Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Ecología austral
versión On-line ISSN 1667-782X
Ecol. austral vol.20 no.2 Córdoba mayo/ago. 2010
SECCIÓN ESPECIAL
Structure and dynamic of food webs in Andean North Patagonian freshwater systems: organic matter, light and nutrient relationships
Beatriz Modenutti1, Ricardo Albariño1, Marcela Bastidas Navarro1, Verónica Díaz Villanueva1, María Sol Souza1, Carolina Trochine1, Cecilia Laspoumaderes1, Florencia Cuassolo1, Gustavo Mariluán1, Leonardo Buria2 y Esteban Balseiro1 *
1Laboratorio de Limnología, INIBIOMA (Universidad Nacional del Comahue-CONICET) Bariloche, Río Negro, Argentina.
2Administración de Parques Nacionales, Bariloche, Argentina.
*Laboratorio de Limnología, INIBIOMA (Universidad Nacional del Comahue-CONICET), Quintral 1250, Bariloche, (8400) Río Negro, Argentina.
Email: e.balseiro@comahue-conicet.gob.ar.
Recibido: 1 de diciembre de 2009;
Fin de arbitraje: 8 de mayo de 2010;
Revisión recibida: 28 de mayo de 2010;
Aceptado: 5 de junio de 2010
ABSTRACT. North Andean Patagonian aquatic systems include a number of deep and shallow oligotrophic lakes with a profuse hydrographic net. In this contribution we present a review of the researches carried out during the last decade in the food webs of North Patagonian water bodies. Food webs in these systems were studied based on field surveys, combining with field and laboratory experiments. Deep and shallow lakes were analyzed in relation to light, organic matter, and nutrient constraints as limiting interacting factors. In addition, we have also extended our knowledge on the structure and dynamics of stream food webs by analyzing different aspects of biotic interactions occurring both through the autotrophic and heterotrophic pathways. Since Andean lakes were defined as high-light low-nutrient environments, and in this scenario high C:P ratios would be expected, food webs of these lakes may be characterized by low food quality for herbivores. We analyzed how this low food quality affects the distribution of zooplankters, and the response at the cellular level. In shallow lakes, macrophytes contributed substantially to the dissolved organic matter content. Also the littoral zone offers higher food availability and refuge against predation. Finally, we analyzed the impact of the introduction of exotic salmonids on food webs of shallow lakes and headwater streams.
Keywords: Bacterioplankton; Phytoplankton; Zooplankton; Fish; Insects; Lakes and rivers.
RESUMEN. Estructura y dinámica de tramas tróficas en sistemas acuáticos andino-norpatagónicos: relaciones con la materia orgánica, la luz y los nutrientes: Los sistemas acuáticos de Patagonia incluyen lagos oligotróficos tanto profundos como someros con una importante red hidrográfica. En este trabajo se presenta una revisión de la información surgida a partir de los trabajos de investigación en redes tróficas de sistemas acuáticos de Patagonia realizados durante la última década. Los estudios se basaron sobre muestreos de campo, experimentos en campo y laboratorio. Los lagos profundos y someros fueron estudiados en relación a la luz, materia orgánica y las restricciones en la disponibilidad de nutrientes como factores limitantes. Por otra parte, también hemos incluido en nuestros estudios a las redes tróficas de arroyos andinos, y analizamos las interacciones tróficas tanto en las vías autotróficas como las heterotróficas. Los sistemas andinos han sido definidos como sistemas de elevada relación luz:nutrientes, y bajo estas condiciones se generan altas relaciones C:P que resultan en redes tróficas caracterizadas por una baja calidad de recursos para los herbívoros. Nosotros analizamos entonces cómo esta baja calidad de alimento restringe la distribución de zoopláncteres y cómo afecta a éstos a nivel celular. En los lagos someros las macrófitas resultaron ser importante en la contribución de sustancia orgánica disuelta. Por otra parte, la zona litoral ofrece mayores recursos alimentarios y refugio a la depredación. Finalmente, analizamos el impacto de la introducción de salmónidos en tramas tróficas de lagos someros y ríos de cabecera.
Palabras clave: Bacterioplancton; Fitoplancton; Zooplancton; Peces; Insectos; Lagos y ríos.
