INTRODUCTION
Human action transforms ecosystems in a greater or lesser degree. The most dramatic type of transformation has taken place in urban habitats (e.g, areas almost completely built-up), agricultural lands and other natural landscapes used for productive-economic purposes. Less intense changes have occurred in areas with few buildings and roads in a natural landscape matrix, acting as a continuum of human influence stretched across the entire land surface (Grimm et al., 2008; McDonnell & Hahs, 2008). This trace of human influence is usually called "human footprint” (Sanderson et al., 2002). Even in well-preserved natural sites, there are microhabitats, with particular physical and biotic characteristics, created and maintained artificially by humans. Examples of these microhabitats are the paths or roads that cross natural areas and the small parks and gardens that surround isolated houses. Human transformation of landscapes is one of the main factors threatening most groups of animals, but little is known about how the anthropization of natural landscapes affects some insect communities.
The evaluation of ecosystem functioning should focus on insects, taking into account the variety of roles that they play (Maleque et al., 2006). This is especially true for Diptera, which is one of the four megadiverse insect orders. Among Diptera, the family Sarcophagidae displays extraordinarily diversified life histories including parasitic species (e.g, parasitoids, kleptoparasites, myiasis producers) and groups whose larvae are scavengers in a wide variety of substrates of animal origin (e.g, dung feeders, large carrion of vertebrates, or small remains of invertebrates) (Brown et al., 2010; Marshall, 2012). The latter groups of species, typically called sarcosaprophagous, are usually more intensely sampled than the parasitic species, because they are more abundant in ecological studies that use baited traps to collect them and because of their role as forensic indicators or pest species (e.g., Linhares, 1981; Mulieri et al., 2011; Pereira de Sousa et al., 2016; Dufek et al., 2020). By contrast, parasitic species of
Sarcophagidae, and particularly kleptoparasites, are strikingly neglected in the literature.
Kleptoparasitism is a form of competition that involves the theft of already-procured items. Thus, kleptoparasitic species develop at the expense of another host organism, through the misappropriation of its food, and end up killing the host either directly or indirectly (Eggleton & Belshaw, 1992; lyengar, 2008). Although kleptoparasitism of food is the most common example, the stolen item may be another type of resource. Sarcophagid flies belonging to the subfamily Miltogramminae are mainly kleptoparasites of bees and solitary wasps (Piwczynski et al., 2017).
Indeed, the scarce information available on kleptoparasitic flies was traditionally generated by hymenopterists, when studying the nesting behaviour of solitary wasps and bees (Wcislo, 1986; Spofford & Kurczewski, 1990), or found in lists compiled by host-parasite associations (Krombein, 1967; Spofford et al., 1989; O’Neill, 2001). Particularly, no studies have focused on environmental factors or the way in which landscape transformation affects kleptoparasitic miltogrammines. In South America, the species diversity of Miltogramminae is lower than in other regions but this subfamily has also been historically less explored or studied for their biodiversity and biological aspects (Pape, 1989, 1996). The far Southern Andean of the continent is no exception although it comprises several large areas of dry shrublands and semi-desert environments where the potential hosts reach higher diversity, providing higher amounts of resources and niche dimensions than the northern and eastern humid tropics/subtropics of South America (Bohart & Menke, 1976; Michener, 2007). Consequently, the
Miltogramminae are poorly represented in local entomological collections; and the composition, diversity patterns, and spatial ecology of their communities remain practically unknown.
Understanding how basic environmental variables influence the spatial distribution of this group of flies is a key issue to understand kleptoparasite ecology, especially since anthropogenic changes in the landscape are affecting the distribution and incidence of most species. In this study, we disentangle the role of habitat types and anthropogenic influence to account for the spatial variation in the species richness, composition and abundance of local fly assemblages. Many miltogrammine flies are strongly associated with sites with a great diversity of solitary aculeate wasps and bees and their nesting aggregations; these sites are open dry habitats with patches of loose soil without vegetation (O’Neill, 2001). Furthermore, solitary wasps are less abundant in highly modified areas, such as the large paved or built areas in the urban core (Christie & Hochuli, 2009). Hence, miltogrammine are presumably largely absent from urban areas. The present study was performed in the context of explorations of the biodiversity of such flies inhabiting temperate habitats in southern South America, where the only available information of Miltogramminae was mentioned in a few taxonomic works, and nothing is known about their occurrence and spatial ecology. Thus, we conducted an intensive sampling programme in relatively preserved areas, as those most probably suitable habitats for these flies.
