Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Ecología austral
versión On-line ISSN 1667-782X
Ecol. austral v.11 n.2 Córdoba jul./dic. 2001
Filling the groove: energy flow to seabirds in the Beagle Channel, Tierra del Fuego, Argentina
Andrea Raya Rey* & Adrián Schiavinin
Centro Austral de Investigaciones Científicas (CADIC) - CONICET, Ushuaia, Tierra del Fuego, Argentina
* CADIC - CONICET; CC 92; 9410 Ushuaia, Tierra del Fuego, ARGENTINA. Email: arayarey@ciudad.com.ar
Received: 13 June 2001;
Revised: 11 October 2001;
Accepted: 16 October 2001
ABSTRACT. We assessed the energy requirements and the food consumption of the seabird community of the Beagle Channel in summer. We evaluated differences in energy flow among a priori established zones in the study area and among species. We converted data on densities of seabirds in the channel (February and March 1997) into energy demands. Energy flow to seabirds in shallow waters was twice that of in deep waters. Average energy flow in the whole study area was 9.46×104 kJ.km-2.day-1. Energy intake rates ranged 4–13×104 kJ.km-2.day-1, being higher in zones that include breeding sites or close to them. Imperial Cormorant was the major contributor to energy flow in all the zones followed by the Black-browed Albatross. Kelp Gull was the major contributor in areas where food from human origin was available. Energy flow was similar in four zones, which may represent a saturation point for seabird biomass in the Beagle Channel. The factors that explain the observed energy flow distribution deal with the distribution of shallow water areas in the channel, the presence of seabird's colonies, the proportion of diver and surface feeders, and the possible segregation of, at least, the most abundant species. Finally, food consumption varied from 1.71–3.42 ton/day, being higher near breeding colonies and lower in areas with more open waters.
RESUMEN. Saturando el canal: flujo energético de aves marinas en el Canal Beagle, Tierra del Fuego, Argentina: Se determinaron los requerimientos energéticos y el consumo de alimento de la comunidad de aves marinas del Canal Beagle durante el verano. Se evaluaron las diferencias en el flujo energético entre seis zonas establecidas a priori en el área de estudio y entre especies. Se transformaron los datos de densidad de aves marinas del canal (febrero y marzo de 1997) en demanda energética. El flujo energético hacia las aves marinas en las aguas someras fue el doble del que se encontró en aguas profundas. El flujo energético promedio en toda el área de estudio fue de 9.46×104 kJ.km-2.día-1. El rango en las tasas de energía en las distintas zonas fue de 4–13×104 kJ.km-2.día-1, siendo mayor en aquellas zonas con grandes concentraciones de colonias de aves marinas o cerca de ellas. El Cormorán Imperial fue el que más contribuyó al flujo energético en todas las zonas, seguido por el Albatros Ceja Negra. Por su parte, la Gaviota Cocinera fue quien más contribuyó al flujo energético en Bahía Ushuaia, en donde esta especie encuentra alimento extra proveniente de residuos de origen humano. El flujo energético fue similar en cuatro zonas, lo que sugiere que la biomasa de aves marinas en el Canal Beagle se encuentra en un nivel de saturación. Los factores que pueden explicar este valor constante a lo largo del Canal y la variación en la proporción de especies en cada zona son la presencia de áreas de aguas someras, la presencia de colonias de aves marinas, la proporción de especies buceadoras y las que se alimentan en superficie, y la segregación de, al menos, las especies más abundantes. Finalmente, el consumo de alimento fue de 1.71–3.42 ton/día, siendo mayor en zonas cercanas a colonias de nidificación y menor en áreas de aguas abiertas.
