Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista de la Sociedad Entomológica Argentina
versión impresa ISSN 0373-5680versión On-line ISSN 1851-7471
Rev. Soc. Entomol. Argent. vol.79 no.3 La Plata set. 2020
http://dx.doi.org/https://doi.org/10.25085/rsea.790304
Artículo
https://doi.org/10.25085/rsea.790304
First record of Cloeon dípterum (L.) (Ephemeroptera: Baetidae) in Buenos Aires, Argentina
Primer registro de Cloeon dipterum (L.) (Ephemeroptera: Baetidae) en Buenos Aires, Argentina
Bárbara P. BANEGAS1 *
Juan I. TÚNEZ2
Carolina NIETO3
Agustina B. FAÑANI1
María A. CASSET2
Luciana ROCHA2
1Departamento de Ciencias Básicas, Universidad Nacional de Luján. Luján, Buenos Aires, Argentina. *E-mail: pame_lb3@hotmail.com
2Departamento de Ciencias Básicas, Universidad Nacional de Luján. INEDES (CONICET-UNLu). Luján, Buenos Aires, Argentina.
3Instituto de Biodiversidad Neotropical, CONICET, Universidad Nacional de Tucumán, Facultad de Ciencias Naturales. Tucumán, Argentina.
Received 01 - V- 2020
Accepted 26 - VIII - 2020
Published 28 - IX - 2020
ABSTRACT. Cloeon dipterum (L.) (Ephemeroptera: Baetidae) is widely distributed in temperate areas of Eurasia, whereas, in North America, it is restricted to three states of the USA and Canada, and, in South America to Chile. To confirm our hypothesis that C. dipterum has been recently introduced in Argentina, the aim of the present study was to identify the species based on molecular analyses and morphological features. To this end, nymphs were collected from three artificial habitats located in different localities of Buenos Aires province and two tributary streams of the Luján River, northeast of Buenos Aires, Argentina. Nymphs were all identified as belonging to C. dipterum thus confirming the presence of the species in Argentina. The distribution of C. dipterum in Buenos Aires, together with its absence from previously sampled sites, would indicate that the individuals recorded are introduced and already well established in the study area.
KEYWORDS. Aquatic insects. Neotropical waterbody. Non-native species.
RESUMEN. Cloeon dipterum (L.) (Ephemeroptera: Baetidae) está ampliamente distribuido en las zonas templadas de Eurasia, sin embargo, está restringido en América del Norte a tres estados de Estados Unidos y Canadá, y en América del Sur a Chile. Con el fin de confirmar nuestra hipótesis de que se introdujo recientemente en Argentina, el objetivo del presente estudio fue identificar la especie en base a análisis moleculares y caracteres morfológicos. Para ello se recolectaron ninfas de tres hábitats artificiales en diferentes localidades de la provincia de Buenos Aires y de dos arroyos afluentes del Río Luján, al noreste de Buenos Aires (Argentina). Todas las ninfas fueron identificadas como C. dipterum, lo que confirma la presencia de la especie en Argentina. La distribución de C. dipterum en Buenos Aires junto a su ausencia en los sitios previamente muestreados de manera regular, indicarían que los individuos registrados son introducidos y están bien establecidos en la zona de estudio.
PALABRAS CLAVE. Cuerpos de agua neotropicales. Especie exótica. Insectos acuáticos.
INTRODUCTION
The aquatic insect order Ephemeroptera has approximately 400 genera and over 3,000 species described worldwide (Barber-James et al., 2008). In South America, there are records of 671 species distributed in 14 families. In particular, in Argentina, there are records of 13 families, with most species belonging to the family Baetidae (Domínguez et al., 2019). In the province of Buenos Aires, only the genera Americabaetis Kluge and Callibaetis Eaton of this family have been reported (Hubbard et al., 1992; Ocon & Rodrígues Capítulo, 2004; Rigacci, 2009). Although Baetidae is a cosmopolitan family, its genera or species are not. In that sense, Cloeon Leach is almost an exception within the family. This genus was described by Leach in 1815, and, since then, numerous species, mainly from the Palearctic, Afrotropical Oriental realms, have been described (Leach, 1815; Salles et al., 2014). Rutschamnn et al. (2014) suggested that Cloeon is a monophyletic group, by reconstructing phylogenetic relationships with the genus Baetis. Du e to their long imaginal stages (Degrange, 1960; Oehme, 1972), Cloeon species seem to be excellent candidates to colonize new habitats. Such is the case of Cloeon dipterum (L.), one of the most common and abundant mayflies distributed throughout the temperate areas of Eurasia, a region that includes Europe, Siberia, Mongolia and Northeast Asia (Sowa, 1975; Bae & Park, 1997), and oceanic islands, such as Azores and Canary Islands (Rutschmann et al., 2014). Cloeon dipterum is currently considered a species - complex that includes seven genetic groups as it is shown by genetic data from samples from East Asia, North America and Europe (Yano et al., 2019). In addition, it is a eurytopic species, with a wide range of tolerance to environmental conditions (Velasco et al., 1998; Menetrey et al., 2008; Lock & Goethals, 2011), such as water pH (Vilenica et al., 2016) and temperature (McKee & Atkinson, 2000). It can also survive at low concentrations of oxygen by using an anaerobic mechanism, after long-term acclimatization in waters at temperatures close to 0 °C (Kamler, 1971; Nagell & Fagerstrom, 1978).
