INTRODUCTION
The red palm mite, Raoiella indica Hirst (Acari: Tenuipalpidae), is a pest that attacks economically important crops such as coconut palm Cocos nucifera L., bananas and plantains Musa spp., some species of ornamental palms and heliconias (). In the Neotropical region, the red palm mite was first detected in 2004 on the leaves of the coconut palm and Christmas palms, Veitchia merrillii (Becc.) H.E. Moore, on the Caribbean island of Martinique ( ). In subsequent years, major infestations of R. indica have been found in the Caribbean islands, Mexico, Florida (USA), Venezuela, Colombia and Brazil (). This species was first detected in Mexico in November 2009 (). To prevent its rapid dispersal in the country, the Mexican government implemented an operational program involving exploration, sampling, diagnosis and chemical control of population outbreaks in commercial nurseries and orchards. For the chemical control of this pest, spraying of the acaricides abamectin, acequinocyl, amitraz, spirodiclofen and elemental sulfur is recommended (). It is also indicated not to spray the same active ingredient more than twice consecutively and to rotate products with different modes of action to avoid resistance of this pest. Recurrent use of pesticides not only leads to pest resistance, but also causes harm to human health and the environment in general; in agricultural production systems, it can collaterally affect natural enemies and cause an ecological imbalance (). Therefore, the use of plant extracts, with proven acaricidal efficacy, may be a lower risk and environmental impact option for chemical control of this pest ().
In the search for alternatives to the use of organosynthetic pesticides, interest in plants containing bioactive secondary metabolites has increased ( ). The presence of these metabolites in plants is the consequence of an evolutionary process that has led to the selection of specimens with better defense mechanisms against microbial attack or predation by insects and other arthropods (). Bioactive metabolites include phenolic compounds, terpenes and steroids, alkaloids and flavonoids ( ; ). The effects of these compounds on arthropods are manifested in inhibition of feeding or chitin synthesis, behavior modification, reduction or inhibition of growth, development or reproduction and mortality, among others (Ascher, 1993; ). In integrated pest management, there is great potential for plants that produce secondary metabolites, since they can be used as a biological barrier in cultivation, incorporated as plant residues or have their extracts used with bioactive compounds (). Some plant extracts are highly effective against insects and mites that are resistant to organosynthetic insecticides and acaricides, due to the content of several metabolites with different modes of action (), which could be used as a replacement or complement to the use of organosynthetic pesticides, the price, availability and application technology of which are beyond the reach of poor farmers (Abdullahi et al., 2019).
The invasion of R. indica in the Neotropical region () has led to the study of the acaricidal activity of plant extracts from native plant species, as part of the search of alternatives to the use of organosynthetic acaricides (; ;; ; Vásquez et al., 2018; ). In Cuba, the application of essential oil from Melaleuca quinquenervia (Cav.) S.T. Blake (Myrtaceae) leaves, diluted to 2.5%, caused 100% mortality in R. indica females (). Likewise, in Venezuela, ethanolic extract of lemon grass
Cymbopogon citratus (D.L) Stapf at 7.5% caused 92.5% mortality and oviposition of surviving females was reduced to zero (), while ethanolic extract of Tanacetum cinerariifolium (Trevir.) Sch. Bip. leaves, diluted from 0.25 to 2%, caused 85 to 100% mortality in R. indica females (). In Brazil, ethanolic extract of Myrciaria dubia (Kunth) McVaugh (Myrtaceae) seeds, in solutions from 1 to 8%, showed acaricidal effect, causing 40 to 100% mortality in R. indica females at 72 hours after exposure ( ). In another bioassay, ethanolic extract of leaves from Spilanthes acmella (L.) R.K. Jansen, a species of the family Asteraceae native to the tropics of Brazil and Peru, drastically reduced the growth rate of R. indica at concentrations from 0.13 to 0.88% (). In Yucatán, Mexico, ) reported that ethanolic extract of Mexican mint Plectranthus amboinicus (Lour.) Spreng. leaves, in dilutions from 0.3 to 13%, caused low mortality in R. indica females (12%); however, with the highest dilution, a repellency effect was observed in 61% of the treated mites, 96 h after exposure. Given the importance of the phytosanitary problem and the limited number of studies on the subject, the objective of this work was to evaluate the toxicity of leaf extracts of Mexican oregano Lippia berlandieri Schauer (Verbenaceae), neem Azadirachta indica A. Juss. (Meliaceae), Mexican mint Plectranthus amboinicus (Lour.) Spreng.
