Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Ecología austral
On-line version ISSN 1667-782X
Ecol. austral vol.16 no.1 Córdoba Jan./June 2006
COMUNICACIONES BREVES
Energy density of freshwater Patagonian organisms
Javier Ciancio* & Miguel Pascual
Centro Nacional Patagónico (CENPAT, CONICET), Puerto Madryn, Chubut, Argentina
*Centro Nacional Patagónico (CENPAT, CONICET), Boulevard Brown s/n, Puerto Madryn (CP 9120), Chubut, Argentina. Tel.: 54-2965-451024 Fax: 54-2965-451543. Email: uncianci@cenpat.edu.ar
Recibido: 11 de noviembre de 2004;
Fin de arbitraje: 30 de mayo de 2005;
Revisión recibida: 19 de enero de 2006;
Aceptado: 10 de mayo de 2006
ABSTRACT: We assessed by using a bomb calorimeter the energy density of the main species of Patagonian freshwater ecosystems, including fish, crustaceans, gastropods, oligochaetes, and insects. Fish (5048-5789 Cal/g) were the most energy density group, followed by insects (5062- 5232), crustaceans (3364-3994), oligochaetes (3471) and gastropods (1143). These data consist on the first direct energy density estimations of freshwater species and are intended as a baseline information for modelling the energy fluxes in Patagonian freshwater ecosystems.
Keywords: Bomb calorimeter; Bioenergetic model.
RESUMEN: Densidad energética de los organismos Patagónicos de agua dulce. La densidad energética de los organismos puede ser utilizada con distintos fines como evaluar la calidad de la dieta, comparar la importancia de distintas presas para un predador, corregir las tasas de evacuación gástrica de modelos, explicar el comportamiento de forrajeo de ciertos predadores o determinar su estado fisiológico. Consiste en un parámetro fundamental para el modelado bioenergético de ecosistemas. En este trabajo estimamos la densidad energética de los principales grupos de los ecosistemas de agua dulce de la Patagonia, incluyendo peces, crustáceos, gasterópodos, oligoquetos e insectos. Encontramos que los peces (5048-5789 Cal/g) es el grupo energéticamente más denso seguido de los insectos (5062-5232), crustáceos (3364-3994), oligoquetos (3471) y finalmente los gasterópodos (1143).
Palabras clave: Calorímetro de bomba; Modelo bioenergético.
INTRODUCTION
Energy density has been used to evaluate diet quality (Wanless 2005), compare relative importance of prey items (Harris & Hislop 1978), correct gastric evacuation rates in models (Pedersen & Hislop 2001), explain foraging behaviour of predators (Benoit-Bird 2004) and determine the physiological status of organisms. During the last 20 years bioenergeticallybased food web models, when coupled with direct sampling for diet, growth, size structure, thermal experience and estimates of relative or absolute abundance, have provided an effective method for quantifying trophic interactions in a temporal, spatial, and ontogenetic framework. Bioenergetics models have also been used to investigate ecological problems such as the potential effects of fish introductions (Ruzycki et al. 2003), global warming (Hill & Magnuson 1990), predation as a factor in recruitment failure (Hartman & Margraf 1993), and several other problems in fish ecology (Brandt 1993; Hayes et al. 2000; Trudel & Rasmussen 2001; Harvey et al. 2002). Bioenergetic models require the estimation of individual size and growth rate, water temperature, energy density in prey and predator, and 10–30 parameters to represent food consumption, metabolic costs and waste production (Trudel et al. 2004).
Adiabatic bomb calorimeter (direct) and proximal composition (indirect) are frequently used methods to assess energy density in organisms (Craig et al. 1978). In Patagonia, few attempts have been made to estimate energy density of freshwater aquatic ecosystems organisms (only indirect measured by the proximal composition as described by Baez 1988 and Dorscht 1988), in spite of an increasing demand of information to feed models that explore different fisheries and conservation managing actions (Shuter & Meisner 1992; Koen- Alonzo & Yodzis 2005). In this paper we used and adiabatic bomb calorimeter to present the first data based on energy density of freshwater aquatics animal species of Patagonian waters (including mollusks, arthropods, insects and fish). The results presented in this work are the first step of ‘bioenergetic modelling'; one way to assess the ecological effects of diverse human actions that are threatening freshwater diversity such as salmonid introduction (Pascual et al. 2002).
