboratory activities
All collected adults of T. infestans were analyzed in the laboratory within three days after collection. Each bug was individually weighed in a Mettler precision balance ± 0.001 g and photographed in dorsal view with a reference scale using a digital camera. The length of the insect body, from the clipeous to the end of the last abdominal segment, was measured using the freely-available Image J program (Noireau & Dujardin, 2001; Ceballos et al., 2005). Then, the insects were dissected to extract the promesenteron; in females, spermathecae and ovarioles were analyzed.
Nutritional and reproductive status
The nutritional status (NS) was calculated as the ratio of weight (mg) to body length (mm). In the case of females, this parameter was adjusted according to the amount of chorionated oocytes present in ovaries and oviducts (see reproductive status). The NS was corrected for females, considering that each chorionated oocyte has a weight of 2.3 mg (Montenegro & Pasina, 1984; Hernández et al., 2011), as follows: NS- (2.3 mg * number of chorionated oocytes). Triatoma infestans bugs were classifed into three categories based on the NS value obtained and following Schofeld (1982): 1. Hunger threshold (NS < 8 mg/mm), triatomines with high probability of taking another meal; 2. Not fully satiated (NS between 8 and 15 mg/mm), triatomines that consumed the blood intake required for metabolic needs but less than that achieved at repletion, with 0.5 probability of taking another meal; 3. Fully engorged (NS > 15 mg/mm), bugs fed to repletion and with no probability of taking another meal. In turn, NS of 8 mg/mm has been frequently used as an indicator of the probability of fight dispersal in search of a new blood source (Lehane et al., 1992; Ceballos et al., 2005), whereas NS of < 15 mg/mm has been considered an indicator of walking dispersal (Abrahan et al., 2011).
The reproductive status of triatomine (RS) was determined in females. The presence of sperm in the spermathecae was recorded, considering females with empty spermatheca as virgin and females with at least one of the spermathecae full as fertilized. Furthermore, both ovaries were dissected to record the number of chorionated oocytes present in the basal follicles of each of the seven ovarioles per ovary, along with chorionated oocytes present in the oviducts. From these data, the number and proportion of females with chorionated oocytes and the average number of chorionated oocytes per female were calculated as indicators of potential fertility (López et al., 1999; Payet et al., 2009).
Feeding Source
The source of blood in the promesenteron of insects collected from each peridomestic structure was determined by immunochemistry, according to Pinto et al. (2008) with some modifcations. IRDye 800CW polyclonal goat anti-rabbit IgG was purchased from LiCor Biosciences (Lincoln, NE, USA). The following whole sera developed in rabbits were used: anti-goat (code G5018), anti-dog (code D4908), anti-human (code H8765) and anti-chicken (code C1036); all of them were from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of analytical grade. Briefy, the promesenterons from male and female insects were dissected in cold phosphate buffered saline (PBS, 6.6 mM Na2HPO4/KH2PO4, 150 mM NaCl, pH 7.4) and individually impregnated onto flter papers (S&S no. 604), and the data of each specimen was recorded. Then, the content of each flter paper was eluted with 150 µl PBS using a disposable pellet pestle (KimbleTM KontesTM, Thermo Fisher Scientifc) and the resulting material was centrifuged at 8,000 x g for 5 min. The procedure was repeated twice and the supernatants were pooled and employed to test the feeding source by dot-blot. For the assays, spots containing 15 µl of each supernatant were applied onto nitrocellulose membrane strips and then blocked with TBS-0.1% Tween 20 containing 5% of non-fat milk at room temperature. Each membrane was washed twice with 50 mM Tris, 150 mM NaCl, pH 7.5 buffer (TBS) and then individually incubated with anti-dog, anti-chicken, anti-goat and anti-human whole antisera (dilution 1:2000 each) for 1 h at room temperature. Then the membranes were rinsed twice with TBS for 10 min and incubated with the secondary antibody (Li-Cor IRDye 800CW polyclonal goat anti-rabbit IgG, 1∶15,000) at room temperature for 1 h. The blots were washed as described and then scanned and analyzed with the Odyssey quantitative western blot near-infrared system (Li-Cor Biosciences, Lincoln, NE, USA) using default settings. Each test had positive and negative controls, which were performed using dog, chicken, goat and human sera, as appropriate. The antisera tested by dot-blots were chosen based on the characteristics of the areas analyzed, with chickens and goats being the main peridomiciliary animals and dogs being the principal blood meal source of domiciliary triatomines as well as the main reservoir of T. cruzi(Gürtler et al., 1996; Gürtler & Cardinal, 2015). For the analysis, T. infestansthat fed on more than one host were grouped in two categories: (a) the mammalian-bird category, comprising insects that fed on chicken and on at least one mammalian host (dog, goat and/or human); (b) the mammalian-mammalian category, comprising insects that fed on different mammalian hosts. This criterion was based on the extensive bibliography that demonstrated that the quality of blood from the selected hosts infuenced the biological requirements of triatomines (Diotaiuti & Dias, 1987; Gomes et al., 1990; Aldana et al., 2009; Nattero et al., 2011).
