INTRODUCTION
ver the past two decades the poultry industry has experienced a rapid growth, with chicken meat and eggs providing high-quality protein, vitamins and oligoelements (Farrell, 2013). The warm and humid environment in which the birds are reared favours the development of different organisms including the darkling beetle Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae) (Cecco et al., 2005). This cosmopolitan beetle causes serious economic losses, affecting birds, breeding facilities and farm workers. Larvae and adults act as mechanical vectors of bacteria, virus, and fungi, including Bacillus spp., Campylobacter spp., Escherichia coli, Pseudomonas aeruginosa, Salmonella spp., and Staphylococcus spp. (Roche et al., 2009; Agabou & Alloui, 2010; Ou et al., 2012;Crippen et al., 2018; Silva Soares et al., 2018), thus favouring the dispersion of pathogenic microorganisms. In addition, when they found in large numbers, injure the birds’ skin causing a traumatic effect (Silva et al., 2005; Uemura et al., 2008). Furthermore, this insect damages breeding facilities (Japp et al., 2010), and causes allergies to people working on farms (Schroeckenstein et al., 1988).
The use of synthetic insecticides is the most common method to control A. diaperinus, with these products being applied by spraying the floor and walls before the replacement of the litter for the next breeding cycle to avoid direct contact with birds (Salin et al., 2003). However, loss of field efficacy of conventional insecticides and resistant A. diaperinus populations have been reported (Lambkin & Furlong, 2011; Arena et al., 2018; Hickmann et al., 2018), which has led to a great interest in the development of alternatives for managing this pest.
Plants produce several secondary metabolites which play an important role in defence against pathogens and herbivores. These compounds affect the behaviour of insects by acting as a deterrent or repellent, or alter development by affecting the digestion and assimilation of food, and some of them have a toxic effect (War et al, 2012). The use of botanical products as biopesticides have certain advantages over synthetic insecticides: they are biodegradable, have low persistence in the environment, and their toxicity to mammals and other vertebrates is relatively low (Gonpalves Marques et al., 2013). In addition, plant extracts are complex mixtures of compounds with different mechanisms of action, which reduces the probability of generating resistance (Akami et al., 2019).
Little is known about the effects of botanical extracts on A. diaperinus. Aqueous, acetonic, and ethanolic extracts obtained from Ipomea fistulosa Mart. ex Choisy (Convolvulaceae), Datura fastuosa L. (Solanaceae), Eucalyptus citriodora (Hook.) K.D. Hill & L.A.S. Johnson (Myrtaceae), Helitropium indicum L. (Boraginaceae), Hedyotis corymbose L. (Rubiaceae), and Sapium indicum Willd. (Euphorbiaceae) from Bangladesh have shown antifeedant effect on A. diaperinus
(Kamruzzaman et al., 2005). In addition, when adult insects were sprayed with an aqueous extract obtained from Azadirachta indica A. (Meliaceae) and an ethanolic extract from Ruta graveolens L. (Rutaceae), mortalities of 98 and 61%, respectively, were produced (Marcomini et al., 2009).
Modifying the environment by providing inadequate sources of food could help reduce the harmful effects of A. diaperinus (Szczepanik et al., 2005). For example, the addition of natural compounds that affect the feeding behaviour of this insect could contribute to the decline of its populations on poultry farms. The aim of this work was to evaluate the effects of exposure of A. diaperinus to food treated with different extracts obtained from plants native from central region of Argentina on the feeding behaviour, development, and survival of this beetle.
MATERIAL AND METHODS
Insects
All the insects used in this study came from laboratory-reared colonies that were started with field-collected individuals from Colonia Caseros, Entre Ríos, Argentina. The coleopterans were maintained in plastic container under controlled temperature and relative humidity (25 ± 1 °C and 60 ± 5% RH) in darkness (Del Valle et al., 2016). A high-density polystyrene sheet was placed in the containers, which served as a site for the larvae to pupate. Insects were provided with a mixture of wheat bran and poultry feed (Rice & Lambkin, 2009).