INTRODUCTION
Traditional studies on trophic ecology consider that food web can be aggregated into different trophic levels, and according to the hypothesis of Hairston, Smith and Slobodkin (HSS) (Hairston et al. 1960) the relative importance of competition and predation alternates between these trophic levels. Similar ideas have been developed in freshwater ecology, since studies on aquatic environments provide some of the most complete investigations on the structure, dynamics, and energetic pathways of food webs encompassing organisms from bacteria to vertebrates (Straile 2005). Early studies of Hrbacek (Hrbacek 1962) provided seminal observations on trophic level dynamics in lakes that contributed to the final definition of the Size Efficiency Hypothesis (SEH) (Brooks & Dodson 1965). These first attempts were followed by many studies on trophic level dynamics that contributed to the enunciation of the trophic cascade concept (Carpenter et al. 1985) with great applicability in aquatic ecosystem management (Shapiro 1990). However, when considering the multiple pathways of matter and energy that species interactions can build up (Morin 1999), is important to consider simplicity and unifying principles (Elser & Hessen 2005). In that sense, food web ecologists can use biochemical networks and any chemical entity as a node in a food web. So, C-based charts can be combined with supply and availability of P, because P is a conservative element that will be normally recycled and may thus bind C in stoichiometric proportions a number of times over a season (Sterner & Elser 2002; Elser & Hessen 2005). Interestingly, food web interactions in temperate lakes depend on the seasonal overlap of prey, competitor or predator species and this mismatch in the overlap of interacting species depends strongly on the physical environment (temperature, light availability, mixing, among others). Therefore, physical, chemical, and biological relationships emerge as interacting factors explaining the functioning of aquatic ecosystems.
North Andean Patagonian aquatic systems include a number of deep and shallow oligotrophic lakes with a profuse hydrographic net. These freshwater systems can be characterized by the presence of endemic species in their food web structures and by the introduction of higher trophic levels (salmonids) that started a hundred years ago. In an earlier contribution (Modenutti et al. 1998a) we have described the food webs from oligotrophic Andean lakes and streams of Bariloche region (41° S). These studies were mainly focused on the ideas of the HSS and the SEH, emphasizing the planktonic endemic species composition, in particular of invertebrate predators (Balseiro 1992; Modenutti 1993, Diéguez & Balseiro 1998) and the effect of vertebrate predation controlling crustacean populations (Modenutti et al. 1993; Modenutti & Balseiro 1994). In these early contributions, we also indicated that deliberated or accidental introductions of species, as well as other practices, are likely to change trophic web structure, and thus produce unexpected effects on the whole system (Modenutti et al. 1998a). Additionally, the extended lake's euphotic zone with an high UVR impact was noticed (Morris et al. 1995) where a particular structure of pelagic food web with a prevalence of mixotrophs develops as a common feature of these lakes (Modenutti 1997). Finally, it was observed that the benthic food web of forested mountain small streams was dominated by detritivores and that leaf litter decay of the main riparian tree Nothofagus pumilio by the biotic compartment was slow. However, when litter was composed by needles from the introduced Pinus ponderosa, the processing rate was significantly reduced (Albariño & Balseiro 2002).
More recently, we have focused our research on the effect of trophic cascade on different food web compartments (microbial and autotrophic food webs), and also in several aspects of the ecological stoichiometry. Trophic efficiency is a key aspect of food webs, and planktonic systems are among those with higher trophic efficiency (Hairston & Hairston 1993). However, when photosynthetic rates are high due to high light intensity but P availability is low, C is accumulated in biomass out of proportion of P (Sterner et al. 1997; Hessen et al. 2002; Urabe et al. 2002). This causes an increase in C:P ratio of the food that would become limiting for certain zooplankters like Daphnia (Elser et al. 2001; Balseiro et al. 2007). Andean lakes were defined as high-light low-nutrient environments (Balseiro et al. 2007). Under this scenario high C:P ratios would be expected (Sterner et al. 1997) and food webs of these lakes may be characterized by low food quality for herbivores. In this contribution we analyze the food webs in North-Patagonian Andean lakes (deep and shallow ones) in relation to light, organic matter, and nutrients constraints as limiting interacting factors. In addition, we describe the structure and dynamics of stream food webs by analyzing different aspects of biotic interactions occurring both through the autotrophic and heterotrophic pathways.