Since C. dipterum can be the main component in the diet of young predatory fish (Cianciara, 1979a; Mc Kee & Atkinson, 2000), its prevalence and abundance, particularly in spring and summer, can be of great ecological importance as an essential link in freshwater trophic networks. The nymphs of C. dipterum are swimmers, they colonize all kinds of still and standing waters and can be found among the riparian vegetation or bottom mud in ponds, lakes (Beketov & Lies, 2005; Jeffries, 2005) and peat bogs (Vilenica et al., 2016), as well as in artificial habitats (Gaino & Rebora, 2005; Salles et al., 2014; Vilenica et al., 2019).
Until a few years ago, C. dipterum was considered the only representative of the genus in the Western Hemisphere, restricted to the temperate zones of North America, in Illinois (Burks, 1953), Ohio (Traver, 1962), Pennsylvania (Sweeney et al., 2018) and Canada (Randolph et al., 2002). In South America, the first record of Cloeon was that by Salles et al. (2014) in the State of Espírito Santo in southeastern Brazil, where these authors recorded Cloeon smaeleni Lestage. Based on morphology and molecular evidence, they concluded that this species had been recently introduced in the country. Later, Vera et al. (2015) recorded C. dipterum in Limari and Maipo, Chile. They studied nymphs as well as male and female imagos, but omitted the evidence to confirm the first record of the species and date of collection.
Considering that C. dipterum had not been previously recorded in Argentina, the aim of the present study was to confirm the presence of this non-native species through molecular and morphological studies.
MATERIAL AND METHODS
Sampling of individuáis
Nymphs were collected from three swimming pools, one located in Navarro (St1) and two in General Rodríguez (St2 and St3), and two tributary streams of the Luján River, located northeast of Buenos Aires province (Carabassa stream -St4- and Burgueño stream -St5-). The geographical records of the species were mapped with QGIS (version 3.4.9) (Fig. 1).
The swimming pools from which the nymphs were collected were located in private homes and were of different dimensions: two of base dimensions 4.45 x 2.25 m and 0.80 m deep and one of base dimensions 4 x 7 m and 1.5 m deep; the bottoms of the three swimming pools were covered with leaf litter ofPinus sp., Populus sp., Juglans sp., Liquidambar sp. and Laurus nobilis L. The Carabassa and Burgueño streams had slow or zero current sectors, and were colonized by submerged (Egeria densa Planch, Potamogeton sp.), floating (Polygonum sp., Hydrocotile sp., Ludwigia sp., Sagittaria sp.) and emerging (Alternathera sp.) macrophytes. Both streams had riparian vegetation with trees and shrubs. All collection sites were characterized by low current velocity, slow levels of nitrates and low dissolved oxygen values, except St5 (Table I).
Nymphs were collected using a hand net of 1 mm pore size, while the adults were obtained by maintaining nymphs and breeding in the laboratory. All voucher specimens are deposited in the Colección de Invertebrados del Laboratorio de Ecología, Departamento de Ciencias Básicas de la Universidad Nacional de Luján, Buenos Aires, Argentina.
Material examined
A total of 160 individuals (111 nymphs and 49 adults) collected from three private swimming pools (St1, St2 and St3) and two tributary streams of the Luján River (St4 and St5) were examined (Table II).