Statistical analysis
The data obtained were analyzed using descriptive statistics. Because the studied variables did not present normal distribution (Test Shapiro-Wilk W) and homogeneity of variance (Levene Test), comparisons between sexes and between groups of insects belonging to different peridomiciliary structures were analyzed using non-parametric tests (test Mann-Whitney, Kruskal Wallis, Spearman correlation and Fisher's exact test). The difference of proportions test was used to compare percentages. Differences were considered statistically signifcant at p values < 0.05.
RESULTS
Triatomines were found in 26.7% (16) of the 60 peridomestic structures surveyed, where 200 adult specimens were collected. Of the infested peridomestic structures, 75% (12/16) were chicken coops (Table I).
Nutritional status (NS)
The median NS of the 71 analyzed females was 11.69 mg/mm, which was signifcantly higher than that of the 102 analyzed males (10.66 mg/mm) (U = 2436, p < 0.001). The NS of females and males did not show signifcant differences among types of peridomestic structures where they were collected (H = 5.83, df = 3, p = 0.16 and H = 3.42, df = 3, p = 0.54 for females and males, respectively).
The triatomines collected in chicken coops varied in the proposed NS categories, as shown in Figure 2.
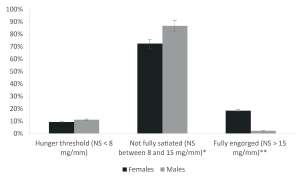
Fig. 2. Percentage of adult Triatoma infestansmales and females collected in chicken coops (axis Y) according to their nutritional status (axis X). * Signifcant differences between sexes (p = 0.02); ** Signifcant differences between sexes (p < 0.001).
Feeding source
Dot-blot assays were carried out to test the feeding source of insects taken from each peridomestic structure. The feeding profle results are summarized in Figure 3. Of the total evaluated specimens (65), 83% (54) were positive to some of the antisera tested. Specimens ingesting a single food source were 68.52% (37/54), being signifcantly more abundant than those that consumed a mixed source (31.48%, 17/54; p = 0.0002). No specimens feeding exclusively on humans were found, but 59% of 17 insects (10/17) ingesting mixed blood sources, included human blood.
All bugs that fed on human blood were collected only in chicken coops and storerooms that were located less than 12 meters from the domiciles. Bugs that fed on chicken were the most frequent among bugs that consumed both single and combined blood sources, with 86.5% and 88%, respectively.
On the other hand, we evaluated the total number of positive samples identifed from a given host and its relationship with the peridomestic structures where the bugs were collected (Table II). For this analysis, 89% of samples (73/82) corresponded to insects collected in chicken coops, but 41.1% of them (30/73) were positive for a blood source other than chicken. The Fisher's exact test revealed that the feeding source consumed by the insect was independent of the collection structure (p = 0.48). Although the peridomestic structures described as storerooms and others" did not have a main host or were not built for housing domestic animals, they were positive with the antisera used in this work (Table II).
Relationship between nutritional and reproductive status and food source
Male and female specimens fed on a single blood source showed an NS > 8 mg/mm, regardless of the type of blood source (Fig. 4) (H = 2.63, df = 2, p > 0.05 and U = 11, p > 0.05 for males and females, respectively). In all cases, the median NS of the triatomines feeding on a combined blood source was recorded in the satiated range. Bugs that fed on mammalian-bird blood did not show signifcant differences between sexes (U = 17; p = 0.327) (Fig. 5). Females and males fed on mammalian-mammalian blood were not compared because only one female with NS = 11.48 mg/mm and one male with NS = 9.01 mg/ mm were recorded.