THE STUDIED AQUATIC ENVIRONMENTS
Studies have been carried out in lakes located between 40° 27' S and 42° 49' S in the North Andean Patagonian region (Argentina). The studied lakes correspond to the Glacial lakes district of the Southern Andes (Iriondo 1989). The climate is cold temperate (mean annual temperature: 8.7 °C) with predominance of westerly winds, and annual precipitation of 1500 mm (Paruelo et al. 1998). The area is included within three National Parks: Nahuel Huapi, Puelo and Los Alerces, and is characterized by a profuse hydrographic system including large deep lakes (area >5 km2; Zmax>100 m) and small and shallow lakes (area <5 km2; Zmax <12 m). The main rivers fed from these Andean waters run through the plateau steppe and outflow to the Atlantic Ocean, but there are also other rivers that cross the Andes flowing towards the Pacific Ocean. The vegetation of the area is mainly composed by evergreen and deciduous trees dominated by species of the genus Nothofagus. In particular, the upper extensions of most low order streams are covered by the deciduous tree locally named "lenga" (Nothofagus pumilio which form pure stands above 1100 m.a.s.l. The trophic status of the lakes range from ultraoligotrophic to oligotrophic and most of them are warm monomictic. Nevertheless, small lakes are dimictic or polymictic, depending on depth, and high altitude lakes (~1700 m.a.s.l.) near the timberline, are dimictic remaining freeze for several months.
Besides large and deep lakes, we also analyzed Patagonian shallow lakes (<12 m depth) between 38° 58' to 48° 51' S and 68° 20' to 71° 03' W. Some of these lakes are included in Laguna Blanca and Perito Moreno National Parks. In this region, the climate is dry (200-400 mm/year) and cold, with strong westerly winds (Iriondo 1989). The region is a steppe with small leaved shrubs. A number of these lakes have been successfully colonized by salmonids (Quirós 1997) introduced early the last century.
The studied streams range from first to third order draining to Nahuel Huapi lake. Streams are characterized by large stony bottoms, low nutrient concentrations (associated to the igneous origin of the geological landscape), a bimodal discharge pattern (determined by the autumn winter-rainy and the spring-snowmelt periods), and high-gradient and well-shaded and open channels.
DEEP LAKES
Lower trophic levels
North Patagonian Andean lakes are environments with a simplified pelagic trophic microbial food web (Figure 1). Because Andean lakes are high light:low nutrients environments (Balseiro et al. 2007) mixotrophy appears as a suitable strategy for exploiting the extended euphotic zone of the water column. In particular, mixotrophy due to the presence of large sized (>80 µm) mixotrophic ciliates living autotrophically with internalized symbiotic algae, have been found to be characteristic of ultraoligotrophic Andean Patagonian lakes from Argentina (Modenutti 1997; Modenutti et al. 1998b; Modenutti et al. 2000) and Chile (Woelfl & Geller 2002). Autotrophy provides carbon via photosynthesis, whereas phagotrophy will be necessary to fulfill elemental requirements in such poor environments as North-Patagonian Andean lakes (Modenutti & Balseiro 2002).
This mixotrophic compartment suggests the presence of an important bacterial assemblage. However, the high levels of ultraviolet and photosynthetically active radiation (UVR and PAR) (Morris et al. 1995), high C:P ratios (Balseiro et al. 2008; Corno et al. 2009) and the low temperature (Callieri et al. 2007) generate conditions for a slow growing bacterial community. In fact, bacteria communities exhibited a very low activity (bacterial production up to 0.1 µg C.L-1.h-1), indicating the existence of high limiting conditions for bacterial growth (Bertoni et al. 2008). Nutrient and dissolved organic carbon enrichments were observed to stimulate bacteria activity in laboratory experiments (Bertoni et al. 2008). Differences in vertical profiles of different light wavelengths (UVR and PAR), temperature, nutrients, and bacterivory (by nanoflagellates and ciliates) can influence bacteria diversity and morphological distribution (cocci or rods vs. filaments >7 µm) (Corno et al. 2009). Our results indicated that the overall bacterial community composition analyzed by a fingerprinting method (Denaturating Gradient Gel Electrophoresis: DGGE), was similar among the lakes and at different depths along the euphotic zone of each lake. Nevertheless, the relative proportion of filaments to total bacterial biovolume was higher in upper layers with higher UVR, showing a monotonical decrease with depth (Figure 1). Filament proportion exhibited a strong correlation with UVR, and lakes with lower diffuse extinction coefficient of UV-B (305 nm) showed a greater proportion of filaments (Corno et al. 2009). Therefore, we proposed that UVR plays an important role stimulating bacterial filamentation, particularly in lakes with high UVR penetration.

Figure 1. Water column in Andean North Patagonian oligotrophic deep lakes: Light, temperature, and chlorophyll a profiles, and microbial food web components.
References: DCM, Deep Chlorophyll Maxima.