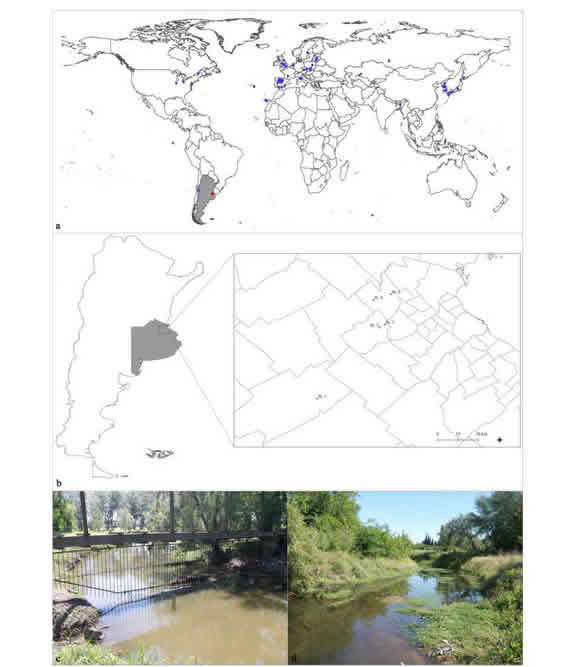
Fig. 1. Distribution of Cloeon dipterum. a. World map (grey, Argentina; blue circles, species distribution)*. b. Map of Argentina with detail of Buenos Aires province and collection stations (red circles) [St1: Navarro, St2 and St3: General Rodríguez, St4: Carabassa Stream (Pilar), St5: Burgueño Stream (Pilar)]. c. Carabassa Stream. d. Burgueño Stream.
* Based on Wingfield, 1939; Burks, 1953; Brown, 1961; Traver, 1962; Kjellberg, 1973; Sowa, 1975; Nagell, 1977; Nagell & Fagerstrom, 1978; Cianciara, 1979b; González del Tánago, 1984; Craig, 1990; Van Wijngaarden, 1993; Silina, 1994; Gupta et al., 1994; Bae & Park, 1997; Velasco et al., 1998; McKee & Atkinson, 2000; Randolph et al., 2002; Jeffries, 2005; Beketov & Liess, 2005; Cayrou & Céréghino, 2005; Gaino & Rebora, 2005; Menetrey et al., 2008; Lupetti et al., 2011; Lee et al., 2013; Camp et al., 2014; Rutschmann et al., 2014, 2016; Salles et al., 2014; Vera et al., 2015; Supina et al., 2016; Vilenica et al., 2016, 2019; Rico et al., 2018; Sweeney et al., 2018; Almudi et al., 2019; Yano et al., 2019.
Table I. Physicochemical variables measured in the sampled sites.
Molecular analyses
For the genetic identificaron of the nymphs, the DNA was extracted from four entire individuáis fixed and preserved in 96% ethanol by the phenol-chloroform method (Sambrook et al., 1989). Fragments of the mitochondrial DNA Cytochrome Oxidase subunit I (COI) gene of approximately 650 bp were amplified using primers LCO 1490 (GGTCAACAAATCATAAAGATATTGG) and HCO 2198 (TAAACTTCAGGGTGACCAAAAAATCA) (Folmer et al., 1994). Each PCR had a reaction volume of 20 pl and contained 2 pl of 10X Pegasus Taq DNA Polymerase Buffer, 0.6 pl of 50 mM MgCl2, 0.4 pl of 10 mM deoxynucleotide triphosphates, 1 pl of 10 pM forward and reverse primers, 0.1 pl of 5 U/pl Pegasus Taq Polymerase (EmbioTec), 5 pl of DNA extract and water to reach the final volume. The amplification protocol consisted of a single denaturation step at 95 °C for 2 min, followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 40 °C for 30 s, extension at 72 °C for 1 min, and a final extension at 72 °C for 5 min.
Amplificaron reactions were carried out in a MasterCycler (Eppendorf) thermal cycler. PCR products were resolved in 1% agarose gel electrophoresis and visualized under UV light. Then, 15 pl of each PCR product was purified using the enzymatic method EXO/ SAP (Werle et al., 1994) and sent to an external laboratory (Macrogen Inc., Korea) for direct sequencing using the same oligonucleotide primers. The sequences obtained for the COI gene were manually edited using Chromas 2.6 software (http://www.technelysium.com.au/ chromas.htm) and aligned using ClustalX 2.1 (Thompson et al., 1997). Then, they were compared with the sequences for different species of the family Baetidae available in GenBank by using the Basic Local Alignment Search Tool (BLAST). Finally, a phylogenetic analysis was performed by the Maximum Parsimony method using the Molecular Evolutionary Genetics Analysis (MEGA-X) software (Kumar et al., 2018) with 1,000 bootstrap replications. The COI sequences obtained were related to sequences representing each of the seven genetic haplogroups described for C. dipterum (Accession numbers: KU757091, KU757111-21-29-30-36, and LC223543; Yano et al., 2019) and sequences belonging to other Cloeon or Baetidae species available in GenBank: Cloeon simile Eaton, C. smaeleni, Cloeon praetextum Bengtsson, and Callibaetis sp. (Accession numbers: KY262243, HG935107, JN299150 and HM917067, respectively). A sequence belonging to Baetis sp. (Accession number HQ152334) was used as outgroup.