INTRODUCTION
In recent years there has been a growing interest in the role of seabirds as consumers in marine ecosystems. Estimates of the marine resources required to maintain those communities have been generated based on the energetic demand of seabird colonies (Furness 1978; Wiens et al. 1979; Croxall & Prince 1980; Furness & Cooper 1982; Woehler 1990). Also, some authors used the energetic requirements of the seabirds' biomass in oceanic regions (Sanger 1972; Idyll 1973; Wiens & Scott 1975; Everson 1977; Hunt et al. 1981; Hunt 1985; Woehler & Green 1992; Woehler 1997). Models based on the abundance of birds in the marine environment include all members of the population and present advantages over models that account only for individuals in colonies (Woehler 1997).
The assessment of the energetic requirements of seabirds was made following different approaches. Some authors transformed the standard metabolic rate of individual birds, estimated through the allometric equations in Kendeigh (1970) or Lasiewski & Dawson (1967), to the daily energy intake. Then, together with the occupancy or density, the seabird biomass can be converted into energetic requirements (Wiens 1984; Schneider et al. 1986; Joiris 1996; Woehler 1997). Other authors used the percentage of biomass as a measure of energetic requirement (Hunt et al. 1981).
The energetic requirement estimations of seabirds may provide a minimum assessment of the stock of marine resources around seabird breeding localities. Moreover, this information is useful in assessing seabird-fisheries competition (Woehler & Green 1992).
Community patterns are usually expressed in terms of numbers of species, densities, or derivatives of these values such as diversity. However, biomass and energy flow is also important criteria for defining community patterns. A focus on the energy dynamics of communities may be especially appropriate for several reasons. First, the availability of energy may limit individuals and populations, and, if community patterns are expressed in terms of energy, they may be related directly to a limiting factor. Second, the demand placed on resources by community members may be estimated quantitatively, which allows an assessment of their role in the trophic dynamics of ecosystems. Also, the species distribution patterns that emerge when communities are considered in terms of biomass may differ from those determined by density calculations, because few individuals of larger species may contribute considerably more standing crop biomass than many individuals of smaller species (Wiens 1989).
In the present study we reanalysed data of seabirds' density in the Beagle Channel, Tierra del Fuego, in terms of biomass. These data were expressed in terms of energy flow to determine areas in the Beagle Channel with regard to energy flow to seabirds. Based on this data, the food consumption in the Beagle Channel was estimated as well as the energy flow to species with different foraging strategies.
STUDY AREA
The Beagle Channel runs in an east–west direction along the southern coast of the Isla Grande de Tierra del Fuego, at about 55°S. It is located in the subantarctic zone and connects the Atlantic and Pacific Oceans. The studied area comprised waters of the central and eastern sector of the Beagle Channel, shared between Argentina and Chile, from the west limit of the Argentine sector to the waters around Isla Martillo, at the east of Isla Gable (Figure 1). Bathymetry defines shallow waters areas where kelp beds (Macrocystis pyrifera) are common. Water depths are variable, increasing from the east to the west, and reaching depths greater than 150 m in the centre of the channel. Surface salinity is strongly affected by fresh water courses (run off and glacial melting), being higher close to the mouth of the channel due to the influence of the Atlantic Ocean (Kloser 1996), and decreasing towards the west or the inner part of the channel due to the presence of glaciers. The exposure to wind and waves due to the westerly prevailing winds changes in relation to the orientation of the coast. Thus, windward coasts generally presents steeper slopes than leeward coasts. The studied area also holds two urban centres, Ushuaia (45000 inhabitants) and Puerto Williams (some thousands inhabitants). Furthermore, the area holds two concentrations of seabird colonies. The first one is located at Islas Bridges, close to the Ushuaia city, and the second one on the East side of the channel close to Isla Gable (Schiavini & Yorio 1995; Raya Rey & Schiavini 2000).
Figure 1. Map of the Beagle Channel showing the six zones considered in this study: Isla Gable (IG), Centre Beagle Channel (CBC), Islas Bridges (IB), Bahía Ushuaia (BU), Bahía Lapataia (BL), and Western part of the Beagle Channel (WBC). Grey areas are urban centres, and the dashed line indicates the international border.
