Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de la Sociedad Entomológica Argentina
Print version ISSN 0373-5680On-line version ISSN 1851-7471
Rev. Soc. Entomol. Argent. vol.78 no.3 La Plata Sept. 2019
http://dx.doi.org/https://doi.org/10.25085/rsea.780301
https://doi.org/10.25085/rsea.780301
Artículo-Article
Evaluation of commercial products based on Isaria fumosorosea and Verticillium lecanii fungi as an alternative in the biocontrol of Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae)
Evaluación de productos comerciales basados en los hongos Isaria fumosorosea y Verticillium lecanii como alternativa en el control biológico de Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae).
GONZÁLEZ-MENDOZA, Daniel1, LEON-JIMENEZ, Eugenia1,4, ESTUDILLO-DIAZ, Enna1,2, MONTES DE OCA, Cristóbal1,2, RODRIGUEZ-HERNANDEZ, Ludwi3, MENDEZ-TRUJILLO, Vianey1,3,*, TZINTZUN-CAMACHO, Olivia1, DURAN-HERNANDEZ, Dagoberto1, GRIMALDO-JUAREZ, Onecimo1 & CECEÑA-DURAN, Carlos1
1 Instituto de Ciencias Agrícolas de la Universidad Autónoma de Baja California (ICA-UABC). Ejido Nuevo León, Baja California, México. * E-mail: ibqvianey2000@yahoo.com
2 Tecnológico Nacional de México/Instituto Tecnológico de Tuxtla Gutiérrez. Tuxtla Gutiérrez, Chiapas, México.
3 Tecnológico Nacional de México/Instituto Superior de Cintalapa. Cintalapa, Chiapas, México.
4 Universidad de Ciencias y Artes de Chiapas. Tuxtla Gutiérrez, Chiapas, México.
ABSTRACT. In the Northwest of Mexico, Phenacoccus solenopsis has been recognized as an aggressively invasive species on cotton plants. Therefore, the aim of the present study was to evaluate the effectiveness of two fungal commercial bioinsecticides (Verticillium lecanii and Isaria fumosorosea) against P. solenopsis under laboratory and semi-felds conditions. Our results showed a high mortality by V. lecanii (100%) followed by I. fumosorosea (40%) and control (0%) after 96 h of exposure. Thirty days after initiating the experiment, we observed a significant reduction of the disease index in cotton plants exposed to I. fumosorosea and V. lecanii (50% and 48%, respectively) compared with control (76%).
The use of V. lecanii and I. fumosorosea did not show significant changes in the physiological parameters of plants (chlorophyll, polyphenol contents, anthocyanins and potential photochemical yield values) compared with the control after 30 days of exposure to entomopathogenic fungi. Currently, the available information about mode of action of commercial formulations using I. fumosorosea and V. lecanii for biocontrol of P. solenopsis is scarce in Mexico compared with other countries. Therefore, studies focused on their effectiveness are necessary.
KEYWORDS. Bioinsecticides. Biological control. Cotton. Entomopathogens. Mealybug.
RESUMEN. En el noroeste de México, Phenacoccus solenopsis ha sido reconocida como una especie agresivamente invasiva en plantas de algodón. Por lo tanto, el objetivo del presente estudio fue evaluar la efectividad de dos bioinsecticidas fúngicos comerciales (Verticillium lecanii e Isaria fumosorosea) contra P. solenopsis en condiciones de laboratorio y semi-campo. Nuestros resultados mostraron una alta mortalidad por V. lecanii (100%) seguida de I. fumosorosea (40%) y control (0%) después de 96 horas de exposición. Después de treinta días de iniciar el experimento, se observó una reducción significativa del índice de severidad en plantas de algodón expuestas a I. fumosorosea y V. lecanii (50% y 48%) comparada con el control (76%). El uso de V. lecanii e I. fumosorosea no mostró cambios significativos en los parámetros fsiológicos de las plantas (valores de clorofla, contenido de polifenoles, antocianinas y eficiencia fotosintética) con respecto al control después de 30 días de exposición a los hongos entomopatógenos. Actualmente, la información disponible sobre el modo de acción de la formulación comercial empleando I. fumosorosea y V . lecanii para el control biológico de P. solenopsis es escasa en México en comparación con otros países. Por lo tanto, son necesarios estudios enfocados en su efectividad.
PALABRAS CLAVE. Algodón. Bio-insecticidas. Cochinilla harinosa. Control biológico. Entomopatógenos.