Morphological features of nymphs and imagos
For morphological identificaron of nymphs, specific publications of the genus and species of Cloeon were consulted (Sowa, 1975; Bae & Park, 1997; Webb & Suter, 2011; Salles et al., 2014) and the following characteristics were examined: number and shape of abdominal gills, presence of rows of lateral spines in segments VIII and IX, color pattern of terminal filaments, and number and shape of segments of the maxillary palps and labial palps. In the imagos, we examined the following: venation of the forewings, male genitalia, shape and coloration of eyes, and coloration of abdomen and terminal filaments.
Nymphs and imagos were measured with a ruler millimeter under a stereoscopic microscope. Length was measured from the apex of the head to the last segment of the abdomen.
RESULTS
Molecular analyses
Mitochondrial DNA COI gene fragments with a length of 620, 450 and 613 bp were obtained from three of the four analyzed samples (Accession numbers: MT786862, MT786863, MT786864). When these haplotypes were compared with those reported by Yano et al. (2019), they showed the highest values of percent identity with the genetic groups CT2-3 (range: 95.33 to 98.87% identity), intermediate values with the groups CT1 and JK (range: 92.01 to 92.43% identity) and the lowest values with the groups IS1-3 (range: 88.42 to 90.31% identity). The Maximum Parsimony phylogenetic tree showed that the haplotypes found in north-eastern Buenos Aires province (C. dipterum I, II and IV) constitute a clade of high reliability (99% bootstrap value) with the genetic groups CT2-3 (Fig. 2). All these sequences were then grouped with the rest of the C. dipterum sequences in another clade, with a high degree of reliability (99% bootstrap value). Moreover, the sequences from north-eastern Buenos Aires province showed a clear differentiation with the other Cloeon species and with Callibaetis, a genus with which it shares similar morphological features.
Morphological features of nymphs and imagos
The main diagnostic characteristics of the nymphs (5-7 mm) were: I) the terminal filaments, which have black rings located interspersed to the middle zone, located next to each other forming three black spots, one in each filament (characteristic of the species) (Fig. 3a), and II) the dorsal surface of the abdomen of mostly dark coloration with two small central yellow spots oriented obliquely backwards (Fig. 3b). Also, the presence of rounded double gills (similar to plates with tracheas) in segments I to VI, simple gills in segment VII (Fig. 3b), and rows of lateral spines in segments VIII and IX (Fig. 3c) were useful to distinguish this species. The morphological features of the labrum (Fig. 4a), hypopharynx (Fig. 4b), mandibles (Fig. 4c, d), and tarsal claws allowed us to separate Cloeon from Callibaetis found in the same collection sites as C. dipterum. Moreover, the maxillary and labial palps were composed of three segments (Fig. 4e, f), which is characteristic of the species of the genus Cloeon present in South America.
The main diagnostic characteristics of adults (6-8 mm) were the forewings, with single intercalary veins and absence of hindwings (Fig. 5a, c). The coloration of the body is orange-yellowish with rust-colored circular spots on the abdomen. The eyes in males are turbinate, from medium to large widened upwards (Fig. 5a), whereas in females the compound eyes are ornamented with two brown longitudinal bands. In the male genitalia the forceps are thin and their basal segment, whitish, is distinctly shorter than the second, being these strongly separated from the third. The base of the third segment has a small spur (Fig. 5b). The penis is a cone bent backwards, more strongly sclerotized on the ventral side, attached to the body with two arms, and can be attached back and move up and down. The terminal filaments of both sexes are clear with black rings.
Cloeon dipterum I Cloeon dipterum IV Cloeon dipterum II Cloeon dipterum (KU757130-CT2) Cloeon dipterum (KU757129-CT3) Cloeon dipterum (KU757111-CTl) Cloeon dipterum (LC223543-JK) Cloeon dipterum (KU757091-IS3) Cloeon dipterum (KU757121-IS2) Cloeon dipterum (KU757136-IS1) Cloeon smaeleni (HG935107)
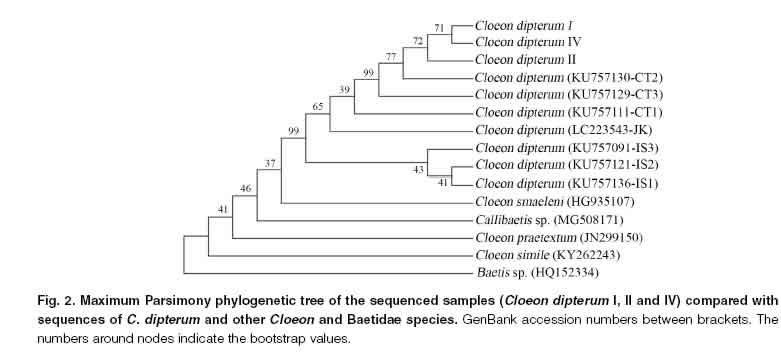

Fig. 3. Cloeon dipterum. a. Nymph. b. Male nymph, dorsal view. c. Detail of the male nymphal tergite IX. Scale bars: 500 pm.