Table I. Number (N) of peridomestic structures examined that were positive for adults of Triatoma infestans and their mean distance from the domicile.

None of the triatomines collected in goat corrals and storerooms were fully satiated (8 mg/ml < NS < 15 mg/ ml). Females collected in the others" peridomestic structure were fully engorged (NS > 15 mg/mm); there was also a male below hunger threshold in this type of structure (NS < 8 mg/mm).
Reproductive status
Of the evaluated females (including females that had not copulated), 35.7% (20/56) presented a median of 12.5 chorionated oocytes. Ninety percent (18/20) of the females with eggs were collected from chicken coops, whereas the remaining ones were collected from other" structures, without signifcant differences in number of chorionated oocytes per female between these peridomestic structures (U = 12; p = 0.44). Of the total of collected females, 56 were evaluated for evidence of copula; of these, 75% (42/56) presented full spermathecae.

Fig. 3. Percentage of Triatoma infestansindividuals feeding on a single or combined blood source.
Table II. Number of positive samples against antisera identified from different hosts, and its relationship with the peridomestic structures where adults of Triatoma infestans were collected (N represents the absolute number of positive samples against antisera). ( a ) = T otal number and percentage of positive samples for chicken, goat, dog and human differ between feeding sources for the same type of peridomestic structures (p < 0.001); (b) = Total number and percentage of positive samples for dogs differ signifcantly between goat corrals and chicken coops (p = 0.008).

Furthermore, no signifcant differences were found in the number of chorionated oocytes per female according to the blood feeding source for both single and combined sources (U = 8.50, p = 0.47). Of the females below hunger threshold collected in chicken coops, only 50% had chorionated oocytes in their ovarioles and/or oviducts (median of chorionated oocytes: 9.67 ± 5.13).
DISCUSSION
Chicken coops were the most frequent structure in this area. In addition, chicken blood was the main food source in single and combined blood meals. The high percentage of combined blood meals recorded in peridomiciliary habitats does not seem to be related to host-feeding source choice and to the main host residing in the peridomicile structure. Nutritional and reproductive status did not seem to be related to the host-feeding sources (one or more than one host or combination of different hosts). The high level of combined blood meals including human blood, within 12 meters of distance, suggests that adults disperse to domiciles and return to peridomiciliary structures.
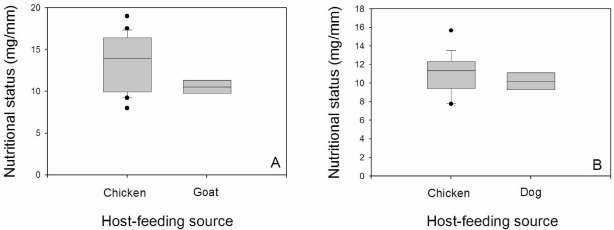
Fig. 4. Box plot for nutritional status of Triatoma infestans according to the single blood source. A: Females. B: Male. The line inside the box represents the median and the box comprises the lower and upper quartiles. The lines above and belowthe box indícate the 90th and 10th percentiles, and dots represent the outliers.
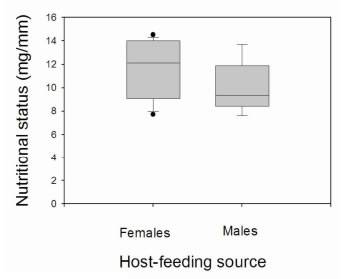
Fig. 5. Box plot for nutritional status of Triatoma infestans females and males according to the combined mammalian-bird blood source. The line inside the box represents the median and the box comprises the lower and upper quartiles. The lines above and below the box indícate the 90th and 10th percentiles, and dots represent the outliers.
In a similar geographic area chicken coops were found to be the main habitat infested with T. infestans populations (López et al., 1999). Moreover, this structure was reported as positive for the presence of triatomines in other rural areas from the Gran Chaco of Argentina (e.g. : Ceballos et al., 2005; Abrahan et al., 2011; Hernndez et al., 2011; Gorla et al., 2013; Gürtler et al., 2014; Ortz et al., 2015). In our work area we demonstrated that chicken coops were the most frequent peridomiciliary structures, where most T. infestans adults included in this study were collected. Therefore, chicken coops showed to be of great importance for the vector control of T. infestans; indeed, although chickens are refractory to T. cruzi (Teixeira et al., 2011), coops support the populations of triatomines that may disperse to other peridomiciliary habitats and to the domiciles. Our results showed that T. infestans adults fed mainly on chicken blood. Chickens are expected to be a single blood source for bugs because of the abundance of chicken coops in these areas and the amount of triatomines collected there. Unlike expected, the presence of chicken blood in combined blood meals shows a low choice by T. infestans.