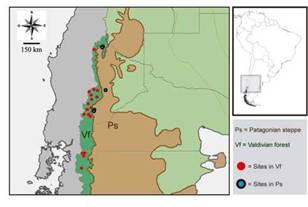
Fig. 1: Map of the study area with location of sampling sites in the Valdivian Temperate Forest and Patagonian steppe ecoregions.
The main aim of this research was to evaluate changes in abundance, species richness and composition between natural and human-created habitats. These comparisons were conducted specifically to evaluate the effect of the human footprint on Miltogrammine community composition in relatively well-preserved areas. In addition, our intensive and specifically-targeted samplings in Andean-Patagonian habitats constitute the first snapshot on the diversity of Miltogramminae for such areas.
MATERIAL AND METHODS
Study area
Samplings were distributed in 26 sites, that are located in Neuquén and Chubut provinces, Argentina (Fig. 1); comprising locations in the eastern slope of the Andean chain in the following preservation areas [National Parks (NP) and Protected Natural Areas (PNA)], as well as their surroundings: NP Lanín, NP Lago Puelo, NP Los Alerces, PNA Batea Mahuida, PNA Domuyo and PNA Epu Laufquen. All surveyed sites were located in two ecoregions: the Valdivian Temperate Forest (n = 21) and the Patagonian steppe (n = 5) (Table I). Samplings were performed during the late spring and summer, which represents a warmer and dry season, the period of highest abundance of flies for the region (Mariluis & Schnack, 1996; Figueroa-Roa & Linhares, 2002, 2004).
SamplingSignificant positive correlation between wasp nest density and number of Miltogramminae flies were previously documented (Wcislo, 1984). For these reasons, sample locations were selected after a careful examination of the site, if the following three conditions were detected: high insolation, patches of bare soil and burrowing bees or wasps nesting.
The active capture method employed for this study involves the capture of all specimens encountered using an entomological net while foraging on flowers or vegetation, resting on soil or stones, or searching for burrows (Fig. 2). These captures took place along transects of approximately 100 m in the selected areas. These captures were done for one to three hours by one to three collectors. Despite this method is a highly collector-dependent method (depending on his ability and expertise to capture flies) and not easily replicable, previous comparisons between collecting methods to establish which one is more appropriate for sampling different Calyptratae guilds demonstrated that active capture by highly trained collectors was the most reliable practice to obtain kleptoparasites (Olea et al., 2017).
Miltogramminae are very delicate flies, hence dried collected flies were mounted with pins or minuten after each capture date. This pinning of samples in the field was done with the use of a Leica ES2 stereomicroscope. Specimens were identified at the species level or, when this was not possible, at the lowest taxon possible, using dichotomous keys and specific taxonomic revisions and descriptions (e.g., Pape, 1987, 1989; Pape & Dahlem, 2010). Voucher specimens of all taxa are housed in the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina.
Environmental variablesIn an effort to deal with the complex factors involved in anthropogenic influence, environmental data were obtained from microhabitat characterization. We divide our sampling points in four broad categories of microhabitats to included human-modified (HM) and natural sites (NS) (Fig. 2).
The HM type categories were as follows:
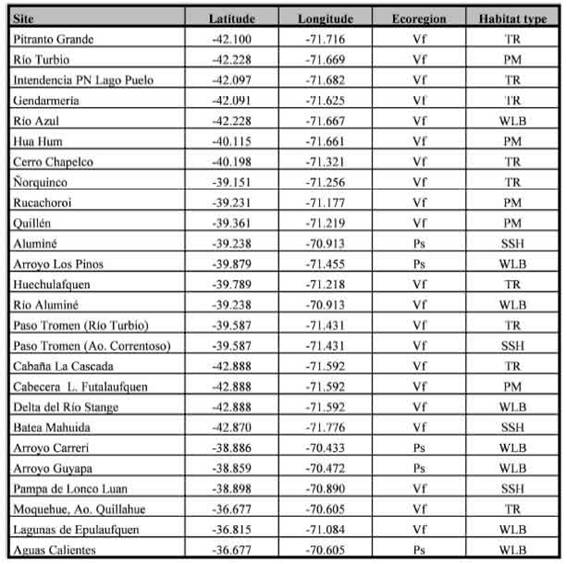
Table I: Locations and habitat type for the sampled sites in the Valdivian forest and the Patagonian steppe, Argentina.