At the same time, other compartments of the microbial food web exhibit a differential vertical distribution in relation to light conditions. In summer, the deep lakes are thermally stratified, and the underwater light climate is characterized by extended euphotic zones which include highly illuminated epilimnetic layers (both UVR and PAR), while dim blue-green light reaches the metalimnion and the upper hypolimnion (Pérez et al. 2002). The vertical variation of chlorophyll a absorption spectra is closely related to this underwater light climate (Pérez et al. 2007). Most of the lakes evidence the development of a Deep Chlorophyll Maxima (DCM) at the metalimnetic layers, near 1% of surface PAR irradiance (Callieri et al. 2007; Pérez et al. 2007). Moreover, the high irradiances at the epilimnetic levels correlate with an increase in the relative concentration of different carotenoids indicating the role of photoprotection of these pigments. On the other hand, we observed an increase in the relative concentration of photosynthetic accessory pigments at deep layers of the euphotic zone that was related to changes in phytoplankton communities between surface and deep layers (Pérez et al. 2007).
Lake stratification enhance light supply through a decrease in mixing depth, while extended epilimnetic layers imply shortage of light, because planktonic producers are frequently dragged down to low light levels (Diehl 2002; Huisman et al. 2004). Thermocline depth and light availability were important factors determining the relative abundance of the two coexisting mixotrophic ciliates Stentor araucanus (Foissner & Woelfl) and Ophrydium naumanni Pejler (Modenutti et al. 2008). Stentor araucanus, a resistant species to UVR (Modenutti et al. 2005), was present in the epilimnion, attaining higher abundances when the thermocline depth was lower and so the mean irradiance higher. The dark cortical pigments confer a high UVR protection, but at the same time, limit the possibility of colonizing deep metalimnetic waters (Figure 1). On the contrary, Ophrydium showed an opposite pattern preferring the metalimnetic layers being more abundant with deeper thermoclines (Figure 1). Ophrydium exhibited a high photosynthetic efficiency at low light intensities (Modenutti et al. 2004), whereas the large and dark S. araucanus needs a high light supply to maintain endosymbiotic algal photosynthesis. Applying the CAtalyzed Reporter Deposition-Fluorescence In Situ Hybridization (CARD-FISH) method, and examining prey inside food vacuoles we observed that the food niche overlap between these two species was negligible (Modenutti et al. 2008). Ophrydium grazed on all the prokaryotic assemblage, including Archea and picocyanobacteria. On the contrary, we did not identify bacteria in food vacuoles of Stentor araucanus, suggesting that small particles are eaten in a very low proportion.
Metalimnetic DCM colonization by phototrophic organisms represents a trade-off between higher survival and lower cell-specific primary production (Modenutti et al. 2004). Although autotrophic species exhibited a positive increase in the net primary production at very low irradiances, at 5 m depth primary production was strongly reduced by PAR+UV radiation. These results evidenced the negative effect of high irradiances (both PAR and UVR) that occurred at the upper levels of the water column, and the net effect on phytoplankton community at these upper levels is a strong photoinhibition with DNA damage (Villafañe et al. 2004).
Other organisms well adapted to the metalimnetic dim light condition are small (<2 µm) autotrophic prokaryotic cells (picocyanobacteria) that have special pigments call phycobilins (phycoerythrin and phycocyanin). Red picocyanobacteria use the pigment phycoerythrin to absorb green light, whereas green picocyanobacteria use the pigment phycocyanin to absorb red light (Callieri 1996; Stomp et al. 2007). In North-Patagonian Andean lakes picocyanobacteria (phycoerithrin- rich cells) are the dominant picophytoplankters at the DCM at 1% of surface PAR (Callieri et al. 2007), because phycoerythrin is especially efficient in utilizing dim blue-green light present at these metalimnetic layers (Pérez et al. 2002). Picocyanobacteria have an important contribution to total primary production especially at the DCM where it raises up to 52% (Callieri et al. 2007). In addition, at very low-phosphorus concentration, typical of North-Patagonian Andean lakes, picocyanobacteria have competitive advantages over larger phytoplankton due to their high surface to volume ratio. Moreover, our data fitted the existing database (Vörös et al. 1998) showing a significant trend towards a decrease of picocyanobacteria biomass but an increase of its relative contribution to total biomass with decreasing trophic state. Low-light and low TDP may interact to create the most favorable conditions for the smaller photosynthetic organisms. Thus, in deep North-Patagonian Andean lakes picocynobacteria achieve higher photosynthetic efficiency than the larger autotrophic organisms (Callieri et al. 2007).