(Lamiaceae), rue Ruta graveolens L. (Rutaceae) and Persian lime Citrus x latifolia Tanaka (Rutaceae) against the red palm mite R. indica under laboratory conditions.
MATERIAL AND METHODS
Biological materials
Coconut palm C. nucifera leaflets naturally infested by R. indica were collected in the Experimental Field of the Academic Division of Agricultural Sciences of the Universidad Juárez Autónoma de Tabasco (Juarez Autonomous University of Tabasco), at Km 25 of the
Villahermosa-Teapa highway, Ranchería la Huasteca 2nd section, municipality of Centro, Tabasco, Mexico. The samples were placed in polyethylene bags and transported inside a cooler to the Plant Health Laboratory, located in the university’s aforementioned academic division. In the laboratory, the leaflets were thoroughly examined using a stereoscopic microscope to obtain relatively young adult females, characterized by an oval-shaped body, larger than the other biological forms of their species, with dark spots on the back, rounded opisthosoma and intense carmine red l.al., 2013). In all cases, the extracted females were used in the bioassays on the same day as the collection.
Extracts preparation
Extracts of P amboinicus, A. indica, R. graveolens, and C. x latifolia were prepared from leaves of relatively young, pest- and disease-free plants collected at the Ranchería Güiral y González, 2nd section of
Huimanguillo, Tabasco (17°55'13" N; 93°24'59.5" W) in September 2018. The leaves were washed and then dried at room temperature in the Plant Health Laboratory for a period of 15 days, protecting them from light. The dried leaves of each plant species were ground in a Hammer Mill. industrial blender (Model B2300). The ground material was passed through an 8- inch mesh No. 60 (250 micron) sieve and the plant leaf powder was stored in 300-g portions in one-liter amber flasks at room temperature until use. s et al. (2019), the extraction methodology was as follows: 15 g of vegetable powder and 150 mL of solvent were deposited in a 250-mL Erlenmeyer flask with a screw cap; the mixture was homogenized for 30 s at 4000 rpm with the aid of a digital Ultra-Turrax T25 homogenizer (IKA Works, Inc., Wilmington, NC), then ultrasonically-assisted extraction was performed for 20 min at 40°C using a Cole-Parmer CPX-956-217R heated ultrasonic cleaner (Cole-Parmer. Vernon Hills, IL). The extraction mixture was filtered on Whatman no.1 paper and placed in a 250-mL ball flask with a ground-glass stopper to remove the extraction solvent, at a temperature of 45°C, using a Rotavaporador. R-300 rotary evaporator (BÜCHI Latinoamérica S. de R.L. de C.V, CDMX). This procedure was repeated until the amount of vegetable powder available from each plant species was exhausted. For the leaf powder of C. x latifolia, P amboinicus and R. graveolens, absolute ethanol was used r ed et al., 2018), while for A. indica aceto et al., 2019), both analytical grade (Reactivos Química Meyer., CDMX). The Mexican oregano L. berlandieri extract, obtained by steam distillation, was purchased from the Natural Solutions S.M.I. company, located in Ciudad Jiménez, Chihuahua. The extracts were stored under refrigeration at 4°C, in sterile amber flasks, until use in the bioassays.