Trails and roads (TR) gravelly roadways, borders of roads for vehicles and trails for hikers. These sites are characterized by compacted soil for human use to the nearly complete exclusion of plants, frequently with management regime; Parks and meadows (PM): green habitats surrounding isolated houses, with predominance of lawn cover and with regular disturbance regime of lawn cutting.
On the other hand, NS were categorized in: Watercourses and lake beaches (WLB): sandy or gravel banks adjacent to watercourses or lake beaches; Sandy and shrubby areas (SSH): low vegetation, shrubby areas not associated to water, with open sandy soil.
AnalysisThe number of specimens collected was standardized taking into account the number of flies per hour percollector (capture rate = CR). The comparisons of fly abundance between the four habitat types were assessed by means of the nonparametric Kruskal-Wallis analysis of variance (ANOVA) for independent samples, followed by a posteriori comparison using Tukey's procedure (Zar, 1996). Proportional abundance data were transformed by the arcsine square root transformation to better meet the normality and equal variance assumptions. The comparisons of fly abundance between HM and NS were assessed by means of the nonparametric Mann-Whitney test (Zar, 1996).
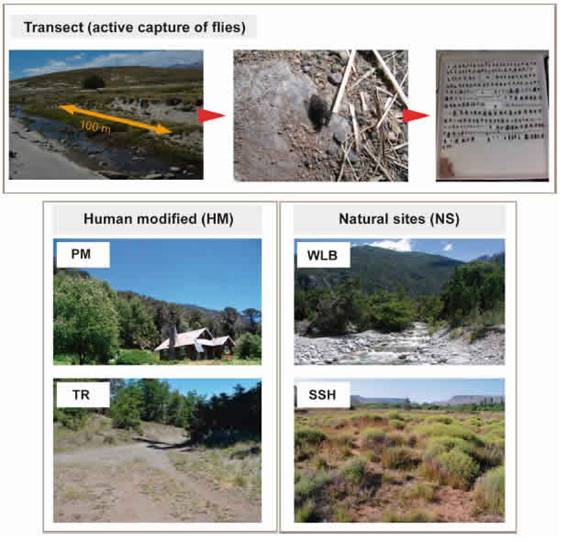
Fig. 2: Trapping methods (transect of capture active) and microhabitat types selected for samplings.
In order to represent the structure of the Miltogramminae communities we performed two non-metric multidimensional scaling (NMDS) analyses, one based on proportional abundance and another with a transformed presence/absence matrix (as 0 for absence and 1 for values > 1 as presence from abundance data). The parameters used were the Bray-Curtis index, with 999 free permutations (PRIMER version 6) (Clarke & Gorley, 2006). Goodness of fit was indicated using the Stress value, which should be less than 0.2 to give an accurate picture (Clarke, 1993).
Species turnover and nestedness components were examined by partitioning B-diversity between environmental conditions (Baselga, 2010). B-diversity patterns may arise from species replacement (turnover) or from species loss or gain (nestedness) and this could be the result of distinct underlying processes (Baselga, 2010). For this purpose, we used the method "beta.multi” of the "betapart” package in R to calculate S0rensen dissimilarities which takes into account all the given samples for producing the mean beta coefficient of pairwise beta coefficients calculated for all possible sample combinations (Baselga & Orme, 2012). S0rensen dissimilarities (Bser) were partitioned into turnover (Bsim) and nestedness (Bnes) components. S0rensen dissimilarity is bounded between zero and one, indicating that two samples share the same or no species, respectively.
RESULTS
A total of 338 flies, belonging to 17 species, were captured throughout this study. The flies obtained belonged to the following genera: Opsidia Coquillett (n = 216; 2 spp.), Amobia Robineau-Desvoidy (n = 79; 2 spp.), Senotainia Macquart (n = 25; 6 spp.), Macronychia Rondani (n = 10; 4 spp.), and Metopia Meigen (n = 8; 2 spp.). Opsidia intonsa Aldrich in this study accounts for 60% of total captures (n = 160), being the dominant species. This species was captured in all microhabitat types and its occurrence was the highest with presence recorded for 21 sites (85%), all other species exhibited a noticeable very low occurrence pattern (Table II).