Plant extractsThe extracts were provided by Fine Chemistry Laboratory of the Catholic University of Córdoba (UCC), which had been obtained from the aerial part of plants belong to families Asteraceae (Gaillardia megapotamica (Spreng.) Baker (UCCOR 127), Trichocline reptans
(Wedd.) Hieron (UCCOR 244), Flourensia oolepis Blake (UCCOR 135), Vernonanthura nudiflora (Less.) H. Rob (UCCOR 129), Baccharis artemisioides Hook. & Arn. (UCCOR 142), B. coridifolia DC (UCCOR 147), Ambrosia artemisiifolia L. (UCCOR 215), Polygonaceae
(Ruprechtia apetala Wedd) (UCCOR 151), and Anacardiaceae (Lithraea molleoides (Vell.) Engl) (UCCOR 183). The specimens were collected in the province of Córdoba, Argentina, in the region of Chaco Serrano (30° 25' to 31° 59' S; 64° 21' to 65° 00' W), and deposited in the Herbarium of the School of Agricultural Sciences, "Marcelino Sayago”, UCC. The vegetal material was dried at room temperature, crushed, and macerated during 48 hours with ethanol (95%); later it was filtered and the solvent evaporated under vacuum. The extracts were conserved at -5 °C (Diaz Napal et al., 2015). The extracts were diluted in ethanol to obtain the 10% concentration used in the assays, which generally has effects in insects (Marcomini et al., 2009).
Effects of plant extracts on A. diaperinus adultsAdults of A. diaperinus were fed with treated food in order to evaluate the effects of plant extracts consumption on feeding behaviour and survival. Wheat wafer discs (3 cm diameter) were used as test food (Kamruzzaman et al., 2005). The discs were treated with 150 pl of the extracts at 10% or ethanol (control) and dried for 24 hours at room temperature, after which the weight of each one was recorded (initial weight). Every disc and five unsexed adults 2-3 weeks old from the laboratory colony were placed in a Petri dish (6 cm diameter). All treatments were replicated six times, under controlled temperature (25 ± 1 °C) and relative humidity (60 ± 10%) in darkness. Mortality was checked daily and insects were considered dead when they showed no movement when touched with tweezers (Arena et al., 2020). After seven days the wafers were weighed again (final weight) to determine the amount of food consumed (FC). The antifeedant indexes (AI) were calculated as
where FCC is the amount of food consumed in control and FCt is the amount of food consumed in the extract treatments (Diaz Napal et al., 2015). Extracts with AI > 75% were considered strong antifeedants, while extracts with AI > -75% were considered strong phagostimulants (Hassanali & Bentley, 1987).
Effects of plant extracts on A. diaperinus larvaeThe extracts of G. megapotamica and B. artemisioides were used to evaluate the effects of ingestion on larvae of A. diaperinus. This assay was conducted following the methodology described above. All treatments were replicated 14 times using a 0.8 cm larva (corresponding to the fifth larval stage according to Hosen et al. (2004)) per dish in order to avoid cannibalism. Mortality and number of exuviae were registered daily for 28 days; in addition, larval weight and wafer weight were recorded every seven days. The FC and AI were also calculated for each extract.
Statistical analysesThe variables amount of food consumed, larval weight and number of exuviae were subjected to one-way analysis of variance (ANOVA) followed by Tukey’s test, with a significance level used of P < 0.05, using the statistical software InfoStat (Di Rienzo et al., 2018). When the assumptions of normality and/or variance homogeneity were not achieved, Kruskal-Wallis tests were performed. The survival probabilities were estimated by the Kaplan-Meier method using the "survival" package (Therneau & Lumley, 2015) with the statistical software R version 3.5.1 (R Core Team, 2018).
RESULTS
Effects of plant extracts on A. diaperinus adultsAdults of A. diaperinus fed on ethanol-treated wafers consumed more food than those who were forced to feed on wafers treated with the extracts, with significant differences between treatments (H = 42.81; P< 0.0001) (Fig. 1). Beetles fed on wafers treated with extracts of G. megapotamica and V nudiflora consumed 98 and 97% less, respectively, compared to the control. Insects forced to feed on food treated with A. artemisiifolia, L. molleoides, B. artemisioides, and B. coridifolia recorded intermediate consumes. Although feed consumption in the treatments with extracts of R. apetala, T. reptans, and F oolepis was numerically lower than in the control, the differences were not significant.
The extracts of G. megapotamica, V nudiflora, A. artemisiifolia, L. molleoides, and B. artemisioides had strong deterrent effects on A. diaperinus adults, while de B. coridifolia, T. reptans, and F oolepis showed moderate deterrent effects. On the other hand, R. apetala extract caused a weak deterrent effect (Table I).
None of the evaluated extracts significantly affected the survival of the adults during the seven days of experimentation. The recorded mortality was 0.8% in the treatments with B. coridifolia, G. megapotamica, and A. artemisiifolia.