Interestingly, at the DCM of deep lakes competition and predator-prey relationships co-occurred. At these levels, autotrophs (both, prokaryotes and eukaryotes) compete for light and nutrients, but at the same time mixotrophs (i.e., O. naumanni) prey actively upon picocyanobacteria, other Eubacteria and Archaea (Modenutti & Balseiro 2002; Modenutti et al. 2004; Modenutti et al. 2008). We also found that light was a decisive factor determining the ciliate's clearance rate, and that the vertical distribution of both predator and prey showed similar profiles, with higher densities at dim light around the 30 m depth.
Large mixotrophic ciliates such as O. naumanni share and compete for food resources with nanoflagellates and cladocerans in these high light:low nutrient environments. We observed that slight differences in this ratio are related to different crustacean and zooplankton compositions so the net effect on the bacterial food web would substantially change (Balseiro et al. 2004). Under high light:nutrient ratio, the contribution to total bacterivory of the ciliate O. naumanni and the cladoceran Ceriodaphnia dubia, was observed to increase at 30 m depth, while the grazing rates of the nanoflagellate assemblage, dominated by the mixotrophic Chrysochromulina parva, were considerably high and did not change along the water column. On the contrary, under relatively lower light:nutrient ratios, nanoflagellate grazing rates were lower and the relative impact of the nanoflagellate assemblage was comparable to those of O. naumanni and Daphnia cf commutata. The observed difference in clearance rates of the nanoflagellate assemblage would reflect an increase in the phagotrophy where light energy is higher relative to phosphorus (Balseiro et al. 2004).
Upper trophic levels
The upper levels of the trophic food webs of the Andean lakes, commonly known as the traditional trophic food web, are also rather simple and composed by very few species (Figure 2). The assemblage is dominated by calanoid copepods of the Family Centropagidae, with different species of the genus Boeckella, although in large and deep lakes seldom more than one. The predaceous calanoid Parabroteas sarsi, is present in some lakes with fish at deep layers, though is very common in fishless lakes (Balseiro & Vega 1994; Modenutti et al. 2003). Among cladocerans, most of the lakes have daphnids, as Ceriodaphnia dubia, while some of these lakes can be inhabited by large Daphnia (D. cf commutata), and many by the small Bosmina, with two species, B. longirostris and B. chilensis. Except for the presence of P. sarsi, these large lakes almost lack of invertebrate predators (Modenutti et al. 2003; Reissig et al. 2004), as cyclopoids are very scarce (Modenutti et al. 1998a), being abundant only in lake Puelo.

Figure 2. Planktonic food web in Andean North Patagonian oligotrophic deep lakes. Dotted lines indicate that these species are not present in all lakes. Left hand arrow indicates that zooplankton performs Diel Vertical Migration (DVM). Arrows point in the direction of the negative effect in the predator-prey interaction.
Among the pelagic copepods, Boeckella michaelseni or Boeckella gracilipes are present in all large lakes and many small lakes, though we never found both species in the same lake. These two species are the smallest calanoids of Andean-Patagonian lakes, being very similar in size and shape, with a total length less than 1mm (Bayly 1992). Mouth appendices are quite similar suggesting that both species feed on similar particle size (Balseiro et al. 2001). Mouthpart morphology analysis indicated that B. gracilipes has an omnivorous diet, and the range of ingested sizes was broad (3.9-33 µm of equivalent spherical diameter) but all selected particles were motile ones with distinctive movements, which would enhance the copepod particle detection (Balseiro et al. 2001). In field experiments, we also demonstrated that B. michaelseni preyed upon the same prey type and size range (Modenutti et al. 2003)
In some lakes, zooplankters with very different feeding modes coexist (i.e., B. michaelseni and P. sarsi coexist with the large cladoceran Daphnia commutata and rotifers). Through a series of field experiments manipulating different zooplankton structures, we determined the effect of zooplankton predation on the pelagic microbial food web (Modenutti et al. 2003). The presence of B. michaelseni and rotifers depressed ciliates and nanoflagellates, but did not affect autotrophic picoplankton and total bacteria abundances. In contrast, the presence of Daphnia was decisive in decreasing autotrophic picoplankton abundances.
Trophic cascade effects were generated by fishes (Reissig et al. 2006), and invertebrate predators (Modenutti et al. 2003). The invertebrate predator P. sarsi significantly affected the survival of the copepod Boeckella michaelseni both in laboratory and field experiments. In lakes, P. sarsi showed a vertical distribution towards deep layers of the water column both at midday and at night, indicating that the copepod had an effective refuge against visual fish predation (Reissig et al. 2004). The impact of P. sarsi predation on intermediate trophic levels resulted in a weak cascade effect on the lower trophic levels and microbial fraction (Modenutti et al. 2003).