Mexicali valley is located in the municipality of Mexicali, Baja California and has an arable land surface and irrigated area of 210,930 hectares (ha) of which 184,283 ha belong to Mexicali and 26,648 ha to San Luis Río Colorado. Agricultural production has a great economic relevance in the Mexicali valley; for example, over 15,000 people are employed in the local agricultural sector producing mainly cotton (González-Soto et al., 2017). Particularly, the cotton crop sown in Mexico has been genetically modifed (GM) over twenty years (Rocha-Munive et al., 2018). GM cotton was authorized in Mexico since 1996 and the crop contains cry genes from Bacillus thurigensis (Bt) that confer resistance to larval stages of different lepidopteran pests (Terán-Vargas et al., 2005). The use of GM cotton has increased in Mexicali valley due to its high-yield production and management of pest control with reduced insecticide application (González-Soto et al., 2017).
Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) have been reported as a severe pest of different crops in Mexico (García-Espinoza et al., 2017). This pest is a polyphagous insect with wide physiological and ecological adaptations in different localities around the world (Nagrare et al., 2009; Wu et al., 2014). The pest management strategies include the combination of chemical insecticides and the culture practices to degrade the complex layer of wax of P. solenopsis (Jhala et al., 2010; Ahmad et al., 2011). Although the insecticides provide high efficiency against P. solenopsis these may cause resistance (Noureen et al., 2016). In this sense, the use of entomopathogenic fungi in the agriculture represents a viable alternative to reduce the chemical pesticides. Currently, the employment of entomopathogenic fungi such as Verticillium lecanii and Isaria fumosorosea has a great relevance due to the fact that they are environmentally friendly and they do not use toxic chemicals in their formulation (Huang et al., 2012). In addition, entomopathogenic fungi as Metarhizium anisopliae and Beauveria bassiana have been previously tested against the mealybug P. solenopsis under laboratory conditions (Ram & Saini, 2010; Ujjan et al., 2015). However, studies about the use of entomopathogenic fungi for control of invasive GM cotton mealybug are scarce in Mexico. Therefore, this study was conducted to evaluate the effectiveness of two fungal commercial bioinsecticides (V. lecanii and I. fumosorosea) against P. solenopsis under laboratory and semi-felds conditions.
Biological material
Phenacoccus solenopsis individuals were originally collected from infested GM cotton plants of Bollgard II variety of Deltapine 1044 B2RF during August 2018 in the Mexicali valley, Mexico. Branches of cotton plants infested with reproducing females of mealybugs were collected from cotton-felds located in Mexicali, Baja California, Mexico (Fig. 1). Identification of P. solenopsis in the samples was determined following the taxonomic keys of Hodgson et al. (2008). Insects were maintained on individual cotton leaves in a climate-controlled chamber (25 ± 3 °C, 60-70% RH, 12:12 photoperiod).
Laboratory bioassays
Two bioassays were conducted using commercial entomopathogenic formulae of I. fumosorosea (PAE-TRON®) and V. lecanii (VERTITRON®), respectively. For the frst bioassays, stock suspensions of conidia were prepared according to the manufacturer's recommendations: PAE-TRON (5 x 106 conidia/mL) and VERTITRON (1 x 109 conidia/mL). Cellular concentration was determined through serial dilutions (0.05% Tween 80 solution) using a Bio-Rad TC20 automated cell counter (Hercules, CA, USA). Stock suspensions were used for virulence bioassays with P. solenopsis and the percentage of viable conidia was determined prior to bioassay. Both commercial entomopathogenic fungi showed more than 90% of viable conidia using the trypan blue dye (Bio-Rad Laboratories, Hercules, CA, USA) and following the manufacturer´s protocol in a Bio-Rad TC20 automated cell counter. In the frst bioassays, the insecticidal activity of both commercial entomopathogenic fungi was evaluated and compared with the control (only water). Thirty insects of adult females (15 days old) were selected and carefully transferred to Petri dishes containing a cotton leaf (Fig. 2). Then, 2 mL aqueous solution containing PAE-TRON (5 x 106 conidia/mL) was sprayed on insects using a small hand atomizer (treatment 1). In the second treatment, the insects were sprayed with 2 mL VERTITRON (1 x 109 conidia/mL); and the third treatment, 2 mL of water was only sprayed (control). All experiments were randomized using triplicates by treatment. The number of P. solenopsis survivors (mortality percentages) was counted after 96 h of initial application. Insect mortality in the frst bioassays was calculated applying the following formula:
Insect Mortality (%) = (Dead insects/Total insects) x 100
which was corrected following Abbott (1925) if any mortality was observed in the control due to natural death.