Figura 1. Mapa del Canal Beagle mostrando las seis zonas consideradas en el estudio: Isla Gable (IG), Canal de Beagle Central (CBC), Islas Bridges (IB), Bahía Ushuaia (BU), Bahía Lapataia (BL) y Canal de Beagle Occidental (WBC). Las áreas grises corresponden a centros urbanos y la línea discontinua señala la frontera internacional.
METHODS
The studied area was stratified a priori in six zones and two water depths, based on the physical factors that vary at a local level (bathymetry, surface salinity, exposure to wind and waves and currents). We took into account the human factor, based on the closeness to the cities. The stratification of water depths was based on a limit of 20 m (shallow waters: less than 20 m depth, deep waters: more than 20 m depth) to include those waters where Macrocystis pyrifera was rooted (Santelices 1991; Raya Rey & Schiavini 2000). The six zones identified were (Figure 1): 1) Bahía Lapataia and adjacent waters, an area of protected waters with inlet waters due to the influence of Lapataia river; 2) Western Beagle Channel, an area of exposed and unprotected coasts, with indented rocky shores with coastal forests (it includes shallow waters area of a bay near Ushuaia city); 3) Bahía Ushuaia, comprising waters close to Ushuaia city and the commercial and navy ports, and including the mouths of three important watercourses; 4) Islas Bridges, an area of islets with a large proportion of shallow waters and a seabird breeding colony with high diversity; 5) Centre Beagle Channel, an area of unprotected coasts without islets, with coastal forests and shrubs, and pocket gravel beaches in some places; and 6) Isla Gable and waters around, with a high proportion of shallow waters (there is a colony of Magellanic penguins there).
We reassessed data on densities of seabirds foraging in the Beagle Channel for each zone (Raya Rey & Schiavini 2000) in terms of biomass, accordingly to the average mass per species (Humphrey et al. 1970; Schiavini 1990), and then into energy flow. Density was estimated in summer (February and March) 1997 by using the strip transect method widely used in seabird studies (Tasker et al. 1984). For more details about estimation of seabird density see Raya Rey & Schiavini (2000). At the moment of the estimation, seabirds were rearing chicks; therefore, their food requirements were larger. We included in the analysis breeding as well as non-breeding species occurring in the study area: Magellanic Penguin (Spheniscus magellanicus), Black-browed Albatross (Thalassarche melanophris), Southern Giant Petrel (Macronectes giganteus), Rock Cormorant (Phalacrocorax magellanicus), Imperial Cormorant (P. atriceps), Kelp Gull (Larus dominicanus), Dolphin Gull (L. scoresbii), Chilean Skua (Catharacta chilensis), and South American Tern (Sterna hirundinacea). These were the most abundant seabird species in summer. There are some other seabirds, although they are rare and their density and body mass are very low (e.g., Pelecanoides sp.).
The energy flow to pelagic birds was estimated from relationships describing daily individual energy requirements as a function of the standard metabolic rate and body mass, following Hunt et al. (1981), Schneider & Hunt (1982), and Schneider et al. (1986). The standard metabolic rate (SMR) was calculated from the allometric formula of Lasiewski & Dawson (1967). The daily intake of active birds was estimated using a conversion of 2.8 SMR (Kooyman et al. 1982) and an assimilation efficiency of 75% (Cooper 1978). Daily intake (in kJ/day) was estimated as 1216 M 0.723, where M is body mass (in kg). The daily occupancy was assessed as the density of each species in each zone. Daily energy flow to each species was estimated as the product of occupancy and intake. Daily energy flow in each of the six zones was the sum of these products. To compare the biomass of seabirds in the different zones we estimated the biomass per area unit as far as each one has different surface.
Food consumption was estimated assuming that individuals consume 20–40% (minimum and maximum) of their body mass in prey per day (Hunt et al. 1981). The total biomasses of species in each zone were used to estimate the food consumption. Taking into account the foraging strategies, we evaluated the energy flow to surface feeders (Black-browed Albatross, Southern Giant Petrel, Kelp Gull, South American Tern and Chilean Skua) and to diver feeders (Imperial Cormorant, Rock Cormorant and Magellanic Penguin).