Significant differences were found in the capture rate of Miltogramminae among habitats (Kruskal-Wallis H = 9.98, P = 0.019). Higher mean capture rates were obtained for TR (CR = 1.57), WLB (CR = 1.76) and SSH (CR = 2.4) compared to PM (CR = 0.22); although differences were significant only between PM with TR, and PM with SSH in pairwise comparisons (Fig. 3a). Also, TR, WLB and SSH exhibited higher variability in the capture rate than PM. The proportional abundance of the dominant species, O. intonsa, differed among habitats (Kruskal-Wallis H = 14.80, P = 0.002) (Fig. 3b) with higher values in both human modified habitats compared to the wild habitat types, although differences were significant only between PM with WLB, and PM with SSH in pairwise comparisons.
Taking into account simplified comparisons in two categories, between human-modified and natural sites, the proportional abundance of O. intonsa were significantly higher in HM (0.93 ± 0.078) habitats than in NS (0.34 ± 0.078) (Mann-Whitney U = 1.45, P < 0.001). Conversely, species richness of Miltogramminae was significantly higher in NS (2.77 ± 0.43) compared to HM (1.38 ± 0.43) (Mann-Whitney U = 3.80, P < 0.016).
The ordination analysis (NMDS) based on proportional abundance showed that human-modified (HM) microhabitats were well separated from the natural sites (NS) (Fig. 4a). On the other hand, the NMDS analysis carried out with presence/absence data showed that species assemblages in the Patagonian steppe were well separated from the Valdivian Temperate Forest. Some human-modified (HM) and natural sites (NS) formed a single group of samples that did not differ greatly in their community composition (Fig. 4b). In both cases, the low stress values indicate a great representation of the overall variance.
Species turnover was the main process shaping Miltogramminae assemblages (turnover = 0.48, nestedness = 0.29) (Fig. 5). Species replacement between microhabitats (Bsim = turnover) explained the community composition, with values ranging from 0.33 to 0.53. The nestedness component of beta diversity was higher between the PM and the other microhabitats because the two species captured in this microhabitat were present in the other habitat types.
Comparisons between the surveyed ecoregions indicated that Miltogramminae belonging to the genus Opsidia did not show significant differences between the Patagonian steppe (CR: 0.63 ± 0.53) and the Valdivian forest sites (CR: 1.13 ± 0.29) (Mann-Whitney U = 5.1, P = 0.949). Whereas, specimens of Senotainia were significantly more abundant in the Patagonian steppe (CR: 0.42 ± 0.09) than in the Valdivian forest (CR: 0.05 ± 0.04) (Mann-Whitney U = 1.75, P= 0.019). The results obtained at the specific level indicated no significant difference for the dominant O. intonsa (Patagonian steppe CR: 0.63 ± 0.59, Valdivian forest CR: 0.90 ± 0.29) (Mann-Whitney U = 4.4, P= 0.613).
DISCUSSION
Human influence on natural ecosystems leads to negative effects on native insects, promoting a decline or homogenization of diversity or biological invasions (Kim, 1993; Knop, 2016; Sánchez-Bayo & Wyckhuys, 2019). Assessing this human influence on natural ecosystems and how it affects each animal community is essential for the implementation of effective conservation measures. Kleptoparasitic dipteran communities are no exception, and this work represents the first approach to this topic. Giraldeau & Caraco (2000) defined three forms of kleptoparasitism: overt aggression
(kleptoparasite uses force to gain exclusive access to resource), competitive scramble (passive individuals can exploit discovered resources by active individals), and stealth (avoid interaction with host). A stealthy kleptoparasite takes the resource from a host but avoids interaction with the host. Miltogramminae adopt different behaviours of nest attack and selection, and therefore, the way in which they find a host and insert larvae in its nests differs among species. The characteristic conditions for most kleptoparasitic interactions were enumerated by Iyengar (2008) as follows: kleptoparasite species must be opportunistic feeders; the transportation or storage of food is made by the host; kleptoparasites can detect cues from appropriate hosts; typical habitats of kleptoparasite and host are congruent; host habits are strongly associated with a certain habitat or area; in such cases, hosts are aggregated in large concentration. Regardless of the species involved and its behaviour, Miltogramminae are considered stealthy kleptoparasites in all cases. To our knowledge, the human pressure on a community of stealthy kleptoparasites has not been evaluated yet.