Fig. 1. Cotton-field from Mexicali valley where Phenacoccus solenopsis was collected (A); Detail of damage in cotton plants by P. solenopsis (B).

Fig. 2. Selection and transferred of Phenacoccus solenopsis to Petri dishes
n mealybug
Semi-field bioassays
In the second bioassays, the efficacy of PAE-TRON® and VERTITRON® products against P. solenopsis population was evaluated under feld conditions on September, 2018. This test was carried out on a plot of cotton plants untreated with pesticides and located at Mexicali valley (32o 33' 33.6'' N; 115o 17' 35.2'' W). Three replicates were performed per treatment using 11 m long rows, 0.80 m row spacing and 0.30 m plant spacing. Four grades of mealybug damage severity were considered in the cotton plants according to Prabhakar et al. (2013): grade 11: scattered appearance of few mealybugs on the plant (<25%); grade 22: severe infestation of mealybug on any one branch or on less than half of the plant (25-49%); grade 33: severe infestation of mealybug on more than one branch or half portion of the plant (50-75%) and grade 44: severe infestation of mealybug on the whole plant (>75%). The experiments were conducted in plants with grade 3 of mealybugs damage severity and these were classifed as follows: PT) plants treated with PAE-TRON® (15 mL per plant containing 5 x 106 conidia/mL); VT) plants exposed to VERTITRON® (1 x 109 conidia/mL); C), plants treated with 15 mL of sterile water and 0.02% T ween 80 per plant (control). The plants were sprayed with suspensions of each treatment in the morning every other week for a month. The disease index (DX) and percentage of protection (PP) were calculated according to Zhu et al. (2013) based on the presence of mealybugs using one to four scales of mealybug infestation from ffty infested plants within each treatment and described as follows:
Disease index (DX) = [(0n0+1n1+2n2+3n3+4n4)/4n] × 100
Protection (%) = [DX (control) - DX (treatment)]/ DX (control)] × 100
where n0-n4 were the numbers of plants with each of the corresponding grade of mealybug infestation, and n was the total number of plants tested.
On the other hand, entomopathogenic fungi are well known for their pest control potential. However, new roles have been reported especially in relation to their ability to improve the physiological status of plants (e.g. : chlorophyll) (Yong-Seong & Kim, 2019). Therefore, the determination of physiological parameters as leaf chlorophyll (Chl), polyphenol contents (EPhen), anthocyanins (Anth) and potential photochemical yield (Fv/Fm) from cotton leaves of each treatment were evaluated in this study. Chl, EPhen, Anth and Fv/Fm were analyzed using the Dualex® scientific sensor (FORCE-A, Orsay, France) and Chlorophyll Fluorometer (OS-30p, OPTI-SCIENCE, USA), according to Gonzalez-Mendoza et al. (2017). Determinations were taken at 30 days after exposure to PAE-TRON® and VERTITRON® products using ffty plants of each treatment.
Data analysis was conducted using the Statistics software package Version 9.0. Differences between thetreatment's means were compared using Tukey's test (p = 0.05), and significant differences were accepted if p = 0.05, for each bioassay (laboratory and feld bioassays).
RESULTS
Laboratory bioassays
Figure 3 shows the changes in the mortality (%) of P. solenopsis after 96 h of the initial application of commercial product of I. fumosorosea (PAE-TRON®) and V. lecanii(VERTITRON®), respectively. The highest mortality (%) was recorded by V. lecanii(100%) followed by I. fumosorosea (40%) and the control (0%) after 96 h of exposure. The results showed significant toxicity of VERTITRON® against P. solenopsis after 96 h of exposure compared with the commercial product PAE-TRON® and the control.
Semi-field bioassays
On the other hand, after thirty days of the establishment of second experiment the lowest disease index of P. solenopsis was observed for the treatments compared with the control (Fig. 4). The results showed a significant reduction of insects exposed to I. fumosoroseaand V. lecanii(50% and 48%, respectively) compared with the control (76%). In this sense, the use of commercial VT and PT products showed a significantly increase of percentage of protection of cotton plants after 30 days of aplication of products (Fig. 5).