DISCUSSION
Our results from molecular and morphological analyses confirmed the hypothesis that C. dipterum was introduced in the province of Buenos Aires (Argentina), representing the first record of the species in freshwaters habitats of slow current or stagnant waters in the country. The features of the body observed in nymphs and imagos, as well as the description of each of the mouthparts made in a previous study (Banegas et al., 2020), agree with those belonging to C. dipterum described by Brown (1961) and Sowa (1975). It is known that C. dipterum belongs to a complex of species, including species such as Cloeon cognatum Stephens, Cloeon inscriptum Bengtsson, Cloeon rabaudi Verrier, Cloeon saharense Soldán & Thomas and Cloeon peregrinator Gattolliat & Sartori (Rutschmann et al., 2016). Due to this, some authors have included some of these species within C. dipterum in studies of reconstruction of the phylogenetic relationships of Ephemeroptera (Rutschmann et al., 2014, 2016; Yano et al., 2019).
The haplotypes obtained through molecular analyses showed a high percentage of identity with the haplogroups CT2-3 described by Yano et al. (2019) for C. dipterum, which indicates that the samples analyzed belong to that species. The sequences of C. dipterum used in the phylogenetic tree were part of the same clade, with a high support (99% bootstrap value), which would indicate the monophyly of C. dipterum. It is remarkable, though, that Callibaetis sp. was recovered within the clade of the genus Cloeon. This could have been caused by an identification error in the only available sequence of Callibaetis, so this material should be reviewed to verify its identity, but this exceeds the aim of our work. It should also be noticed that nymphs of both genera have similar morphological features (Domínguez & Fernández, 2009; Webb & Suter, 2011; Salles et al., 2014): gills on abdominal segments I-VI with double and rounded lamellae and tarsal claws pointed towards the apex with two rows of cylindrical denticles. Moreover, they share the same habitats: low current bodies of water (Nieto, 2008), and are both currently distributed in Buenos Aires province; they were found in the same sampling sites. However, there are not previous studies in which the phylogenetic relationships between Callibaetis and Cloeon are reconstructed. Therefore, we consider that the inclusion of a greater number of species is necessary to reconstruct the evolutionary histories between the different mayflies.
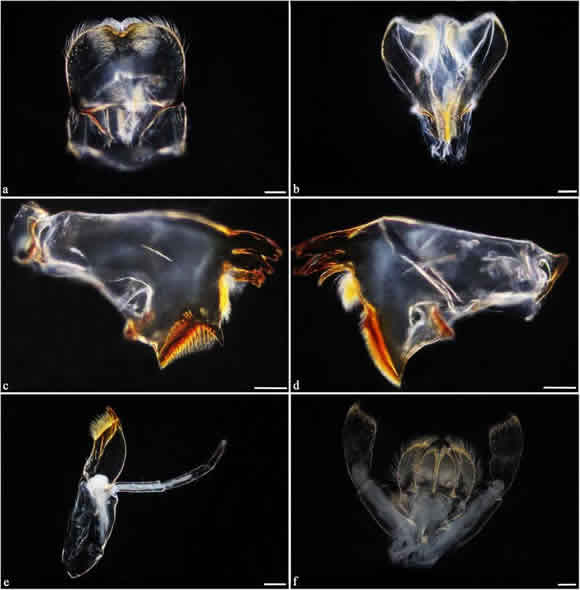
Fig. 4. Cloeon dipterum, dorsal view. a. Labrum. b. Hypopharynx. c. Left mandible. d. Right mandible. e. Right maxilla. f. Labium. Scale bars: 100 pm.
The distribution of C. dipterum in Buenos Aires and its absence from previously sampled habitats would indicate that the individuals collected are introduced. Yet, it is still unknown how and where this introduction has occurred. We could speculate that the ovoviviparity, the viability of female imagos and the great tolerance of C. dipterum nymphs to environmental factors would allow their establishment even in temporary water bodies, facilitating their dispersión (Sowa, 1975). In addition, female imagos may often be found quite far from the water body where they developed as nymphs and are able to actively disperse into new habitats, all of which makes them a true colonizing species (Menetrey et al., 2008). Cloeon dipterum is abundant in temporary ponds, slow-moving streams, pools and peat bogs (Beketov & Liess, 2005; Jeffries, 2005; Vera et al., 2015; Vilenica et al., 2016), and imagos have even been collected from windows inside rooms (Vera et al., 2015).