Results showed that the blood source agrees with the peridomestic structure from which triatomines were collected. As described by Gürtler et al. (2014), the fact that bugs fed on chickens were found in peridomiciliary structures other than chicken coops could be explained by the dispersion of adults among habitats. However, a possible walking dispersion of triatomines, as well as in a host as a passive carrier, cannot be neglected within the house context (Abrahan et al., 2011). The presence of a combined blood source is evident considering the dispersion of T. infestans adults between domestic and peridomestic habitats (e.g. : Wisnivesky-Colli et al., 1987; Salvatella et al., 1994; Pinto et al., 2008). The results obtained from the food profle indicate that one third of the triatomines evaluated had fed at least on two different hosts. Furthermore, we found specimens that consumed the four types of blood sources identifed in this work: chicken, goat, dog and human. Studies on T. infestans under experimental (Rabinovich, 1972; Schofeld, 1980) and natural conditions (Ceballos et al., 2005) suggest that the dispersion of T. infestans is regulated by the nutritional status, which in turn depends on population density and host availability. The results obtained in this work indicate a good nutritional status in T. infestans adults (8-15 mg/mm), as those obtained for other regions within the Argentine Chaco (e.g. : Ceballos et al., 2005; Abrahan et al., 2011). Although these nutritional status values suggest a low probability of fight initiation and further dispersion in search of new food sources (Ccere et al., 1997; Hernndez et al., 2011), walking dispersion cannot be discarded. However, the intake of combined blood sources in triatomines with a good nutritional status might be related to opportunistic dispersion strategies and blood feeding behavior. Castillo-Neyra et al. (2015) noticed dispersal events of T. infestansin the presence of a host and with easy access to it. The authors suggest a new approach to the understanding of T. infestansdispersal strategies, which allow bugs to fnd and colonize new areas, maximizing the overall survival of the offspring in the case of fnding an appropriate environment.
This work showed a low incidence of dog blood in both combined and single blood meals. However, the presence of dog blood is a warning signal, since these animals are frequently present in houses and sometimes sleep within domiciles. Dogs are also considered the main hosts that support and maintain the domestic transmission of T. cruzi (Wisnivesky-Colli et al., 1982; Gürtler et al., 1991, 1996). On the other hand, our results indicate that goats were scarcely represented in single blood meals, but highly represented in combined ones. Therefore, although the triatomines collected in the peridomiciliary structures of the Argentine Chaco are rarely infected with T. cruzi, goats and dogs can contribute as reservoirs and amplifers of this parasite. In the case of their introduction into the house system, they might reactivate the vectorial transmission of the parasite by the arrival of infected triatomines to the domicile from the peridomicile (Gürtler & Cardinal, 2015). We also identifed human blood in combined blood source consumed by T. infestans that were collected in chicken coops and storerooms, suggesting that, besides infestation events occurring from peridomiciliary structures to domiciles, there may be a fow of bugs in both directions. Similar results were found in rural areas of the Gran Chaco by Wisnivesky-Colli et al. (1982) and Gürtler et al. (2014). Human blood was found in bugs collected in those peridomiciliary structures that were less than 12 meters away from houses. This result confrms the proposal of López et al. (1999), who suggest the construction of peridomiciliary structures at distances greater than 12 meters from domiciles.
Under natural habitat conditions, nutritional status was not signifcantly related to blood source. The number of chorionated oocytes did not show differences between females feeding on a single or combined blood source. These results were in disagreement with those proposed by several authors, who mention differences in nutritional quality and reproductive parameters between bugs feeding on mammalian versus avian hosts (Diotaiuti & Dias, 1987; Gomes et al., 1990; Guarneri et al., 2000; Nattero et al., 2011). In turn, the ingestion of combined blood sources did not result in signifcant differences in the number of chorionated oocytes present in females, in contrast to what was proposed by Aldana et al. (2009). In this context, our fndings are in line with the ones described by Jiron & Zeledón (1982) for T. infestans, who suggest a similar weight gain between the bugs being offered one or multiple sources of blood under experimental conditions, regardless of the host-feeding source.