The use of VERTITRON® (V. lecanii) and PAE-TRON® (I. fumosorosea) did not show significant changes on Chl, EPhen and Anth values compared to the control (Table I). Measurements of chlorophyll a fuorescence did not show a significant decrease on potential photochemical yield (Fv/Fm) value of cootton plants exposed to VERTITRON® and water (control) after 30 days of treatments (Table I).
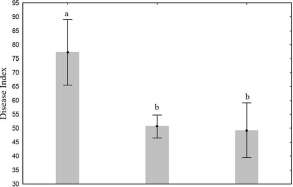
Water (control)
/. fnmosorosea (PT) Entomopathogenic fimgus
V.iecami (VT)
Fig. 4. Disease index in cotton plants exposed to Isaria fumosorosea and Verticillium lecanii after 30 days of treatment
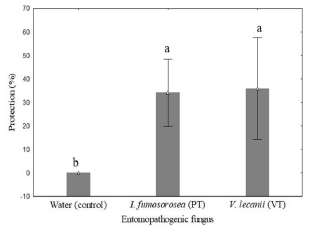
Fig. 5. Protection (%) in cotton plants exposed to Isaria fumosorosea and Verticillium lecanii after 30 days of treatment
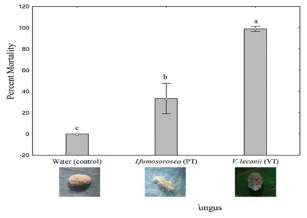
Entomopathogenic 1
Fig. 3. Insect mortality of Phenacoccus solenopsis by application of commercial product of Isaria fumosorosea and Verticillium lecanii after 96 hours of treatment.
DISCUSSION
In the present study, the commercial formulation of V. lecanii at 1 x 109 conidia/mL showed significant toxicity against P. solenopsis after 96 h of exposure compared with other commercial products of this entomopathogen. In this sense, Banu et al. (2010) reported that V. lecanii (2 x 108 CFU/g) showed less inhibitory effect against P. solenopis (42.22%) after 48 h of treatment. In addition, Kumar et al. (2012) reported a mortality maximum of 45.66% of this pest in the formulation containing V. lecanii (1 x 108 conidia/g) after fve days of exposure. On the other hand, I. fumosorosea has also shown a great potential to control different insect pests. However, the efficacy of commercial formulation for the biocontrol of adults of mealybug was lower (40%) in the present study.
Table I. Effects of commercial product based on Isaria fumosorosea (PT) and Verticillium lecanii (VT) on physiological parameters of cotton plants. Results are expressed as mean ± standard deviation of values from triplicate experiments. Values with the same letter within each line are equal according to the Tukey test at p = 0.5
| Treatments | Leaf chlorophyll Chl | Polyphenols EPhen | Anthocyanins Anth | Photochemical yield Fv/Fm |
| PAE-TRON® (PT) | 24.16 ± 0.41a | 1.89 ± 0.32a | 0.15 ± 0.08a | 0.60 ± 0.03a |
| VERTITRON® (VT) | 24.12 ± 0.58a | 1.93 ± 0.23a | 0.15 ± 0.01a | 0.57 ± 0.06a |
| | Control (water) | 24.32 ± 0.39a | 1.95 ± 0.68a | 0.16 ± 0.06a | 0.58 ± 0.05a |
Few data are available on the use of the commercial formulation of I. fumosorosea against mealybug in cotton plants in Mexico. However, recent studies indicated that I. fumosorosea has the potential to control the whitefy infestation of beans crops in nymphal stage, but not with the adults, which showed a very low susceptibility (Flores-Macas et al., 2013). Moreover, Huang et al. (2012) found that the chemical control caused an enhancement in the mortality of P. solenopis mainly on the frst instars but it did not occur for adult females after 5 days under laboratory conditions. In this sense, the present study showed that both myco-insecticides had a significant impact against P. solenopsis in comparison with some commonly chemical insecticides used as fubendamide, chlorantraniliprole and imidacloprid (Nagrare et al., 2016).
On the other hand, the commercial formulations of I. fumosorosea (5 x 106 conidia/mL) and V. lecanii (1 x 109 conidia/mL) had a significant positive impact on the reduction of the disease index as well as the increase of protection of cotton plants after 30 days of aplication. Although the mechanism of reduction of the pest is not known yet, some studies suggest that the insects could cause a significant loss of plant nutrients through their feeding damage. In addition, the plants seem to have a mechanism to recover those nutrients, through the entomopathogenic fungi (Raya-Díaz et al., 2017).