Effects of plant extracts on A. diaperinus larvaeAccording to the results observed in adults and the availability of extracts, we decided to evaluate the effects of two of them on the larvae. Thus, extracts of G. megapotamica and B. artemisioides, which had strong deterrent effects in adults, were used to assess the effects of long-term exposure of larvae to treated food. Larvae fed on wafers treated with B. artemisioides and G. megapotamica extracts consumed 83 and 62% less, respectively, compared to the control at seventh day of the experiment.
The consumption of larvae exposed to food treated with the extracts was statistically different from the control (day 7: H= 19.26; P< 0.0001; day 14: H= 12.06; P = 0.002; day 21: H = 17.44; P = 0.0001). At day 28 only significant differences were observed between the consumption of larvae fed with B. artemisioides extract and the control (F(2,23) = 5.88; P= 0.009) (Fig. 2).
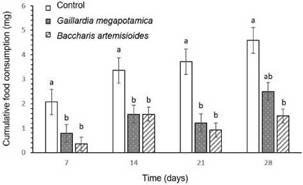
Fig. 1: Mean consumption (± SE) of wafers treated with different extracts or ethanol (control) by adults of Alphitobius diaperinus for 7 days (N = 6). Bars marked by different letters are significantly different according to LSD test (P < 0.05).

Fi g 2: Accumulated consumption (mean ± SE) by Alphitobius diaperinus larvae fed w i th wafers treated w i th extracts of Gaillardia megapotamica and Baccharis artemisioides or ethanol (control) (N = 14). Bars with the same letters at the same observation time do not differ significantly according to LSD test (P> 0.05).
After the first week of assay, the extract of B. artemisioides generated a strong deterrent effect, while that of the extract of G. megapotamica was moderate (Table II). During the time of assay, the deterrent effect of the B. artemisioides extract was attenuated, while that of G. megapotamica remained moderate after 28 days.
Although the larvae consumed the food offered, they experienced a decrease in weight throughout the experiment in both the treatments and the control. It is possible that the wheat wafers did not meet the nutritional requirements to ensure the growth of the larvae. However, differences in the larval weight were detected, being the larvae fed with wafers treated with
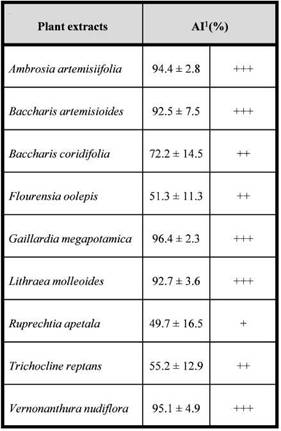
Fig. 3: Mean weight (± SE) (bars) and mortality (lines and/ or dots) of Alphitobius diaperinus larvae fed on wafers treated with extracts of Gaillardia megapotamica and Baccharis artemisioides or ethanol (control). Different letters at the same observation time indícate significant differences in larval weight among treatments according to Tukey’s test (P < 0.05).
DISCUSSION
received food treated with G. megapotamica extract was significantly lower than those that consumed wafers treated with ethanol and B. artemisioides extract on day 14 (F(2,36) = 6.72; P = 0.003). At day 21 both extracts affected the larval weight showing significant differences with the control (F(2,27) = 5.87; P = 0.008). Nevertheless, at day 28 the larvae that consumed B. artemisioides extract experienced an increase in weight, reaching values similar to the control. Larvae fed with wafers treated with G. megapotamica kept losing more weight in relation to the control and the treatment with B. artemisioides extract although no significant differences among treatments was recorded (F(2,13) = 1.73; P = 0.22).
None of the extracts evaluated affected the larval survival during the first 14 days of experimentation (Fig. 3). Afterwards, the B. artemisioides extract was the most harmful, with 36% more dead larvae in relation to the control at the end of the assay. There were significant differences in the probability of survival being lower in the treatments with extracts in relation to the control (Chisq(2) = 9.3; P = 0.009) (Fig. 4). No significant differences were registered between the two extracts evaluated (P = 0.7).
The number of larval exuviae in the control was twice that of the extracts of G. megapotamica and B. artemisioides (H = 7.99; P = 0.009) (Fig. 5).