Extracts evaluation
Dilutions at 0.25, 0.50, 0.75 and 1% (v/v) were prepared for each of the five extracts, using distilled water as a diluent. The experimental unit was a 2.5 x 4 cm leaf blade portion of palm leaflet, immersed for 5 s in the plant extract solution or distilled water as a control. The portions were dried for 20 min at room temperature, placed with the abaxial face on a 5 x 5 cm acrylic plate, with a 2.5 cm diameter hole in the center, and their edges were fixed with adhesive tape to the plate. Then 10 R. indica females were placed on the abaxial area of the leaf blade bounded by the hole in the acrylic. To confine the mites to this space, another acrylic plate (without a hole) of the same size was superimposed and the edges of both plates were sealed with adhesive tape (Sánchez-). This experimental unit represented a replicate and was placed on a layer of damp cotton inside a Petri dish. Each treatment had 10 replicates for a total of 100 mites per concentration of plant extract. The total number of living and dead mites was quantified at 24, 48 and 72 h after exposure. Mites that did not move when disturbed for 5 s with a No. 000 brush () were considered dead. All bioassays were carried out in a rearing chamber at a temperature of 28 ± 2 °C, 50 ± 10% relative humidity and 12 h photoperiod.
Statistical analysis
Mortality data were corrected with respect to observed mortality in the control (Abbott, 1925). When the mortality in the control was greater than 10%, the bioassay was discarded. Comparison of mortality rates was made by analysis of variance between extracts of the same concentration and exposure time. Previously, data were transformed to arcsine values to satisfy the normal distribution of errors (). The multiple comparison of means was performed using Tukey's method. The analyses were performed with the GLM procedure of the SAS statistical package ( ). In all cases, P < 0.05 was considered significant.
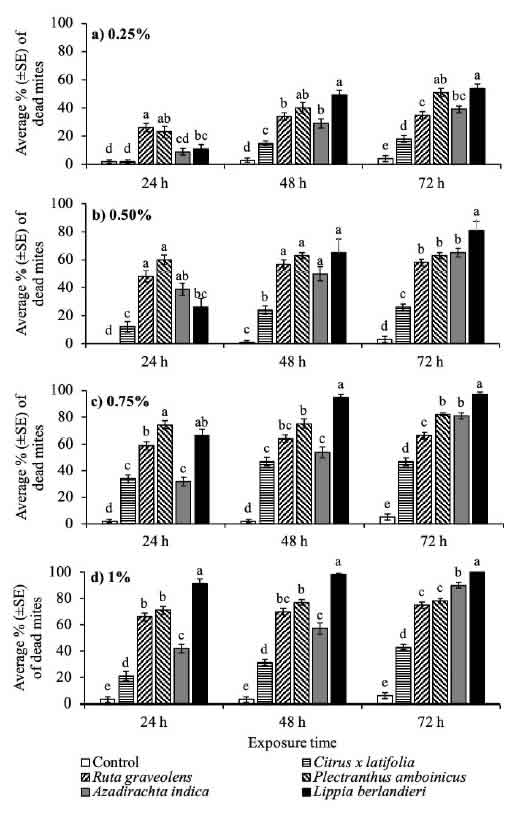
Fig. 1: Accumulated mortality of R. indica females per exposure time to the leaf extracts of five plant species, in concentrations from 0.25 to 1%, under laboratory conditions. Bars with the same letter per exposure time are not significantly different (Tukey, P > 0.05).
The mortality rate of adult red mite females rose due to the increase in the extract concentration and exposure time. The strongest acaricidal activity against R. indica was observed in the extracts of L. berlandieri, A. indica and P amboinicus at dilutions of 0.75 and 1%, whose mortalities were > 78% at 72 h of exposure (Fig. 1c, d). Exposure of R. indica to L. berlandieri extract for a period of 72 h, at dilutions of 0.25 (F = 57.5, df = 54, P
Acaricidal activity of plant extracts has been reported in other species of the genus Lippia (Cavalcanti et al., 2010; Sivira et al., 2011). Leaf extracts from four accessions of L. sidoides Cham. ca o-e Tetranychus urticae Koch (Cavalcanti et al., 2010), with toxicity attributed to the high content of the essential oils thymol (70.3%) as well .1%). Likewise, Sivira et al. (2011) found that ethanolic extract of wild oregano L. origanoides H.B.K., at concentrations of 5 to 20%, 43.7 to 96.6% mortality in adult Tetranychus cinnabarinus (Boisduval) mites. In contrast, Castillo-Sanchez et al. (2018) reported that ethanolic extract of P amboinicus leaves, diluted to 13%, produce for R. indica adults.