. b. Male genitalia. c. Female imago, lateral view. Scale bars: 500 pm
Fig. 5. Cloeon dipterum. a. Male imago, lateral view (a-c); 30 pm (b).
In the swimming pools sampled, we found the nymphs exclusively among organic substrates such as leaf litter, and were very abundant in spring and summer.
As concluded by Salles et al. (2014), it is difficult to predict the impact caused by the presence of non-native species on the coast of Brazil or Argentina. However, it is clear that Cloeon species inhabit the same habitats as Callibaetis ones. Long-term studies of population dynamics are essential to evaluate the effect of non-native species on native species and their habitats.
ACKNOWLEDGMENTS
We are grateful to Soledad Byrne for her collaboration in molecular analyses, Dr. Frederico Salles and Dr. Carlos Molineri for their collaboration in the morphological identification and Agustina Silvera for collecting the nymphs. We thank Dr. Fernando Momo for reading and his comments of the manuscript. This work was funded by Departamento de Ciencias Básicas, Universidad Nacional de Luján (PI4 project, DISPCD-CBLUJ:0000503-18), Buenos Aires, Argentina.
LITERATURE CITED
Almudi, I., Martín-Blanco, C.A., García-Fernández, I.M., López-Catalina, A., Davie, K., Aerts, S., & Casares, F. (2019) Establishment of the mayfly Cloeon dipterum as a new model system to investigate insect evolution. Evo-Devo, 10(1), 6. [ Links ]
Bae, Y.J., & Park, S.Y. (1997) Taxonomy of Cloeon and Procloeon (Ephemeroptera: Baetidae) in Korea. TheKorean Journal of Sistematic Taxonomy, 13(4), 303-314. [ Links ]
Banegas, B.P., Casset, M.A., Silvera, A., & Rocha, L. (2020) Mouthpart morphology and food habits of a pampean population of Cloeon dipterum (Linnaeus, 1761)
(Ephemeroptera: Baetidae). Annales de Limmnologie -International Journal of Límnology, 56, 21.
Barber-James, H.M., Gattolliat, J.L., Sartori, M., & Hubbard, M.D. (2008) Global diversity of mayflies (Ephemeroptera, Insecta) in freshwater. Hydrobiologia, 595, 339-350. [ Links ]
Beketov, M.A., & Liess, M. (2005) Acute contamination with esfenvalerate and food limitation: chronic effects on the mayfly, Cloeon dipterum. Environmental toxicology and chemistry, 24(5), 1281-1286. [ Links ]
Brown, D.S. (1961) The morphology and functioning of the mouthparts of Cloeon dipterum L. and Baetis rhodani (Pictet) (Insecta, Ephemeroptera). Proceedíngs of the Zoological Society of London, 136(2), 147-176. [ Links ]
Burks, B.D. (1953) The mayflies, or Ephemeroptera, of Illinois. Illinois Natural History Survey Bulletin, 26, 1-216.
Camp, A.A., Funk, D.H., & Buchwalter, D.B. (2014) A stressful shortness of breath: molting disrupts breathing in the mayfly Cloeon dipterum. Freshwater Science, 33(3), 695-699.
Cayrou, J., & Céréghino, R. (2005) Life-cycle phenology of some aquatic insects: implications for pond conservation. Aquatic Conservation: Marine and Freshwater Ecosystems, 15(6), 559-571.
Cianciara, S. (1979a) Some study on the biology and bioenergetics of Cloeon dipterum (L.) Ephemeroptera (preliminary data). Proceeding Second International Conference ofEphemeroptera (ed. Pasternak, K., & Sowa, R.), pp 175-219. Kracow, Poland.
Cianciara, S. (1979b) Life cycles of Cloeon dipterum (L.) in natural environment. Poliskie Archiwum Hydrobiologii, 26, 501-513.
Craig, D.A. (1990) Behavioural Hydrodynamics of Cloeon dipterum Larvae (Ephemeroptera: Baetidae). Journal of the North American Benthological Society, 9(4), 346-357.
Degrange, C. (1960) Recherches sur la reproduction des Ephéméroptéres. Travaux du Laboratoire de Písciculture de l'Université de Grenoble, 50-51, 7-193.
Domínguez, E., & Fernández, H.R. (2009) Macroinvertebrados bentónícos sudamericanos: sistemática y biología. Fundación Miguel Lillo, Tucumán.