The lack of reactivity to the antisera found in 17% of T. infestans specimens could be explained by the presence of unknown food sources. According to the objectives of this study, no antisera were considered against wild hosts that potentially arrived at the house. Rodents, opposums (Didelphis spp.) and armadillos (Dasypodidae) have been identifed as wild reservoirs of T. cruzi in countries endemic for Chagas disease (Salvatella, 1993; WHO, 2002; Herrera, 2010). Triatomine bugs have been also detected feeding on these hosts in peridomestic environments (Salvatella et al., 1994; Calderón-Arguedas et al., 2001). Wisnivesky-Colli et al. (1982) reported rodents and opposums as hosts of T. infestans specimens from chicken coops and goat corrals in a similar study area to that evaluated in this work. Additionally, domiciliary rodents were found as important reservoirs of T. cruzi and as potential sources of this parasite for T. infestans in the Argentine Gran Chaco (Gürtler & Cardinal, 2015).
The peridomiciliary structures infested with triatomines vary between areas of the Argentine Gran Chaco (Cécere et al., 2004; Ceballos et al., 2005; Chartier & Crocco, 2007; Gorla et al., 2013). This heterogeneity leads to the need to address vector control from the unique characteristics of the local environment. The northwest region of Córdoba province is an area historically endemic for Chagas disease (Moreno et al., 2010). The abundant combined blood source consumption found in this study, even including more than two hosts, suggests that T. infestans adults would move between ecotopes. These movements are greater than expected and evidence the high dispersion of these insects under natural conditions. The presence of human blood in the blood meal of peridomestic T. infestans reveals the participation of humans as hosts and the potential risk of vector transmission of Chagas disease.
This scenario shows the need for further studies addressing active and passive dispersion of triatomines in the peridomicile and domicile contexts, to evaluate the alternative dispersion strategies that might occur in triatomine populations under natural conditions. Furthermore, analyses of food source should include sera of wild hosts that may be present in the dispersion area of triatomines between the different environments (domiciliary, peridomiciliary and wild environments).
We thank the National and Provincial Program of Chagas for the logistic of the feld trip, R. Stariolo and P. Lobbia (Centro de Referencia de Vectores (CeReVe), Ministerio de Salud de la Nación, Argentina) for providing triatomines for controlled experiment; Also thanks to Biol. Ana López, Lic. Florencia Quintanilla, Vet. Gerardo Pérez Nieto, Biol. Daniel Villareal and Florencia Carnicero for their collaboration at different stages of experimentation. Jorgelina Brasca revised the language grammar and style. This study was funded by Secretaria de Ciencia y Tecnología de la Universidad Nacional de Córdoba (SECyT/ UNC), Concejo Nacional de investigaciones Científcas y T écnicas (CONICET) and Ministerio Ciencia y Técnica de la Provincia de Córdoba.