Besides predator-prey and competition interactions, food quality in terms of carbon (C):phosphorus (P) ratio can also constrain the success of some species like highly demanding P herbivores as Daphnia (Sterner & Elser 2002). Because North Andean Patagonian lakes have low nutrient concentrations and well-developed euphotic zones we aimed to investigate the distribution of the large Daphnia commutata in relation with food quality (sestonic C:P ratio) and predation risk (Balseiro et al. 2007). Based on the fish species present (native and introduced) and their relative eye diameter (as a measure of prey detection capability) and transparency of the lake, we estimated the predation risk in each studied lake (Atlantic and Pacific watersheds). The C:P ratios in the lakes were high, varying from 350 to >1200. The lakes with D. commutata had significantly lower C:P ratio than those without these daphnids. On the other hand, those lakes where Daphnia is present have the lower predation risk than those were Daphnia is absent. In addition, we carried out growth experiments with neonates and natural seston of three lakes with different C:P ratio. The growth rates were inversely related with C:P of the food. Combining these field and laboratory studies we demonstrated that food quality and predation risk together determined the success or failure of large Daphnia populations in these Andean clear ultraoligotrophic lakes (Balseiro et al. 2007).
CONNECTION BETWEEN LITTORAL AND PELAGIC ZONES: SHALLOW LAKES
Lower trophic levels
North Andean Patagonian shallow lakes (surface <5 km2 and depth <12 m) have a well developed littoral zone with emergent and submersed macrophytes (Balseiro & Modenutti 1990; Balseiro et al. 1997; Bastidas-Navarro & Modenutti 2007) (Figure 3). Macrophytes could be a substantial source of dissolved organic matter (DOM) generated either through extracellular release or following aging and subsequent release of dissolved and particulate constituents to the surrounding water (Bertilsson & Jones 2003). Macrophyte degradation and leachate production is increased by UV radiation (Denward & Tranvik 1998; Anesio et al. 1999), but at the same time, UVR affects DOM that results in a progressive loss of color, lower average molecular size, and decrease in spectrophotometric absorbance (Osburn et al. 2001; Zagarese et al. 2001). In shallow Andean lakes, macrophytes DOM source may be an important source of organic carbon for bacteria, connecting littoral and pelagic areas (Bastidas Navarro et al. 2009). Molecular weight and size was indicated as an important issue influencing the microbial utilization of DOM, therefore photoalteration caused by short-wavelengths can be an important factor driving resource bioavailability for bacteria growth (Perez & Sommaruga 2007). In laboratory experiments, we observed substantial changes in optical features of lake water and Schoenoplectus californicus and Potamogenton linguatus leachates exposed to UVR: the increase of both the absorbance at short wavelength (<300 nm) and the a250: a365 ratio, indicated a decrease in the mean molecular size of DOM. Consequently, natural bacteria assemblages showed a positive response in abundance and production to leachate addition and UVR exposed DOM (Bastidas Navarro et al. 2009).
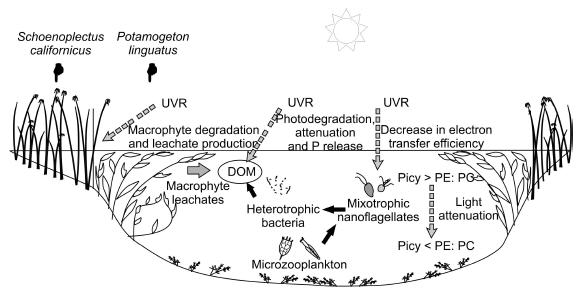
Figure 3. Microbial food web in Andean North Patagonian shallow lakes: dissolved organic matter (DOM) and the effect UVR.
References: Picy: Picocyanobacteria; PE: Phycoerithryn rich cells; PC: Phycocyanin rich cells.
Dissolved yellow substances are the major light-absorbing components in Andean shallow lakes (Pérez et al. 2002). This dissolved organic matter interacts with light climate (Kirk 1996), and at 1% of surface PAR, light is mainly composed by the red end of the spectrum. Under these conditions we observed that the different phytoplanktonic size fractions (< or >2 µm) were differentially affected. Picophytoplankton (<2 µm) exhibited a decrease in the phycoerithrin-phycocyanin ratio that could be related with the underwater light climate (Bastidas Navarro et al. 2009). In these shallow lakes, although PAR is enough to support photosynthesis at any depth, picophytoplankton resulted photosynthetically more efficient than the larger autotrophs. Despite these lakes have higher DOM concentration and light attenuation coefficients than large lakes, summer surface irradiances (PAR and UVR) still cause photoinbition in phytoplankton (Villafañe et al. 2004; Bastidas Navarro et al. 2009) while laboratory experiments showed that ultraviolet radiation has negative effect in the electron transfer efficiency of the Photosystem II of mixotrophic algae (Bastidas Navarro 2009).