METHODS
Organisms were collected from different rivers and lakes of Patagonia from November to April in 2000-2001 (Rivers: Limay (L), Santa Cruz (SC), Tecka (T), Corcovado (C), Lakes: Gutierrez (G), Moreno (M), Nahuel Huapi (N), Strobel Plateau lagoons (S)). Invertebrates were captured using a surber trap, and fish and crayfish using a fishing basket. (Table 1). Samples were placed in bags to avoid dehydration and were frozen. Samples were chopped, homogenized, dried in a stove at 60-80 oC for 24-72 hours and then ground to powder. Chilina spp. were homogenized including shells. Wet and dry weights were registered with precision of 0.001 g and were determined by weighing the homogenate before and after drying. One-gram pellets were made and burned at 30 atmospheres of oxygen in a calorimeter bomb (Parr model 1241) to determine gross energetic content. Fuse wire corrections were determined after each combustion event. One to three pellets of each sample were burned and the mean was used to estimate energy density of the sample. For small individuals, i.e. insects, am-phipods and gastropods, a sampling unit consisted of whole-body samples of numerous in-dividuals. For large individuals such as fish, a sampling unit consisted of a sample of a sin-gle individual from a whole-body homogenate. Energy density (ED) is expressed in calories per gram dry weight and joules per gram wet weight.
In order to validate the results of the calorimetric analyses, the caloric content of several prey was also estimated based on their proximal composition of protein, lipids and carbohydrates (Table 1), as reported elsewhere (Baez et al. 1988; Dorscht 1988), multiplied by the corresponding gross energy contents (protein: 5640 Cal/g DW; lipid: 8700 DW; carbohydrates: 4111 Cal/g DW) (Higgs et al. 1995), or compared with analogous species of North America.
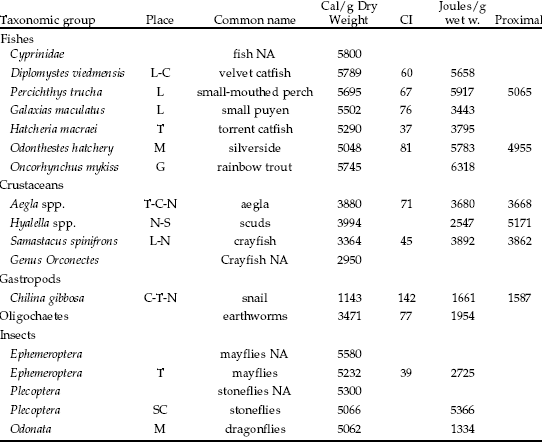
Table 1. Groups included in calorimetric analysis and their gross energy values determined both by bomb calorimeter (Cal/g Dry Weight and Joules/g wet weight ) and proximal composition (Cal/g DW) (Baez et al. 1988; Dorscht 1988) (95% confidence interval for the mean CI). Crayfish NA (Genus Orconectes), Stoneflies NA (Plecoptera), Mayflies NA (Ephemeroptera) and fish NA (Cyprinidae) correspond to homologous species of North America (Probst et al. 1984).
Tabla 1. Densidad energética de los grupos determinada por calorimetría mediante el uso de una bomba adiabática de oxígeno (Cal/g Dry Weight calorías por gramo de peso seco y Joules/g wet w. Joules por gramo de peso húmedo) y por su contenido proximal (Proximal, Expresado en Cal/g peso seco) (Baez et al. 1988; Dorscht 1988). CI: intervalo de confianza del 95% para la media. Crayfish NA (Genus Orconectes), Stoneflies NA (Plecoptera), Mayflies NA (Ephemeroptera) and fish NA (Cyprinidae) corresponden a especies homólogas de Norte América (valores tomados de Probst et al. 1984).
RESULTS
According to their energy densities, prey species can be separated into four groups (Table 1). The most energy-rich prey group (5502-5789 Cal/g DW) contains exclusively fish. The following group (5062-5290 Cal/g DW) is composed by fish and insects. The third group is composed by crustaceans and oligochaetes (3364-3994 Cal/g DW). Snails were the leastcaloric group (1143 Cal/g DW). Calorific contents based on proximal composition match direct measures closely (Table 1). Also, the calorific contents reported are similar to those of homologous groups of North America (Probst et al. 1984), such as fish, crayfish, and insects. Most of the species included in the analysis are conspicuous prey item of salmons in Patagonia (Macchi et al. 1999). For example, the endemic macro-crustaceans Aegla spp. have been regarded as high quality food for trout both by biologists (Burns 1972; Ferriz 1993) and by sport fishermen (several articles from Chile and Argentina in internet search for "Aegla" or "pancora"). This work indicates that the energy density of Aegla spp. (3880 Cal/g DW) is significantly lower than those of fish, insects and amphipods, but significantly higher than that of crayfish both from Patagonia (Samastacus spinifrons, 3364 Cal/g DW) and North America (Orconectes spp., 2950 Cal/g DW). The quality of a particular species as prey is not given exclusively by its energy content. Other factors will also determine its intrinsic value, such as proximal composition, availability, handling time, and individual size.
ACKNOWLEDGEMENTS
We thank P. Macchi for his help on samples collection.