LITERATURE CITED
Abrahan, L.B., Gorla, D.E., & Catalá, S.S. (2011) Dispersal of Triatoma infestansand other Triatominae species in the arid Chaco of Argentina: fying, walking or passive carriage? The importance of walking females. Memórias do Instituto Oswaldo Cruz, 106, 232-239. [ Links ]
Aldana, E.J., Jacome, D., & Lizano, E. (2009) Efecto de la alternación de fuentes sanguíneas sobre la fecundidad y la fertilidad de Rhodnius prolixus Stal (Heteroptera: Reduviidae). EntomoBrasilis, 2, 17-23. [ Links ]
Calderón-Arguedas, O., Chinchílla, M., García, F., & Vargas, M. (2001) Preferencias alimentarias de Triatoma dimidiata (Hemiptera: Reduviidae) procedente de la meseta central de Costa Rica a fnales del siglo XX. Parasitología al día, 25, 78-81. [ Links ]
Castillo-Neyra, R., Barbu, C.M., Salazar, R., Borrini, K., Naquira, C., & Levy, M.Z. (2015) Host-Seeking Behavior and Dispersal of Triatoma infestans, a Vector of Chagas Disease, under Semi-feld Conditions. PLOS Neglected Tropical Diseases, 9, e3433. [ Links ]
Ceballos, L.A., Vazquez-Prokopec, G.M., Cécere, M.C., Marcet, P.L., & Gürtler, R.E. (2005) Feeding rates, nutritional status and fight dispersal potential of peridomestic populations of Triatoma infestans in rural northwestern Argentina. Acta Tropica, 95, 149-159. [ Links ]
Cécere, M.C., Gürtler, R.E., Canale, D., Chuit, R., & Cohen, J.E. (1997) The role of the peridomiciliary area in the elimination of Triatoma infestans from rural Argentine communities. Revista Panamericana de Salud Pública, 1, 273-279. [ Links ]
Cécere, M.C., Vazquez-Prokopec, G.M., Gürtler, R.E., & Kitron, U. (2004) Spatio-temporal analysis of reinfestation by Triatoma infestans (Hemiptera: Reduviidae) following insecticide spraying in a rural community in northwestern Argentina. The American Journal of Tropical Medicine and Hygiene, 71, 803-810. [ Links ]
Chartier, D.I., & Crocco, L.B. (2007) Relevamiento de vectores de la Enfermedad de Chagas en peridomicilios del área rural del Departamento Ayacucho, San Luis, Argentina. Revista de la Sociedad Entomológica Argentina, 66, 181-185. [ Links ]
Chuit, R., Paulone, I., Wisnivesky-Colli, C., Perez, A., Sosa-Estani, S., & Segura, E. (1992) Results of a frst step toward community-based surveillance of transmission of Chagas disease with appropriate technology in rural areas. The American Journal of Tropical Medicine and Hygiene. 46, 444-450. [ Links ]
Coura, J., Viñas, P.A., & Junqueira, A.C.V. (2014) Ecoepidemiology, short history and control of Chagas disease in the endemic countries and the new challenge for non-endemic countries. Memórias do Instituto Oswaldo Cruz, 109, 856-862. [ Links ]
Diotaiuti, L., & Dias, J.C.P. (1987) Estudo comparativo do ciclo evolutivo de Rhodnius neglectus alimentados em pombos ou camundongos. Revista da Sociedade Brasileira de Medicina Tropical, 20, 95-100. [ Links ]
Gomes, J.E.P.L., Azambuja, P., & Garcia, E.S. (1990) Comparative studies on the growth and reproductive performances of Rhodnius prolixusreared on different blood sources. Memórias do Instituto Oswaldo Cruz, 85, 299-304. [ Links ]
Gorla, D.E., Abrahan, L., Hernández, M.L., Porcasi, X., Hrellac, H.A., Carrizo, H., & Catalá, S.S. (2013) New structures for goat corrals to control peridomestic populations of Triatoma infestans (Hemiptera: Reduviidae) in the Gran Chaco of Argentina. Memórias do Instituto Oswaldo Cruz, 108, 352-358. [ Links ]
Guarneri, A.A., Pereira, M.H., & Diotaiuti, L. (2000) Infuence of the blood meal source on the development of Triatoma infestans, Triatoma brasiliensis, Triatoma sordida, and Triatoma pseudomaculata (Heteroptera, Reduviidae). Journal of Medical Entomology, 37, 373-379. [ Links ]
Gurevitz, J.M., Ceballos, L.A., Gaspe, M.S., Alvarado-Otegui, J.A., Enríquez, G.F., Kitron, U., & Gürtler, R.E. (2011) Factors affecting infestation by Triatoma infestans in a rural area of the humid Chaco in Argentina: a multi-model inference approach. PLOS Neglected Tropical Diseases, 5, e1349. [ Links ]