Macrophytes and decaying leaf litter not only affect microorganisms through leachates but also through algae, fungi and bacteria that colonize their surfaces and constitute important food resources for grazers. Microorganisms colonizing submerged leaf litter were observed to be important in the diet and growth of the larvae of the trichopteran Verger cf. limnophilus (Limnephilidae) (Díaz Villanueva & Trochine 2005). The consumption of leaves with algae and microorganisms produced higher growth rates of the insect than leaves without microorganisms, indicating that the combination of detritus with its associated flora resulted in a better food quality. Therefore, insects process relatively large quantities of detritus to obtain sufficient associated resources for growth (Díaz Villanueva & Trochine 2005).
Upper trophic levels
Litoral zones of shallow lakes are colonized by macrophytes, usually with an outer ring of the emergent Schoenoplectus californicus and an inner one of the submerged Potamogeton pectinatus. The interaction between these two sections and the open water was analyzed combining field survey and laboratory and field experimental studies. The presence of macrophytes in the littoral zone of lakes produces particular conditions including high resource availability for consumers. For this reason, the littoral zone is generally the area with the highest diversity. Nanoplankton (mainly nanoflagellates) dominated the lake's pelagic zone while net phytoplankton prevailed in the littoral zone (mainly diatoms, cyanophytes and chlorophytes) (Bastidas Navarro & Díaz Villanueva 2004). The highest number of species and diversity of microzooplankton can be observed in the littoral zones whereas some species were exclusively present in the pelagic zone in relation to the high nanoplankton abundance (Keratella cochlearis, Synchaeta spp., Polyarthra vulgaris and Collotheca mutabilis) (Bastidas-Navarro & Modenutti 2007). Other daphnids like Simocephalus vetulus and S. serrulatus, and chydorids are commonly found, and invertebrate predators are very common. Besides P. sarsi and some cyclopoids, there are other planktonic predators as watermites and turbelarians (Mesostoma ehrenbergii) (Balseiro 1992; Trochine et al. 2009).
Mesostoma ehrenbergii can have high predation rates on cladocerans and copepods (Trochine et al. 2008). In these systems, this predator often coexists with the calanoid copepod Boeckella gracilis, a medium sized, orange colored copepod very common in shallow lakes (Trochine et al. 2005). Boeckella gracilis has a particular mating behavior, with a large proportion of adults participating in copulating pairs that lasted for days. M. ehrenbergii ate significantly more copulating pairs because the use of mucus threads allowed the predator to ingest both members of the pairs instead of only one in most attacks. Larger prey may create more turbulence in the water while swimming, so the hydrodynamic signals produced by pairs should be greater than those produced by single individuals, making them more vulnerable (Trochine et al. 2005).
Spatial heterogeneity affected the predator–prey interaction between Mesostoma and its prey, mainy B. gracilis and the cyclopoid Acanthocyclops robustus (Figure 4). Predation rates of Mesostoma on B. gracilis increased without heterogeneity with the increase in prey abundance, while predation rates reached saturation with horizontal spatial heterogeneity. In consequence, in natural habitats, interaction between extent of macrophyte development and intensity of predation by M. ehrenbergii on copepods species may be expected. The structural complexity given by macrophytes provides a bottleneck for M. ehrenbergii predation reducing its predation rates on copepods (Trochine et al. 2006). In this sense, copepods and cladocerans would react differently to chemical signals produced by macrophytes, predator presence or injured conspecific (Trochine et al. 2009). Our field studies demonstrated the coexistence of M. ehrenbergii and the selected prey in different seasons and that A. robustus and C. dubia choose the vegetated area (a mixed bed of Juncus pallescens and Myriophyllum quitense) over the non-vegetated area. The habitat choice experiments indicated that the presence of M. ehrenbergii may directly affect the habitat selection of B. gracilis, because this zooplankter swam away from the predator. On the other hand, Mesostoma may indirectly affect the habitat selection of the cyclopoid copepod A. robustus and the cladoceran C. dubia, as both zooplankters exhibited a negative response to the alarm signal produced by crushed conspecifics. The presence of the submerged M. quitense did not affect the horizontal movements of any of the zooplankters studied. In contrast, the emergent macrophyte J. pallescens elicited a positive response of B. gracilis, suggesting that this aquatic plant may act as a predation refuge for this prey (Trochine et al. 2009).