Effect of human footprintIn the case of Miltogramminae, host-kleptoparasite interactions include strong evolutionary responses involving complex behaviours among insects, with several stereotyped forms of stealing strategies, and noticeable defence mechanisms by hosts (Pape, 1996). Thus, the human footprint on the environments may locally disrupt parasite-host relationships by directly impacting at the community level (e.g. habitat loss) or by affecting the specific behavioural cues and searching strategies at the species-specific level. In this work, the study area represents the early stages of human modifications in relatively pristine areas, and our results provide evidence on the significant effects of human ¡nfluence on landscapes for kleptoparasitic fly diversity and abundance.
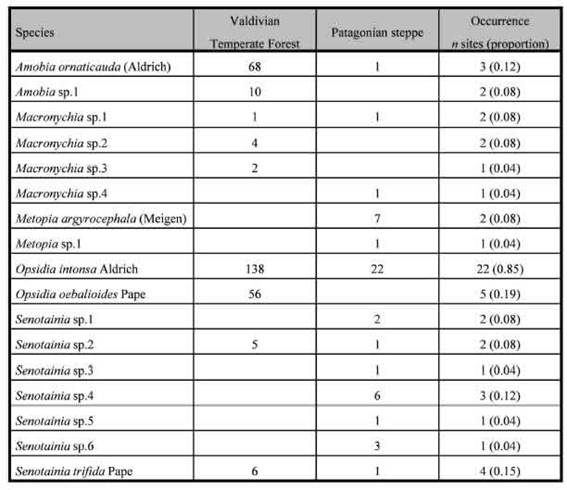
Table II: List of species, total captures and site occurrence of Miltogramminae in the Valdivian forest and the Patagonian steppe, Argentina.
Habitat loss for nesting places of solitary wasps most probably impacts miltogrammines by diminishing host resources, and this likely represents the main cause of their low abundance in disturbed sites. Artificially irrigated green spaces, such as parks and lawns, alter natural cover and strongly affect Miltogramminae occurrence, because this habitat type exhibits the lowest abundance of flies. In the studied area, this kind of habitat transformaron replaces patches of bare soil with lawn green areas and hence prevents the proliferation and aggregation of burrows of sphecid (Sphecidae) or bembecine (Crabronidae: Bembecinae) wasps regarded as usual hosts for many Miltogramminae species (Sppofford et al., 1989; O'Neill, 2001).
However, we also found that some discrete or moderate human activity may open new habitats for colonisation by Miltogramminae. In dry and natural areas, such as desert and semi-desert ecosystems, these flies prefer bare soils or riparian corridors such as dry rivers or stream beds. In temperate environments, this preference is often transferred to the banks of unpaved roads/tracks, regardless of the type of surrounding vegetation (i.e. forest or open habitats).
Variables associated with soil characteristics may represent important factors for the nesting behaviour of wasps. According to our study, the proliferation of roads or trails can alter soil characteristics such as compaction, temperature, and moisture. These features may affect host abundance and composition, and hence their associated kleptoparasites. Further ecological analyses are needed to confirm this possibility by taking into account nest density, host abundance and diversity. Our results indicate that O. intonsa is a broad-niche species since it occurs in all habitats surveyed, and it was highly dominant in the samples. The higher dominance of O. intonsa in gravelly roads and trails and in parks around isolated houses (though in low abundance), than in patches of bare soil along rivers and sandy shrublands, suggests its tolerance to places with high disturbance regimes (e.g, lawn mowing, artificial irrigation, periodic maintenance of roads).
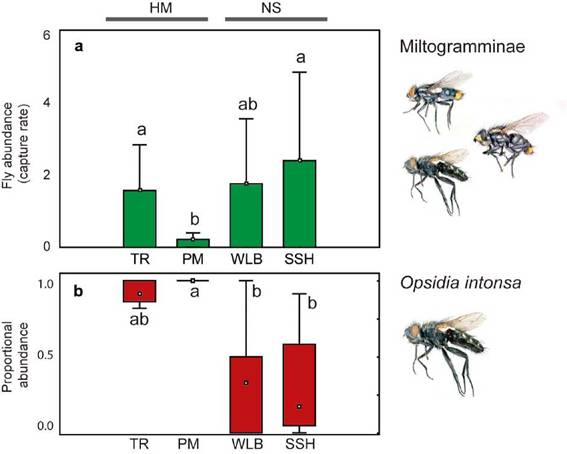
Fig. 3: Comparison of Miltogramminae abundance obtained in four microhabitat types. a. Capture rate for total Miltogramminae. b. Proportional abundance of O. intonsa. References: PM, parks and meadows; SSH, sandy and shrubby areas, TR, trails and roads; WLB, watercourses and lake beaches.