RESULTS
Seabirds' energy flow was not homogeneously distributed between the two water depth strata and among zones. Energy flow to seabirds in shallow waters was almost twice than the one in deep waters (one-way ANOVA, F1,10= 5.95, P = 0.037). Isla Bridges, West part of the Beagle Channel and Isla Gable zones support the highest energy flow to seabird per area unit (Figure 2a). Bahía Ushuaia (which holds the commercial and navy harbors) was the fourth zone in regard to energy flow importance. Bahía Lapataia and Centre Beagle Channel zones held the lowest energy flow per area unit. The total energy flow to birds averaged over all six zones (mean ± SD) was (9.46 ± 3.2)×104 kJ.km-2.day-1. However, Isla Gable, Islas Bridges, and Western Beagle Channel zones presented almost equal energy flow per unit area (Figure 2a). The contribution to energy flow per species also differed between zones (Figure 2b). The Imperial Cormorant was the major contributor in all the six zones, followed by the Black-browed Albatross in almost all zones, except Islas Bridges. This species showed a contribution greater than 10% in each zone. Flow to Kelp Gull as well as to Southern Giant Petrel occurred in the western zones and, specially, in Bahía Ushuaia. In Bahía Ushuaia and Bahía Lapataia, the Kelp Gull was the second dominant species. The Dolphin Gull was the second most important species in Islas Bridges, and was restricted to this zone as well as to the Bahía Ushuaia zone. The remaining species contributed less to total consumption in all the zones, either because of their low masses (e.g., South American Tern) or because their low densities (e.g., Rock Cormorant).
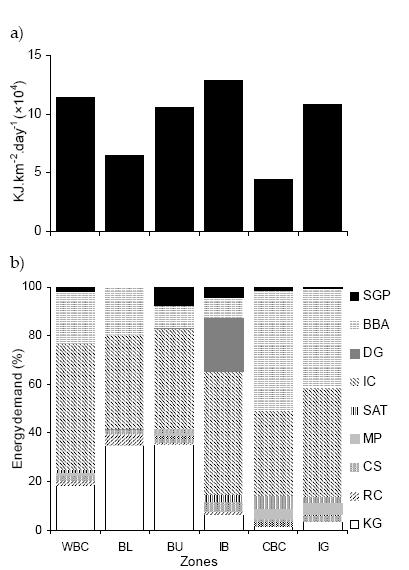
Figure 2. Energy flow to seabirds per area unit (a) and percent energy demand for each seabird species (b) in the six zones of the Beagle Channel. Zone codes are the same as in Figure 1. SGP: Southern Giant Petrel, BBA: Black-browed Albatross, DG: Dolphin Gull, IC: Imperial Cormorant, SAT: South American Tern, MP: Magellanic Penguin, CS: Chilean Skua, RC: Rock Cormorant, KG: Kelp Gull.
Figura 2. Flujo energético hacia las aves marinas por unidad de área (a) y porcentaje de la demanda energética de cada especie (b) en las seis zonas del Canal Beagle. Los códigos de las zonas son los mismos que en la Figura 1. SGP: petrel gigante del sur, BBA: albatros de ceja negra, DG: gaviota austral, IC: cormorán imperial, SAT: gaviotín sudamericano, MP: pingüino de Magallanes, CS: skúa chileno, RC: cormorán de cuello negro, KG: gaviota cocinera.
Western Beagle Channel and Islas Bridges zones showed a higher proportion of energy flow to diver feeders, while Isla Gable zone presented 50% of energy flow to diver feeders and 50% to surface feeders (Figure 3). Likewise, the other three zones showed 60% of energy flow to surface feeders and 40% to diver feeders. The maximum energy flow among the surface feeders went to the Blackbrowed Albatross, while among the diver feeders it went to the Imperial Cormorant (Figure 3).