Domínguez, E., Molineri, C., Nieto, C., & Zúñiga, M.C. (2019) Lista de especies de Ephemeroptera Sudamericanos. (Available at: http://ibn-conicet.nob.ar/) [Last access July 2019].
Folmer, O., Black, M., Hoeh, W., Lutz, R., & Vrijenhoek, R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294-299.
Gaino, E., & Rebora, M. (2005) Egg envelopes of Baetis rhodani and Cloeon dipterum (Ephemeroptera, Baetidae): a comparative analysis between an oviparous and an ovoviviparous species. Acta Zoologica, 86(1), 63-69.
Gonzáles del Tánago, M. (1984) Distribución y biología de la familia Baetidae (Ephem.) en la Cuenca del Duero. Boletín de la Asociación española de Entomología, 8, 73-94.
Gupta, A.B.H.I.K., Gupta, S., & Michael, R.G. (1994) Seasonal abundance and diet of Cloeon sp. (Ephemeroptera: Baetidae) in a northeast Indian lake. Archiv fur Hydrobiologie, 130, 349-349.
Hubbard, M.D., Domínguez, E., & Pescador, M.L. (1992) Los Ephemeroptera de la República Argentina: un catálogo. Revista de la Sociedad Entomológica Argentina, 50, 201-240.
Jeffries, M. (2005) Local-scale turnover of pond insects: intra-pond habitat quality and inter-pond geometry are both important. Hydrobiologia, 543(1), 207-220.
Kamler, E. (1971) Reactions the two species of aquatics insects to the changes of temperatura and oxygen concentration. Poliskie Archiwum Hydrobiologii, 18, 303-323.
Kjellberg, G. (1973) Growth of Leptophlebia vespertina L., Cloeon dipterum L. and Ephemera vulgate L. (Ephemeroptera) in a small woodland Lake. Entomoiogisk Tidskrift, 94, 8-14.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution, 35, 1547-1549.
Leach, W.E. (1815) Entomology. Edinburgh Encyclopaedia (ed. Brewster, D.), pp 57-172. Edimburgh, Scotland.
Lee, C.Y., Kim, D.G., Baek, M.J., Choe, L.J., & Bae, Y.J. (2013) Life History and Emergence Pattern of Cloeon dipterum (Ephemeroptera: Baetidae) in Korea. Environmental Entomology, 42(6), 1149 -1156.
Lock, K., & Goethals, P.L. (2011) Distribution and ecology of the mayflies (Ephemeroptera) of Flanders (Belgium). Annales de Limnologie-International Journal of Limnology, 47(2), 159-165.
Lupetti, P., Mencarelli, C., Mercati, D., Gaino, E., & Dallai, R.
(2011) The spermatodesm of Cloeon dipterum (L.): Fine structure and sperm movement. Tissue and Cell, 43(3), 157-164.
McKee, D., & Atkinson, D. (2000) The influence of climate change scenarios on populations of the mayfly Cloeon dipterum. Hydrobiologia, 441, 55-62.
Menetrey, N., Oertli, B., Sartori, M., Wagner, A., & Lachavanne, J.B. (2008) Eutrophication: are mayflies (Ephemeroptera) good bioindicators for ponds? Hydrobiologia, 597, 125-135.
Nagell, B. (1977) Phototactic and thermotactic responses facilitating survival of Cloeon dipterum (Ephemeroptera) larvae under winter anoxia. Oikos, 29, 342-347.
Nagell, B., & Fagerstrom, T. (1978) Adaptations and resistance to anoxia in Cloeon dipterum (Ephemeroptera) and Nemoura cínerea (Plecoptera). Oikos, 30, 95-99.
Nieto, C. (2008) The larvae of some species of Callibaetis Eaton (Ephemeroptera: Baetidae). Aquatic Insects, 30(3), 229-243.
Ocon, C.S., & Rodrigues Capítulo, A. (2004) Presence and abundance of Ephemeroptera and other sensitive macroinvertebrates in relation with habitat conditions in pampean streams (Buenos Aires, Argentina). Archiv für Hydrobiologie, 159 (4), 473-487.
Oehme, G. (1972) Zur maximalen Lebensdauer von Cloeon dipterum L. (Eph. Baetidae). Entomologische Nachrichten, 16, 131-133.
Randolph, R.P., Mc Cafferty, W.P., Zaranko, D., Jacobus, L.M., & Webb, J.M. (2002) New Canadian records of Baetidae (Ephemeroptera) and adjustments to North American Cloeon. Entomologicalnews, 113(5), 306-309.
Rico, A., Arenas-Sánchez, A., Pasqualini, J., García-Astillero, A., Cherta, L., Nozal, L., & Vighi, M. (2018) Effects of imidacloprid and a neonicotinoid mixture on aquatic invertebrate communities under Mediterranean conditions. Aquatic Toxicology, 204, 130-143.