Species and biology
During host chasing, the stereotyped behaviours exhibited by the miltogrammines are basically offensive, whereas the wasps tend to develop defensive counter-kleptoparasitic behaviours (e.g, freeze-stops and face-offs, chasing flights) (Spofford & Kurczewski, 1992). Based on this information, miltogrammines were classified according to their host-finding behaviour into two main groups: hole-searchers and satellite flies (Evans, 1970; Wcsilo, 1986). The hole-searcher species seek out and larviposit in burrows in the soil. Conversely, the satellites pursue provisioning wasps and larviposit on the prey while the host female is in flight or as it enters the nest. The females of hole-searchers typically have small eyes and enlarged flagellum and presumably orient to potential nests by a combination of visual and olfactory cues, while satellite species most probably use visual cues as females have conspicuously enlarged anterior ommatidia (Wcsilo, 1986; Spofford & Kurczewski, 1990). The modification of microhabitat features is probably a key factor affecting the nesting and provisioning behaviour of hosts or interfering with the seeking behaviour of flies. Consequently, at the specific level, modifications may differently affect the behaviour of satellite and hole-searcher species of flies. According to their morphology (relatively small eyes and enlarged flagellum) and isolated observations made during samplings, both Opsidia species seem to behave as hole-searchers. Our findings suggest that hole-searchers, namely O. intonsa, seem less affected by human traces on landscapes. Conversely, although the number of Senotainia collected is very low, suggestively no specimen was caught in human-modified environments and, perhaps satellite flies are particularly sensitive to these changes.

Fig. 4: NMDS based on Miltogramminae community composition species in the four types of microhabitats surveyed in sites of the Valdivian Temperate Forest (circle dots) and Patagonian Steppe (square dots) ecoregions, Argentina. a. NMDS based on proportional abundance. b. NMDS based in presence/absence data. References: PM, parks and meadows; SSH, sandy and shrubby areas, TR, trails and roads; WLB, watercourses and lake beaches.
Another classification of Miltogramminae considers whether they are parasites of mud nests or nests in stem (generically called twig nesting) or parasites of ground-nesting wasps. Our sampling design and method of capture probably biased the captures toward ground-nesting kleptoparasites. Typically, the genus Amobia is observed as a twig-nest attacking species (Krombein, 1967). Our study recorded two species of this genus, with a total of 79 specimens, and Amobia ornaticauda (Aldrich) was the second species in abundance.
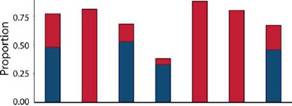
Fig. 5: Partitioning of incidence beta diversity into the turnover (blue) and nestedness (red) components for Miltogramminae assemblages between the four microhabitats surveyed in Argentina. References: PM, parks and meadows; SSH, sandy and shrubby areas; TR, trails and roads; WLB, watercourses and lake beaches.
However, all except one specimen of this species were obtained in a single locality of a deep forested site in the Valdivian ecoregion. In addition, ten specimens belonging to a probably undescribed species were also captured in Valdivian sites. These results suggest twig-nest kleptoparasites, or at least that these two Amobia, are probably more strongly associated with forested areas than with shrublands or steppes.
The species of Senotainia are rare; our samples recorded 25 specimens belonging to seven species occurring in eight sites in both surveyed ecoregions. The occurrence of this genus is specifically associated with open sites in the Valdivian Forest ecoregion (e.g, Batea Mahuida or Pampa de Lonco Luan) and to dry shrublands in the Patagonian steppe.
Finally, in our study, several species of Macronychia were registered in low numbers. Species of this genus have been considered as accidental in wasp nests as they are presumably introduced accidentally into host cells when the wasp prey is a previously parasitised Diptera (Spofford & Kurczewski, 1990), but so far we have no evidence at all that Macronychia spp. parasitise adult flies. In contrast, Verves & Khrokalo (2006) considered the species of Macronychia as kleptoparasites of wasps nesting in twigs, stems and holes in branches.