Previous studies have revealed that some entomopathogenic fungi (e.g.: Lacanicillium psalliotae) increase the leaf chlorophyll content compared with untreated plants (Kumar et al., 2012). In contrast, our results showed that the commercial formulations of I. fumosorosea and V. lecanii did not change the leaf chlorophyll, polyphenol contents, anthocyanins and potential photochemical yield in cotton plants infested with P. solenopsis. These results could be explained by the length of infestation time, the effect of different environmental conditions on herbivore activity, the herbivore densities, or the interaction with biological agents of control (Huang et al., 2013).
Currently, the available information about mode of action of commercial formulations using I. fumosorosea and V. lecanii for biocontrol of P. solenopsis is scarce in Mexico compared with other countries. Therefore, studies focused on their effectiveness are necessary.
ACKNOWLEDGEMENTS
Authors are thankful to the Secretary of Agricultural Development (SEDAGRO), Government of Baja California, Mexico, for providing fnancial assistance to develop this research project.
LITERATURE CITED
Abbott, W.S. (1925) A method for computing the effectiveness of an insecticide. Journal of Economic Entomology, 18, 265-267. [ Links ]
Ahmad, F., Akram, W., Sajjad, A., & Imran, A. (2011) Management practices against cotton mealybug, Phenacoccus solenopsis (Hemiptera: Pseudococcidae). International Journal of Agriculture and Biology, 13, 547-552 [ Links ]
Banu, J.G., Surulivelu, T., Amutha, M., & Gopalakrishnan, N. (2010) Laboratory evaluation of insecticide and biopesticides against Phenacoccus solenopsis and Paracoccus marginatus infesting cotton. Journal of Biopesticides, 3, 343-346. [ Links ]
Flores-Macías, A., Pucheta Díaz, M., Ramos-López, M., Rodríguez Navarro, S., Ramos-Espinosa, G., & Juárez-Ruiz, D. (2013) Estudio del hongo entomopatógeno Isaria fumosorosea como control microbiológico de la mosquita blanca Bemicia tabaci. Interciencia, 38, 523-527. [ Links ]
García-Espinoza, F., Salazar-Flores, C., Valdés-Perezgasga, M.T., López-Hernández, J., & Hernández-Hernández, V. (2017) Presencia de piojo harinoso (Hemiptera: Pseudococcidae) en Talipariti tiliaceum (L.) Fryxell (Malvaceae) en la comarca lagunera de Coahuila. Entomología Mexicana, 4, 363-368. [ Links ]
Gonzalez-Mendoza, D., Mendez-Trujillo, V., Grimaldo-Juarez, O., Ceceña-Duran, C., Tzintzun-Camacho, O., Gutierrez-Miceli, F., Sanchez-Viveros, G., & Aviles-Marin, M. (2017) Changes of photochemical efficiency and epidermal polyphenols content of Prosopis glandulosa and Prosopis juliforaleaves exposed to cadmium and copper. Open Life Sciences, 12, 373-378. [ Links ]
González-Soto, T .E., Moreno-Ramírez, L., T roncoso-Rojas, R., González-Mendoza, D., Sánchez-Estrada, A., Grimaldo Juárez, O., Tzinzun-Camacho, O., & Ceceña-Durán, C. (2017) Inoculation of Trichoderma longibrachiatum in transgenic cotton: change in the phenolics compounds and enzymes of oxidative stress. Idesia, 35, 19-24. [ Links ]
Hodgson, C., Abbas, G., Arif, M.J., Saeed, S., & Karkar, H. (2008) Phenacoccus solenopsis Tinsley (Sternorrhyncha: Coccoidea: Pseudococcidae), an invasive mealybug damaging cotton in Pakistan and India, with a discussion on seasonal morphological variation. Zootaxa, 1913, 1-35. [ Links ]
Huang, J., Zhang, J., Yu, Y.M., Lu, Y.B., & Luan, J.B. (2012) Biological characteristics and chemical control of the invasive mealybug, Phenacoccus solenopsis (Hemiptera: Pseudococcidae) on tomato in the laboratory. Journal of the Kansas Entomological Society, 85, 179-185. [ Links ]