Gürtler, R.E., & Cardinal, M.V. (2015) Reservoir host competence and the role of domestic and commensal hosts in the transmission of Tr ypanosoma cruzi. Acta Tropica, 151, 32-50. [ Links ]
Gürtler, R.E., Cécere, M.C., Rubel, D.N., Petersen, R.M., Schweigmann, N.J., Lauricella, M.A., & Wisnivesky-Colli, C. (1991) Chagas disease in north-west Argentina: infected dogs as a risk factor for the domestic transmission of T rypanosoma cruzi. Transactions of the Royal Society of T ropical Medicine & Hygiene, 85, 741-745. [ Links ]
Gürtler, R.E., Cécere, M.C., Vazquez, D.P., Chuit, R., & Cohen, J.E. (1996) Host-feeding patterns of domiciliary Triatoma infestans (Hemiptera: Reduviidae) in northwest Argentina: seasonal and instar variation. Journal of Medical Entomology, 33, 15-26. [ Links ]
Gürtler, R.E., Cohen, J.E., Cécere, M.C., & Chuit, R. (1997) Shifting host choices of the vector of Chagas disease Triatoma infestans and the availability of hosts in houses in north-west Argentina. Journal of Applied Ecology, 34, 699-715. [ Links ]
Gürtler, R.E., Cécere, M.C., Fernández, M., Vazquez-Prokopec, G.M., Ceballos, L.A., Gurevitz, J.M., & Cohen, J.E. (2014) Key source habitats and potential dispersal of Triatoma infestans populations in Northwestern Argentina: implications for vector control. PLOS Neglected T ropical Diseases, 8, e3238. [ Links ]
Hernández, M.L., Abrahan, L.B., Dujardin, J.P., Gorla, D.E., & Catalá, S.S. (2011) Phenotypic variability and population structure of peridomestic Triatoma infestans in rural areas of the arid Chaco (western Argentina): spatial infuence of macro-and microhabitats. Vector-Borne and Zoonotic Diseases, 11, 503-513.
Herrera, L. (2010) Una revisión sobre reservorios de Trypanosoma (Schizotrypanum) cruzi (Chagas, 1909), agente etiológico de la Enfermedad de Chagas. Boletín de Malariología y Salud Ambiental, 50, 3-15. [ Links ]
Jirón, L.F., & Zeledón, R. (1982) Preferencias alimentarias de tres especies de Triatominae (Hemiptera: Reduviidae) en condiciones experimentales. Revista de Biología Tropical, 30, 151-159. [ Links ]
Justi, S.A., & Galvão, C. (2017) The evolutionary origin of diversity in Chagas disease vectors. Trends in Parasitology, 33, 42-52. [ Links ]
Karlin, M.S. (2013) Características físicas y ambientales: El clima. El Chaco Árido(ed. Karlin, M.S., Karlin, U.O., Coirini, R.O., Reati, G.J., & Zapata, R.M.), pp. 25-31. Encuentro grupo editor, Córdoba, Argentina. [ Links ]
Lehane, M.J., McEwen, P.K., Whitaker, C.J., & Schofeld, C.J. (1992) The role of temperature and nutritional status in fight initiation by Triatoma infestans. Acta Tropica, 52, 27-38. [ Links ]
López, A., Crocco, L.B., Morales, G., & Catalá, S.S. (1999) Feeding frequency and nutritional status of peridomestic populations of Triatoma infestans from Argentina. Acta Tropica, 73, 275-281. [ Links ]
Mc Ewen, P.K., & Lehane, M.J. (1993) Factors infuencing fight initiation in the triatomine bug Triatoma sordida(Hemiptera: Reduviidae). Insect Science and Its Application, 14, 461-464.
Montenegro, S.S., & Pasina, L. (1984) Consumo y utilización del alimento en adultos de Triatoma infestans Klug, 1834 (Hemiptera, Reduviidae). Physis, 42, 127-133.
Moreno, M.L., Moretti, E., Basso, B., Céspedes, M.F., Catalá, S.S., & Gorla, D.E. (2010) Seroprevalence of Tr ypanosoma cruzi infection and vector control activities in rural communities of the southern Gran Chaco (Argentina). Acta Tropica, 113, 257-262.
Moreno, M.L., Hoyos, L., Cabido, M., Catalá, S.S., & Gorla, D.E. (2012) Exploring the association between Tr ypanosoma cruzi infection in rural communities and environmental changes in the southern Gran Chaco. Memórias do Instituto Oswaldo Cruz, 107, 231-237.
National Chagas Program Report (2017) Situation diagnosis. Available at: http://www.msal.gob.ar/chagas/index.php/ institucional/diagnostico
Nattero, J., Leonhard, G., Rodríguez, C.S., & Crocco, L.B. (2011) Infuence of the quality and quantity of blood ingested on reproductive parameters and life-span in Triatoma infestans(Klug). Acta Tropica, 119, 183-187.