The acaricidal effect of neem extract on R. indica observed in the present study is similar to that reported for other mite species. In adult females of the two- spotted spider mite T urticae, the aqueous extract of neem leaves at 1 and 5% caused between 83.9 and 85.2% mortality (Castiglioni et al., 2002), while the ethanolic extract of fruits, at concentrations of 1 to 20%, produced betwee.5% mortality (Carrillo- Rodríguez et al., 2011). In another study, the population growth rate of the mite Polyphagotarsonem linearly with an increasing concentration of neem extract, becoming negative at an exposure dose of 0.13 g of active ingredient per liter (Venzon et al., 2008). According to Castiglioni et al. (2002), A. indica is one of the most studied plants with inseal properties i. The of isolated neem compounds can be classified as follows (Ascher, 1993): a) mortality of eggs, juveniles or adults, b) total or partial reduction in fertility, c) oviposition, d) antifeedant effect and e) growth regulator, among others. Due to this variability in the modes of action of the neem extract compounds, the possible development of pest resistance is minimal.
The poorest acaricidal effect was observed with leaf extracts of R. graveolens and C. x latifolia, where the maximum concentration of 1% and 72 h exposure (F = 178.3, df = 54, P < 0.0001) resulted in 75 and 43% mite mortality, respectively (Fig. 1d). Previous studies of the acaricidal or insecticidal activity of extracts leaves of R. graveolens and Citrus spp. differ from each other. Potenza et al. (2006) reported that 1% aqueous extract of R. graveolens sprayed on T urticae specimens .9% mortality. In adults of the Mediterranean fruit fly Ceratitis capitata (Wiedemann) (Tephritidae), the ethanolic extracts of R. graveolens at 3.6 and 6% caused a 100% mortality (Ghabbari et al., 2018). Likewise, this ethanolic extracts at 0.1% produced 78% mortality on neonate larvae of the fall armyworm
Spodoptera frugiperda Walker (Noctuidae) (Ayil- Gutiérrez et al., 2015). Regarding the mortality re of Persian lime C. x latifolia, it is known that exposing at 8% of ethanolic extracts leaves of Citrus sinensis Osbeck and Citrus aurantium L. against second instar mealybug nymphs of Drosicha mangiferae Stebbins (Margaroridae) caused 17 and 27% mortality, respectively (Majeed et al., 2018). Similarly, larvae of the red flour beetle Tribolium castaneum (Herbst) (Tenebrio, 10 and 15% methanolic extracts of C. aurantium leaf caused 7.8, 15.7 and 24.7% mortality (Ali et al., 2019).
One problem for chemical control of phytophagous mites is their high potential to develop resistance to acaricides (Whalon et al., 2008). In this context, the incorporation of plant extracts with acaricidal activity in an integrnt scheme has great potential (Pino et al., 2013). It has been seen that some plant extracts with metabolites of different modes of action areagainst pest species that are resistant to insecticides and organosynthetic acaricides (Rosado-Aguilar et al., 2017), which could be used as a replacement or complement to pest management, at e reach of poor farmers (Abdullahi et al., 2019). Based on our results, leaf extracts of Mexican oregano L. berlandieri, neem A. iint P amboinicus are highly promising to be included in a management program for the red palm mite R. indica. However, it should be noted that it would be important to determine the appropriate doses to apply in the field, since a laboratory determination does not consider losses due to entrainment, photodecomposition, thermoregulation and pest escape (Lagunes-Tejeda et al., 2009).
ACKNOWLEDGMENTS
Karen Z. Ruíz Jiménez thanks the scholarship granted by the National Council of Science and Technology (CONACYT) of Mexico. The authors are grateful for the support provided by the Scientific and Technological Research Support Program (PAICYT; CT 570-18) of the Autonomous University of Nuevo León.












 uBio
uBio 