The effects of the exposure of A. diaperinus to food treated with extracts obtained from the nine selected plant species are reported for the first time. The results of this study revealed that the extracts affect the feeding behaviour of adults and larvae, as well as the weight, development, and survival of larvae. The reduction in food consumption and the low toxicity for adults suggest that these compounds may be unpalatable to this coleopteran (Glendinning, 2002; War et al., 2012). The effects of extracts from the plant species used in this study have been previously evaluated against other insects. For example, extracts of F oolepis, G. megapotamica, and L. molleoides generated a total foraging inhibition of Acromyrmex lundi Mayr (Hymenoptera: Formicidae) in choice tests; whereas R. apetala, B. artemisioides, V nudiflora, A. artemisiifolia produced intermediate foraging values, and B. coridifolia and T. reptans did not inhibit foraging activity (Diaz Napal et al., 2015). Plants from family Asteraceae have been particularly well studied due to the variety of secondary bioactive metabolites they possess, which makes them interesting sources of natural products to be used as bioinsecticides (Verdi et al., 2005; García et al., 2007; Padin et al., 2013). Contrary to what was observed in our study, compounds from F oolepis had a strong antifeedant effect (98%) on Xanthogaleruca luteola Müller (Coleoptera: Chrysomelidae) (Diaz Napal et al., 2009), and, when applied by contact, caused 33% of mortality on adults of Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) (García et al., 2007). The strong antifeedant activity of V nudiflora recorded in this work against A. diaperinus adults could be attributed to the presence of sesquiterpene lactones, compounds with have shown marked deterrent effect on lepidopterans (Burnett Jr. et al., 1974).
Larvae of A. diaperinus consumed less food when it was treated with the plant extracts, mainly during the first week of experimentation. During the course of the assay, the antifeedant effect of B. artemisioides was weakened while that of G. megapotamica was maintained. In agreement with our results, larvae of Epilachna paenulata Germar (Coleoptera: Coccinellidae) avoided food treated with B. artemisioides extract in choice tests after 24 hours of experimentation, evidencing a strong deterrent effect (Del Corral et al., 2014). Similar results were obtained with larvae of this coccinellid when evaluating the extract of F oolepis (Diaz Napal et al., 2009).
The extracts affected A. diaperinus larval weight, with a decrease observed throughout the experience in relation to the control. However, larvae exposed to wafers treated with B. artemisioides extract showed a weight gain after 21 days, reaching similar values to those of the control. According to these results, these individuals may have activated a metabolic pathway through which enzymes responsible for the detoxification of secondary metabolites are expressed (Glendinning, 2002; War et al., 2012), or the active compounds may have degraded over time (Dayan & Duke, 2009). Larvae of T castaneum fed with extracts obtained from other plant families behaved similarly to those recorded in this work (Jbilou et al., 2006).
Fi g 4: Surv ival probab i l i ty of larvae fed on wafers treated w ith extracts of Gaillardia megapotamica and Baccharis artemisioides or ethanol (control). Different letters indicate significant differences among treatments according to Log-Rank test (P< 0.05).
The probability of survival of A. diaperinus larvae was lower in the treatments with extracts than in the control, which could be caused by starvation. Nevertheless, a toxic effect of the extracts cannot be dismissed since compounds from other plant families have adversely affected the larval survival of coleopterans, including A. diaperinus (Zorzetti et al., 2015; Ahumad et al., 2019).
The mean number of exuviae recorded was higher in the control than in the extracts of G. megapotamica and B. artemisioides, which indicates that these extracts would be affecting larval development, as observed by Jbilou et al. (2006).

Fig. 5: Mean number of larval exuviae (± SE) obtained in tests with larvae fed on wafers treated with extracts of Gaillardia megapotamica and Baccharis artemisioides or ethanol (control). Different letters indicate significant differences among treatments according to Tukey’s test (P < 0.05).
Secondary metabolites are biodegradable and of low toxicity to vertebrates, so they could be applied during the different stages of poultry farming, unlike synthetic insecticides. The extract of G. megapotamica
maintained its deterrent activity throughout the experience, and that of B. artemisioides affected larval survival, both having promising characteristics as bioinsecticides.
In the future, evaluations of the extracts should be conducted using the birds’ breeding litter and/or feed to assess survival and oviposition and feeding behaviours of A. diaperinus under conditions closer to those occurring on poultry farms. At the same time, consideration should be given to whether the food treated with these compounds affects the weight gain and oviposition capacity of the birds, as well as the organoleptic characteristics of the meat and eggs.
ACKNOWLEDGMENTS
This research was supported by Secretaría de Ciencia y Teconología (SECyT-UNC) (33620180100129CB). MTD is a researcher at the Universidad Nacional de Córdoba and SMP is a researcher of CONICET. JSA has a fellowship from CONICET.












 uBio
uBio 