Noireau, F., & Dujardin, J.P. (2001) Flight and nutritional status of sylvatic Triatoma sordida and Triatoma guasayana. Memórias do Instituto Oswaldo Cruz, 96, 385-389.
Ortíz, V., Rodríguez, C.S., López, A., Nattero, J., Soria, C., Carnicero, F., Lizarraga, M.A., & Crocco, L.B. (2015) Anexos peridomiciliarios potencialmente riesgosos para la presencia de triatominos en comunidades del oeste de la provincia de Córdoba, Argentina. In: Actas del IX Congreso Argentino de Entomología, 2015, Posadas. Pp. 10-35.
Payet, V., Ramirez-Sierra, M.J., Rabinovich, J., Menu, F., & Dumonteil, E. (2009) Variations in sex ratio, feeding, and fecundity of Triatoma dimidiata (Hemiptera: Reduviidae) among habitats in the Yucatan Peninsula, Mexico. Vector-Borne and Zoonotic Diseases, 9, 243-251.
Pinto, J., Cáceres, A.G., Vega, S., Martínez, R., & Náquira, C. (2008) Fuentes de alimentación de Panstrongylus herreri (Hemiptera: Triatominae) capturados en Utcubamba, Amazonas - Perú. Revista Peruana de Medicina Experimental y Salud Pública, 25, 179-184.
Rabinovich, J.E. (1972) Vital statistics of Triatominae (Hemiptera: Reduviidae) under laboratory conditions. I. Triatoma infestansKlug. Journal of Medical Entomology, 9, 351-370.
Salvatella, R. (1993) Los ciclos de transmisión de Tr ypanosoma cruzi(Chagas, 1909) (Protozoa, Mastigophora) en Uruguay. Revista Médica del Uruguay, 9, 55-64.
Salvatella, R., Calegari, L., Puime, A, Basmadjian, R.R., Guerrero, J., Martinez, M., Mendaro, G., Briano, D., Montero, C., & Wisnivesky-Colli, C. (1994) Perfl alimentario de Triatoma rubrovaria (Blanchard, 1843) (Hemiptera, Triatominae) en ámbitos peridomiciliarios, de una localidad rural de Uruguay. Revista do Instituto de Medicina Tropical de São Paulo, 36, 311-320.
Schofeld, C.J. (1980) Nutritional status of domestic populations of Triatoma infestans. Transactions of the Royal Society of Tropical Medicine and Hygiene, 74, 770-778.
Schofeld, C.J. (1982) The role of blood intake in density regulation of populations of Triatoma infestans (Klug) (Hemiptera: Reduviidae). Bulletin of Entomological Research, 72, 617-629.
Schofeld, C.J., Jannin, J., & Salvatella, R. (2006) The future of Chagas disease control. Trends in parasitology, 22, 583-588.
Teixeira, A.R., Hecht, M.M., Guimaro, M.C., Sousa, A.O., & Nitz, N. (2011) Pathogenesis of chagas' disease: parasite persistence and autoimmunity. Clinical Microbiology Reviews, 24, 592-630.
WHO -World Health Organization Expert Committee on the Control of Chagas Disease- (2002) Control of Chagas disease: second report of the WHO expert committee. Available at: http://www.who.int/iris/handle/10665/42443.
Wisnivesky-Colli, C., Gürtler, R.E., Solarz, N., Salomón, D., & Ruiz, A. (1982) Feeding patterns of Triatoma infestans (Hemiptera: Reduviidae) in relation to transmission of American Trypanosomiasis in Argentina. Journal of Medical Entomology, 19, 645-654.
Wisnivesky-Colli, C., Ruiz, A. M., Ledesma, O., Gütler, R.E., Lauricella, M., Salomon, D.O., & Segura, E.L. (1987) Ecología doméstica de la tripanosomiasis americana: perfl alimentario del Triatoma infestans en un área rural de la provincia de Santiago del Estero, Argentina. Revista da Sociedade Brasileira de Medicina Tropical, 20, 31-39.
Zeledón, R. (1976) Pan American Health Organization: New approaches in American trypanosomiasis research. Proceedings of an international symposium. Belo Horizonte Minas Gerais, Brazil, 18-21 March 1975. Scientifc Publication, Pan American Health Organization, 12, 326-329.