Huang, J., Zhang, P.J., Zhang, J., Lu, Y.B., Huang, F., & Li, M.J. (2013) Chlorophyll content and chlorophyll fuorescence in tomato leaves infested with an invasive mealybug, Phenacoccus solenopsis (Hemiptera: Pseudococcidae). Environmental Entomology, 42, 973-979. [ Links ]
Jhala, R.C., Patel, M.G., & Bharpoda, T.M. (2010) Evaluation of insecticides for the management of mealybug, Phenacoccus solenopsis (Tinsley) in cotton. Karnataka Journal of Agricultural Sciences, 23, 101-102. [ Links ]
Kumar, R., Mukesh, N., Rahul, C., Vijender, P., & Kranthi, K.R. (2012) Evaluation of ecofriendly control methods for management of mealybug, Phenacoccus solenopsisTinsley in Cotton. Journal of Entomology, 9, 32-40. [ Links ]
Nagrare, V.S., Kranthi, S., Biradar, V.K., Zade ,N.N., Sangode, V., Kakde, G., Shukla, R.M., Shivare, D., Khadi, B.M., & Kranthi, K.R. (2009) Widespread infestation of the exotic mealybug species, Phenacoccus solenopsis (Tinsley) (Hemiptera: Pseudococcidae), on cotton in India. Bulletin of Entomological Research, 99, 537-541. [ Links ]
Nagrare, V.S., Kranthi, S., Kranthi, K.R., Naik, V.C.B., Deshmukh, V., Naikwadi, B., & Dahekar, A. (2016) Relative toxicity of insecticides against cotton mealybug Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) and its fortuous parasitoid Aenasius bambawalei Hayat (Hymenoptera: Encyrtidae). Journal of Applied and Natural Science, 8, 987-994. [ Links ]
Noureen, N., Hussain, M., Samman, F., & Mobeen, G. (2016) Cotton mealybug management: a review. Middle-East Journal of Scientific Research, 24, 2424-2430.
Prabhakar, M., Prasad, Y.G., Vennila, S., Thirupathi, M., Sreedevi, G., Rao, G.R., & Venkateswarlu, B. (2013) Hyperspectral indices for assessing damage by the Phenococcus solenopsis mealybug (Hemiptera: Pseudococcidae) in cotton. Computers and Electronics in Agriculture, 97, 61-70.
Ram, P., & Saini, R.K. (2010) Biological control of solenopsis mealybug, Phenacoccus solenopsis Tinsley on cotton: a typical example of fortuitous biological control. Journal of Biological Control, 24, 104-109.
Raya-Díaz, S., Sánchez-Rodríguez, A.R., Segura-Fernández, J.M., del Campillo, M.dC., & Quesada-Moraga, E. (2017) Entomopathogenic fungi-based mechanisms for improved Fe nutrition in sorghum plants grown on calcareous substrates. PLoS ONE,12, e0185903.
Rocha-Munive, M.G., Soberón, M., Castañeda, S., Niaves, E., Scheinvar, E., Eguiarte, L.E., Mota-Sánchez, D., Rosales-Robles, E., Nava-Camberos, U., et al. (2018) Evaluation of the Impact of Genetically Modifed Cotton After 20 Years of Cultivation in Mexico. Frontiers in Bioengineering and Biotechnology, 6, 82.
Terán-Vargas, A.P., Rodríguez, J.C., Blanco, C.A., Martínez-Carrillo, J.L., Cibrián-Tovar, J., Sánchez-Arroyo, H., Rodríguez-Del-Bosque, L.A., & Stanley, D. (2005) Bollgard cotton and resistance of tobacco budworm (Lepidoptera: Noctuidae) to conventional insecticides in the Southern Tamaulipas, Mexico. Journal of Economic Entomology, 98, 2203-2209.
Ujjan, A.A., Khanzada, M.A., & Shahzad, S. (2015) Efficiency of Metarhiziumspp. (Sorokīn) strains and insecticides against cotton mealybug Phenacoccus solenopsis (Tinsley). Pakistan Journal of Zoology, 47, 351-360.
Wu, F.Z., Ma, J., Hu, X.N., & Zeng, L. (2014) Homology difference analysis of invasive mealybug species Phenacoccus solenopsisTinsley in Southern China with COI gene sequence variability. Bulletin of Entomological Research, 105, 32-39.
Yong-Seong, L., & Kim, Y.C. (2019) Tobacco Growth Promotion by the Entomopathogenic Fungus, Isaria javanica pf185. Mycobiology, 47, 126-133.
Zhu, H.Q., Feng, Z.L., Li, Z.F., Shi, Y.Q., Zhao, L.H., & Yang, J.R. (2013) Characterization of two fungal isolates from cotton and evaluation of their potential for biocontrol of Verticillium wilt of cotton. Journal of Phytopathology, 161, 70-77.