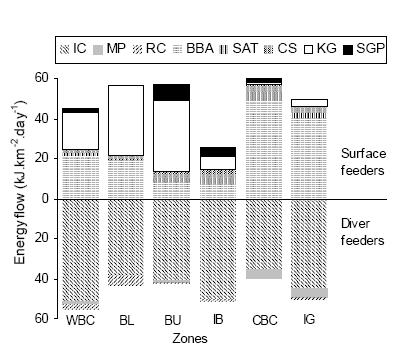
Figure 3. Energy Demand flow to surface feeder species (above), and to diver feeder species (below) in the six zones of the Beagle Channel. Zone and species codes are the same as in Figure 2.
Figura 3. Flujo energético hacia cada especie que se alimenta en la superficie (arriba) y hacia cada especie que se alimenta por debajo de la superficie o buceando (abajo) en las seis zonas del Canal Beagle. Los códigos de las zonas y de las especies son los mismos que en la Figura 2.
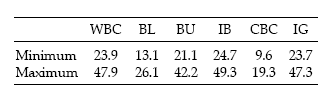
Mean food consumption for the whole study area was estimated at 1.71–3.42 ton/day. The highest values were found in Islas Bridges, while the Centre Beagle Channel zone presented the lowest values (Table 1).
Table 1. Minimum and maximum food consumption of seabirds (in kg.km-2.day-1) in the six zones of the Beagle Channel. Zone codes are the same as in Figure 1.
Tabla 1. Consumo máximo y mínimo de alimento por aves marinas (en kg.km-2.día-1) en las seis zonas del Canal Beagle. Los códigos de las zonas son los mismos que en la Figura 1.
DISCUSSION
This paper reports a comparison of seabird biomass and of food consumption distribution among different zones in a watercourse with particular characteristics as the Beagle Channel. Life histories and foraging strategies might be the most important factors that determine the observed pattern. The factors that explain the observed energy flow distribution deal with the distribution of shallow waters areas in the Beagle Channel, the presence of seabird colonies, the proportion of diver and surface feeders, and the possible segregation of, at least, the most abundant species. These factors might influence the distribution pattern of energy flow at different degrees among the six zones, resulting in apparently confusing patterns.
The fact that shallow waters present the larger biomass of seabirds could be related to the large diversity and abundance of organisms that can be found in the kelp forests (Moreno & Jara 1994; Castilla 1995; BJ Lomovasky, CADIC, public comm.). This kelp forest has been reported as an important feeding habitat for many seabird species (Humphrey et al. 1970; Punta et al. 1993; pers. obs.).
A remarkable point is that there are four zones of the Beagle Channel with a similar value of energy flow (average of [115 ± 1]×104 kJ.km-2.day-1). This rather homogeneous value across different zones of the Beagle Channel suggests that the energy flow to seabirds, and thus the biomass, could be at a saturation point. Even though we may accept the energy flow level as a saturation point, it is remarkable that this level is constant across the Beagle Channel, with changes in the proportion of species. The mechanism associated to this variation in the proportion of biomass of species across zones is not clear to us, although it is likely that the distribution of seabird colonies may play an important role on it.
The energy demand of seabirds was also reported to increase with the closeness to their breeding colonies (Furness 1978; Wiens 1984; Stahl et al. 1985). In this context, it should be expected that the seabird community of the Beagle Channel (excepting the Black-browed Albatross and the Giant Petrel, non-resident breeding species) would not forage far from their colonies without diminishing their breeding success. Actually, the energy intake rate in the Beagle Channel ranges 4–13×104 kJ.km-2.day-1, being larger in zones that include breeding sites, such as Islas Bridges and Isla Gable zones (Schiavini & Yorio 1995).