Rigacci, L.N. (2009) Estudio de invertebrados bentónicos en arroyos con diferente vegetación ribereña. Tesis de grado. Buenos Aires, Argentina, Universidad Nacional de Luján.
Rutschmann, S., Gattolliat, J.L., Hughes, S.J., Baez, M., Sartori, M., & Monaghan, M.T. (2014) Evolution and island endemism of morphologically cryptic Baetis and Cloeon species (Ephemeroptera, Baetidae) on the Canary Islands and Madeira. Freshwater Biology, 59(12), 2516-2527.
Rutschmann, S., Detering, H., Simon, S., Funk, D.H., Gattolliat, J.L., Hughes, S.J., Raposeiro, P.M., DeSalle, R., Sartori, M., & Monaghan, M.T. (2016) Colonization and diversification of
aquatic insects on three Macaronesian archipelagos using 59 nuclear loci derived from a draft genome. Molecular Phylogenetics and Evolution, 107, 27-38.
Salles, F.F., Gattolliat, J.L., Angeli, K.B., De-Souza, M.R., Gongalves, I.C., Nessimian, J.L., & Sartori, M. (2014) Discovery of an alien species of mayfly in South America (Ephemeroptera). ZooKeys, 399, 1-16.
Sambrook, J., Fritsch, E.F., & Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Press, New York, EEUU.
Silina, A.E. (1994) Ecological Peculiarities of the Sympatric Species of Mayflies, Cloeon dipterum L. and C. inscríptum Btss. (Ephemeroptera, Baetidae). Entomological Review, 73(7), 43-49.
Sowa, R. (1975) What is Cloeon dipterum (Linnaeus, 1761)? The nomenclatural and morphological analysis of a group of the European species of Cloeon Leach (Ephemerida: Baetidae). Entomologica Scandinavia, 6, 215-223.
Supina, J., Bojková, J., & Boukal, D.S. (2016) Influence of food
availability, predation risk and initial body size on growth and maturation of Cloeon dipterum (Ephemeroptera: Baetidae). Zoosymposia, 11,53-64.
Sweeney, B.W., Funk, D.H., Camp, A.A., Buchwalter, D.B., & Jackson, J.K. (2018) Why adult mayflies of Cloeon dipterum (Ephemeroptera: Baetidae) become smaller as temperature warms. Freshwater Science, 37(1), 64-81.
Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F., & Higgins, D.G. (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25, 4876-4882.
Traver, J.R. (1962) Cloeon dipterum (L.) in Ohio (Ephemeroptera: Baetidae). Bulletin of the Brooklyn
Entomological Society, 57, 47-50.
Van Wijngaarden, R. (1993) Comparison of response of the mayfly Cloeon dipterum to chlorpyrifos in a single species toxicity test, laboratory microcosms, outdoor ponds and experimental ditches. Science ofthe Total Environment, 134, 1037-1046.
Velasco, J., Suárez, M.L., & Vidal-Abarca, M.R. (1998) Factores que determinan la colonización de insectos acuáticos en pequeños estanques. Oecologia aquatica, 11, 87-99.
Vera, A., Ojeda, P., Orostica, A., & Muñoz, F. (2015) Catálogo actualizado de los Baetidae (Ephemeroptera) presentes en chile y su distribución geográfica. Revista Chilena de Entomología, 40, 37-50.
Vilenica, M., Brigic, A., Kerovec, M., Gottstein, S., & Ternjej, I. (2016) Spatial distribution and seasonal changes of mayflies (Insecta, Ephemeroptera) in a Western Balkan peat bog. ZooKeys, 637, 135-149.
Vilenica, M., Vuckovic, N., & Mihaljevic, Z. (2019) Littoral mayfly assemblages in South-East European man-made lakes. Journal of Limnology, 78(1), 47-59.
Webb, J.M., & Suter, P.J. (2011) Identification of Larvae of Australian Baetidae. Museum Victoria Science Reports, 15, 1-24.
Werle, E., Schneider, C., Renner, M., Volker, M., & Fiehn, W. (1994) Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Research, 22, 4354-4355.
Wingfield, C.A. (1939) The function of the gills of mayfly nymphs from different habitats. Journal of Experimental Biology, 16(3), 363-373.
Yano, K., Takenaka, M., &Tojo, K. (2019) Genealogical Position of Japanese Populations of the Globally Distributed Mayfly Cloeon dipterum and Related Species (Ephemeroptera, Baetidae): A Molecular Phylogeographic Analysis. Zoological Science, 36(6), 479-489.