REFERENCES
BAEZ, V; E DORSCH; M BATTINI & R PAPA. 1988. Consideraciones sobre la composición química de Percichthys spp. y Patagonina hatcheri. Segunda Reunión Argentina de Acuicultura. Puerto Madryn, Chubut. [ Links ]
BENOIT-BIRD, KJ. 2004. Prey caloric value and predator energy needs: foraging predictions for wild spinner dolphins. Mar. Biol. 145:435–444. [ Links ]
BRANDT, SB. 1993. The effect of thermal front on fish growth: a bioenergetics evaluation of food and temperature. Estuaries 16:142-159. [ Links ]
BURNS, JW. 1972. The distribution and life history of South American Freshwater crabs (Aegla) and their role in trout stream and lakes. Trans. Am. Fish. Soc. 4:595-607. [ Links ]
CRAIG, JF; MJ KENLEY & JF TALLING. 1978. Comparative estimation of the energy content of fish tissue from bomb calorimetry, wet oxidation and proximate analysis. Fresw. Biol. 8:585-590. [ Links ]
DORSCHT, E. 1988. Valoración química de los alimentos naturales consumidos habitualmente por Salmo gairdneri. Segunda Reunión Argentina de Acuicultura. Puerto Madryn, Chubut. [ Links ]
FERRIZ, RA. 1993. Some Aspects of four fish species diet from Limay River (Argentina). Revista de Ictiología. 2/3:1-7. [ Links ]
HARRIS, M & JRG HISLOP. 1978. The food of young Puffins Fratercula artica. J. Zool. Lond. 185:213-236. [ Links ]
HARTMAN, KJ & FJ MARGRAFF. 1993. Evidence of predatory control of yellow perch (Perca Flavescens) recruitment in Lake Erie, U.S.A. J. Fish Biol. 42:109-119. [ Links ]
HARVEY, CJ; PC HANSON; TE ESSINGTON; PB BROWN & JF KITCHELL. 2002. Using bioenergetics models to predict stable isotope ratios in fishes Can. J. Fish. Aquat. Sci. 59:115–124. [ Links ]
HAYES, JW; JD STARK & KA SHEARER. 2000. Development and test of a whole-lifetime foraging and bioenergetics growth model for drift-feeding Brown trout. Trans. Am. Fish. Soc. 129: 315-332. [ Links ]
HIGGS, DA; JS MACDONALD; CD LEVINGS & BS DOSANJH. 1995. Nutrition and feeding habits in relation to life history stage. Pp. 159-315 in: C Groot; L Margolis & WC Clarke (eds.). Physiological Ecology of Pacific Salmon. Univ. British Columbia Press. Vancouver. [ Links ]
HILL, DK & JJ MAGNUSON. 1990. Potential Effects of Global Climate Warming on the Growth and Prey Consumption of Great Lakes Fish. Trans. Am. Fish. Soc.119:265–275 [ Links ]
KOEN-ALONSO, M & P YODZIS. 2005. Multispecies modeling of some components of the marine community of northern and central Patagonia, Argentina. Can. J. Fish. Aquat. Sci. 62 (7):1490- 1512. [ Links ]
MACCHI, PJ; VE CUSSAC; MF ALONSO & MA DENEGRI. 1999. Predation relationships between introduced salmonids and the native fish fauna in lakes and reservoirs in northern Patagonia. Ecol. Freshwat. Fish. 8:227-236. [ Links ]
PASCUAL, MA; P MACCHI; J URBANSKY; F MARCOS; C RIVA ROSSI ET AL. 2002. Evaluating potential effects of exotic freshwater fish from incomplete species presence-absence data. Biol. Invasions. 4:101-113. [ Links ]
PEDERSEN, J & JRG HISLOP. 2001. Seasonal variations in the energy density of fishes in the North Sea. J. Fish Biol. 59:380–389. [ Links ]
PROBST, WE; CF RABENI; WG COVINGTON & RE MARTENEY. 1984. Resource use by stream-dwelling Rock Bass and smallmouth Bass. Trans. Am. Fish. Soc. 113:283-294. [ Links ]
RUZYCKI, JR; DA BEAUCHAMP & DL YULE. 2003. Effects of introduced lake trout on native cutthroat trout in Yellowstone Lake. Ecol. Appl.13:23-37. [ Links ]
SHUTER, BJ & JD MEISNER. 1992. Tools for assessing the impact of climate change on freshwater fish populations. Geojournal 28:7-20 [ Links ]
TRUDEL, M & JB RASMUSSEN. 2001. Predicting mercury concentration in fish using a mass balance model. Ecol. Appl. 11:517–529. [ Links ]
TRUDEL, M; DR GEIST & DW WELCH. 2004. Modeling the Oxygen Consumption Rates in Pacific Salmon and Steelhead: An Assessment of Current Models and Practices. Trans. Am. Fish. Soc. 133:326–348. [ Links ]
WANLESS, S; MP HARRIS; P REDMAN & JR SPEAKMAN. 2005. Low energy values of fish as a probable cause of a major seabird breeding failure in the North Sea. Mar. Ecol. Prog. Ser. 294:1–8. [ Links ]