It should be also expected that shallow waters, including the coast, would be more suitable for species with diving foraging behaviour (cormorants) or for species that depend on human induced resources like garbage on the coasts (gulls). Hunt (1985) suggested that, when foraging in inshore waters, surface feeders would be at a competitive disadvantage in relation to sub-surface foragers, due to the large proportion of the water column available to diver feeders. However, as we include surface feeder species that has coastal feeding habits, such as Kelp Gull and Southern Giant Petrel, zones with high percentage of shallow waters (e.g., Bahía Ushuaia) present a large percentage of surface feeders.
The contribution of energy flow to diver feeders was larger in zones with a large proportion of breeding colonies or close to them (Western Beagle Channel and Islas Bridges zones), which coincided with the results found in South Georgia by Croxall & Prince (1980). However, Isla Gable, a zone with breeding colonies, presented equal percentages for both diver and surface feeders. This may be due to the presence of species with large mass among the surface feeders (such as the Black-browed Albatross), in turn due to the closeness of this zone with the mouth of the Beagle Channel, an open water area.
Another explanation could be some kind of segregation as a product of interspecific competition, at least for the most important species in terms of abundance (such as the Blackbrowed Albatross, the Imperial Cormorant and the Kelp Gull). It was already reported that none of the three species has interspecific association with the other two in the Beagle Channel (Raya Rey & Schiavini 2000). These authors reported that Black-browed Albatross and Kelp Gull present differences in distribution between zones, and that Imperial Cormorant is more abundant in shallow waters without differences among zones.
Given that the Western Beagle Channel and the Centre Beagle Channel zones both present similar characteristics, it is notable that the first one has almost twice the energy demand per area unit than the second. The high values for the Western Beagle Channel zone may be explained for several reasons. First, its closeness to the Islas Bridges zone (with many seabird colonies). Second, the inclusion of large areas of shallow waters at the west of the Península Ushuaia. Finally, the closeness of this zone to the Murray Channel, a small waterway that connects the Beagle Channel to the more open waters of the southern Fueguian archipelago close to Cape Horn.
It has been suggested that a large biomass of seabirds on the sea is correlated with a high marine productivity in the Antarctica (Woehler 1990, 1997). Then, the energy flow described in our study area suggests that the Beagle Channel may represent an area of high productivity. Moreover, we found a high-energy flow to non-resident species in the study area: Black–browed Albatross (in all the zones) and Southern Giant Petrel (in Bahía Ushuaia zone). Woehler (1997) pointed out that nonresident species could be better indicators of the availability of marine resources than resident species, because the resident birds would be incapable of feeding far from their colonies.
Total food consumption in the Beagle Channel ranges 1.71–3.42 ton/day. Comparisons with other areas such as Alaska or South Georgia could be interesting, since these areas are geographically similar (presence of fjords, small channels, inlets, etc.). Food intake values in the Beagle Channel were of one order of magnitude greater than the ones found for seabirds around Svalbard (Joiris 1996). Values for South Georgia (Croxall et al. 1984) were five orders of magnitude higher than values for the Beagle Channel. The high density of seabird colonies in this island, especially penguins, may be the reason of the high food consumption in this zone. In contrast, food intake values were of the same order of magnitude as in the Gulf of Alaska (Wiens 1984). However, we have to be careful to make conclusions because data from those studies were based on the reproductive population and ours are based on all foraging seabirds, which includes juveniles.
The process of management of natural resources in the marine environment usually includes the assessment of utilization of the marine environment by top predators, as well as the distribution of breeding colonies. As the Beagle Channel is experiencing a growing pressure for the use of their natural resources (due to tourism, fishing and aquaculture), the assessment of the distribution of foraging effort may represent a useful tool for the zoning and the management of the whole area.
ACKNOWLEDGEMENTS
This study was supported by CONICET and by the project Consolidation and Implementation of the Patagonian Coastal Zone Management Plan (Project ARG 97 G31 GEF/ UNDP/ Ministerio de Relaciones Exteriores, Comercio Internacional y Culto). We are grateful to the captains and the crews of the catamarans Ezequiel MB and Luciano Beta, who helped with the transportation through the channel, and who collaborated beyond all expectation. We also thank J. Calvo, J. Calcagno, J. Lopez de Casenave and A. Chizzini for their help in different aspects of this study.