Other behavioural categorisations specifically refine the classification of Miltogrammine by including behaviours such as stalkers and lurkers or subdividing by the type of twig nest parasite (Wcislo, 1986;Spofford & Kurczewski, 1990). Further studies and observations of the natural and behavioural history of the Miltogramminae should be carried out to typify and clarify the strategies of each species.
Biodiversity
Since Alien (1926) provided the first information of natural history on the New World species of Miltogramminae, several works have added taxonomic and biological information on these flies, nevertheless Neotropical species are still vastly unknown. In fact, Hall (1937) did not record any species in his taxonomic compilation focused on Sarcophagidae from southern South America (reported as "Patagonia and South Chile”). Subsequently, the taxonomic works mentioned only five species for such geographical areas (Pape, 1996; Mulieri & Mariluis, 2011). We confirmed that Miltogramminae diversity is strongly underestimated since 17 species were found during the study. In addition, this research revealed the lack of informative and high-quality taxonomic voucher specimens of miltogrammine flies from large regions of South America and the further need for inventories targeted to these kleptoparasites. Some genera here reported require taxonomic revision and further descriptions of species, such as Senotainia or Macronychia. For instance, four species of Machronychia and six species of Senotainia, which represent new species to be described, were collected during our sampling programme, highlighting the need for biodiversity explorations for this group of flies.
Perspectives and conclusionsExcept for a few taxonomic works, no studies have evaluated the spatial ecology and habitat-association of kleptoparasitic Sarcophagidae as part of their conservation. Studies based on field collections of Miltogramminae do not provide definitive evidence about many general ecological and behavioural aspects. However, while important aspects of their biology, including the host-parasite relationship and the classification of the species according to their searching behaviour, were not specifically studied during this research, our observations on the frequencies of species occurring in relation to different habitats may provide insights into several of these issues.
An emerging question after this study was how each species finds its hosts and its associated niche width in terms of host specificity. We have shown that different habitats lead to changes in the miltogrammine community. The high variability in catches detected in our study is probably associated with local nest density, as observed by Wcislo (1984). We did not measure host density, and this is an important factor to be further analysed.
The fly assemblages differed among habitat types but in most cases indicated a high level of dominance by a single species, O. intonsa. This species, along with O. oebalioides Pape, is presumably a hole-searcher species according to its morphological traits and the preliminary observations performed during this work. Given the dominant role of O. intonsa, represented in almost all samples collected in landscapes modified by humans, we hypothesised that changes in landscapes would likely have a less drastic effect on this relatively common species. By contrast, satellite flies seem to be prone to local extinction on roads and near human settlements.
Previous works suggest that lack of host specificity is related to strong habitat choice in species of Miltogramminae, especially in those observed in bare sandy areas where generalist species were prevalently associated with the diversity and large number of wasp species nesting in such places (Spofford et al., 1989). To conclude, our work revealed that O. intonsa is probably pre-adapted to conditions generated by humans with a wide range of potential hosts. Assessing how the catch frequency and occurrence of kleptoparasitic miltogrammines is related to the type of habitat is of great value not only for their conservation but also for the evaluation of little-known aspects of the history of life, local abundance, niche width, and behavioural traits of the species. However, the communities of kleptoparasites of solitary wasps and bees are not restricted to Miltogramminae and include other Díptera and Hymenoptera (Polidori et al., 2015; Torretta, 2015), and comprehensive studies of the spatial ecology and conservation should be expanded by taking into account these groups.
ACKNOWLEDGMENTS
Special thanks to María Sofía Olea and Luis Compagnucci for their help in the field. Many thanks are also due to the personnel of Administración de Parques Nacionales (APN), to all the staff of the Los Alerces, Lago Puelo and Lanín National Parks, as well as the staff of Batea Mahuida, Epu Laufquen and Domuyo Protected Natural Areas. This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) under Grants PIP 11220090100548, PIP 2015-0431; Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) under Grant PUE 2017-2021; Agencia Nacional de Promoción Científica y Tecnológica under Grants PICT-2008-0094, PICT-2011-0490, PICT 2016-3185.












 uBio
uBio 