REFERENCES
CASTILLA, JC. 1995. Food webs and functional aspects of the kelp, Macrocystis pyrifera, community in the Beagle Channel, Chile. Pp. 407-414 in: WR Siegfred; PR Condy & RM Laws (eds). Antarctic nutrient cycles and food webs. Springer-Verlag, Berlin. [ Links ]
COOPER, J. 1978. Energetic requirements for growth and maintenance of the Cape Gannet (Aves: Sulidae). Zoologica Afr. 13:307–317. [ Links ]
CROXALL, JP & PA PRINCE. 1980. Food, feeding ecology and segregation of seabirds at South Georgia. Biol. J. Linn. Soc. 14:103–131. [ Links ]
CROXALL, JP; C RICKETTS & PA PRINCE. 1984. Impacts of seabirds on marine resources, especially krill, at South Georgia waters. Pp. 285–317 in: GC Whittow & H Rahnn (eds). Seabird energetics. Plenum Press, New York. [ Links ]
EVERSON, I. 1977. The living resources of the Southern Ocean. Food and Agricultural Organization, Southern Ocean Fisheries Survey Programme, Roma. [ Links ]
FURNESS, RW. 1978. Energy requirements of seabirds communities: a bioenergetic model. J. Anim. Ecol. 47:39–53. [ Links ]
FURNESS, RW & J COOPER. 1982. Interactions between breeding seabird and pelagic fish populations in the southern Benguela region. Mar. Ecol. Prog. Ser. 8:243–250. [ Links ]
HUMPHREY, PS; D BRIDGE; PW REYNOLDS & RT PETERSON. 1970. Birds of Isla Grande (Tierra del Fuego). Smithsonian Institution, Washington DC. 411 pp. [ Links ]
HUNT, GL. 1985. A preliminary comparison of marine bird biomass and food consumption between the southeastern Bering Sea and parts of the Southern Ocean. Pp. 487–492 in: WR Siegfred; PR Condy & RM Laws (eds). Antarctic nutrient cycles and food webs. Springer-Verlag, Berlin. [ Links ]
HUNT, GL; B BURGERSON & GA SANGER. 1981. Feeding ecology of seabirds of the eastern Bering Sea. Pp. 629–648 in: DW Hood & JS Calder (eds). The eastern Bering Sea shelf: oceanography and resources. Vol 2. US Gov. Printing Office, Washington DC. [ Links ]
IDYLL, CP. 1973. The anchovy crisis. Sci. Am. 228:22–29. [ Links ]
JOIRIS, CR. 1996. At-sea distribution of seabirds and marine mammals around Svalbard, summer 1991. Polar Biol. 16:423–429. [ Links ]
KENDEIGH, SC. 1970. Energy requirements for existence in relation to size of birds. Condor 72:60–65. [ Links ]
KLOSER, H. 1996. Hydrography of the Beagle Channel. Pp. 18–20 in: W Arntz & M Gorny (eds). Cruise report of the joint Chilean-German-Italian Magellan "Victor Hensen" campaign in 1994. Berichte zur Polarforschung 19. Bremerhaven, Alemania. [ Links ]
KOOYMAN, GL; RW DAVIS; JP CROXALL & DP COSTA. 1982. Diving depths and energy requirements of King Penguins. Science 217:726–727. [ Links ]
LASIEWSKI, RC & WR DAWSON. 1967. A re-examination of the relation between standard metabolic rate and body weights in birds. Condor 69:13–23. [ Links ]
MORENO, CA & HF JARA. 1994. Ecological studies on fish fauna associated with Macrocystis pyrifera belts in the south of Fueguian Islands, Chile. Mar. Ecol. Prog. Ser. 15:99–107. [ Links ]
PUNTA, GE; JRC SARAVIA & PM YORIO. 1993. The diet and foraging behaviour of two Patagonian cormorants. Mar. Ornithol. 21:27–36. [ Links ]
RAYA REY, A & ACM SCHIAVINI. 2000. Distribution, abundance and associations of seabirds in the Beagle Channel, Tierra del Fuego, Argentina. Polar Biol. 23:338–345. [ Links ]
SANGER, GA. 1972. Preliminary standing stock and biomass estimates of seabirds in the subarctic Pacific region. Pp. 589–611 in: AY Takenouti et al. (eds). Biological oceanography of the northern North Pacific Ocean. Idemitsu Shoten, Tokyo. 626 pp. [ Links ]
SANTELICES, B. 1991. Littoral and sublittoral communities of continental Chile. Pp. 347–369 in: AC Mathieson & PH Nienhuis (eds). Intertidal and littoral ecosystems. Ecosystems of the world. Vol. 24. Elsevier, Amsterdam. 564 pp. [ Links ]
SCHIAVINI, AC & P YORIO. 1995. Distribution and abundance of seabird colonies in the Argentine sector of the Beagle Channel, Tierra del Fuego. Mar. Ornithol. 23:39–46. [ Links ]
SCHIAVINI, ACM. 1990 Estudio de la relación entre el hombre y los pinnípedos en el proceso adaptativo humano al Canal Beagle, Tierra del Fuego, Argentina. Tesis doctoral. Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Argentina. [ Links ]
SCHNEIDER, DC & GL HUNT. 1982. Carbon flux to seabirds in waters with different mixing regimes in the southeastern Bering Sea. Mar. Biol. 67:337–344. [ Links ]
SCHNEIDER, DC; GL HUNT & NM HARRISON. 1986. Mass and energy transfer to seabirds in the southeastern Bering Sea. Cont. Shelf Res. 5:241–257. [ Links ]
STAHL, JC; P JOUVENTIN; JL MOUGIN; JP ROUX & H WEIMERSKIRCH. 1985. The foraging zones of seabirds in the Crozet Islands sector of the Southern Ocean. Pp. 478–486 in: WR Siegried; PR Condy & RM Laws (eds.) Antarctic nutrient cycles and food webs. Springer-Verlag, Berlin. [ Links ]
TASKER, ML; P HOPE JONES; T DIXON & BF BLAKE. 1984. Counting seabirds at sea from ships: a review of methods employed and a suggestion for a standardized approach. Auk 101:567–577. [ Links ]
WIENS, JA. 1984. Modeling the energetic requirements of seabirds populations. Pp. 255–284 in: GC Whittow & H Rahnn (eds). Seabird energetics. Plenum Press, New York. [ Links ]
WIENS, JA. 1989. The ecology of bird communities. Cambridge University Press, Cambridge. 315 pp. [ Links ]
WIENS, JA; RG FORD; D HEINEMANN & C FIEBER. 1979. Simulation modelling of marine bird population energetics, food consumption and sensitivity to perturbation. Environ. Assess. Alaskan Cont. Shelf. 1:217–270. [ Links ]
WIENS, JA & JM SCOTT. 1975. Model estimation of energy flow in Oregon coastal seabird populations. Condor 77:430–452. [ Links ]
WOEHLER, EJ. 1990. The distribution of seabird biomass in the Australian Antarctic Territory: implications for conservation. Environ. Conserv. 17:256–261. [ Links ]
WOEHLER, EJ. 1997. Seabird abundance, biomass and prey consumption within Prydz Bay, Antarctica 1980/1981–1992/1993. Polar Biol. 17:371–383. [ Links ]
WOEHLER, EJ & K GREEN. 1992. Consumption of marine resources by seabirds and seals at Heard Island and the McDonald Islands. Polar Biol. 12:659–665. [ Links ]