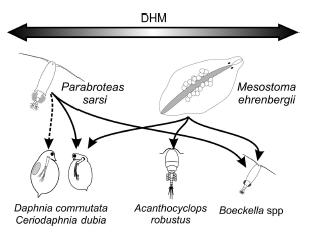
Figure 4. Predator-prey relationships in fishless shallow lakes. Dotted lines indicate weak interactions. Top arrow indicates that zooplankton performs Diel Horizontal Migration (DHM). Arrows point in the direction of negative effect in the predator-prey interaction.
STREAMS
Small streams running through deciduous forests receive a large temporal pulse of organic matter from fallen leaves (Figure 5). Nothofagus pumilio (lenga) leaf shedding occurs between late summer and late autumn (84 g AFDM.m-2.month-1) covering large extensions of stream channel (Albariño et al. in press). A substantial amount of leaf litter may be transported downstream by spates occurring from late autumn to winter and eventually reaches the lake, after traveling more than 9 km from the upper catchment. The remaining fraction of leaves is redistributed and retained along upper catchment channels and may account for >30 g DM/m2 in May (Buria 2008; Albariño et al. in press). This organic matter constitutes the detrital base of the food web. In situ leaf litter processing of lenga in those small forested streams, estimated in field experiments (average k value=0.0067±0.0017) (Albariño & Balseiro 1998, 2002; Buria 2008), appears to be slow as decay rate was at the lower limit of the intermediate decay rate category established by Petersen & Cummins (1974). This is probably a consequence of two main factors. On the one hand, Nothofagus pumilio strongly resorbs nitrogen before leaf abscission resulting in poor N:C leaf litter (Diehl et al. 2003), consequently becoming a low quality resource for detritivores (Albariño & Balseiro 2001; Balseiro & Albariño 2006). On the other hand, low water temperature in winter and low nutrient concentration would determine a harsh environment for decomposer colonization and hence conditioning of leaf litter (Suberkropp & Chauvet 1995). These conditions reduce food quality for detritivores (Graça et al. 2001) and would determine the long larval growth periods (e.g., semivoltine life cycles) (Albariño & Balseiro 2001).

Figure 5. Generalized food web in North-Patagonian Andean streams, showing consumptive links between basal resources, invertebrates and aquatic vertebrates (fishes). Arrows point in the direction of matter flow. Differences between food webs in upstream, forested reaches (UPS) and downstream, open canopy reaches (DWS) are represented in the scheme by different arrow widths, which illustrate the magnitude of the interaction between shredders and coarse particulate organic matter (CPOM). Dashed arrows show relationships between fine particulate organic matter (FPOM) and collectors or invertebrate predation effects which strength has not yet been studied in this area. Fishless stream reaches are not considered in this scheme.
Despite those circumstances, the community structure of riffle habitats in small forested streams of the area is dominated by detritivores both in biomass and density (~75%), with shredders representing ~50% of total biomass year round and collectors with ~50% of community density (Albariño 1999, Albariño & Díaz Villanueva 2006) (Figure 5). In particular, larvae of the plecopteran Klapopteryx kuscheli, the dipteran Tipula sp, two common large sized shredders, and various trichopteran species (sericostomatids and limnephilids) play an important role in the breakdown of N. pumilio leaves (Albariño & Balseiro 1998; Albariño & Valverde 1998; Albariño 1999; Albariño & Balseiro 2002; Albariño & Díaz Villanueva 2006) and in the transference of matter up in the food web (Buria et al. 2007; Buria et al. 2009). The afforestation or invasion of exotic plants with different phenology, chemical and physical characteristics to those of the native riparian species change the timing, quantity and quality of leaf litter standing stock in streams (Naiman et al. 2005). These changes affect community abundance and composition (Allan & Castillo 2007). Needles of the exotic Pinus ponderosa decomposed two-fold slower than leaves of the native lenga (Albariño & Balseiro 2002). Colonization and use by invertebrate detritivores was significantly reduced as they were unable to feed on pine needles after four months in the stream (Albariño & Balseiro 2002). Valdovinos (2001) found similar results by comparing breakdown and invertebrate feeding between lenga leaves and needles of the exotic Pinus radiata in a low order stream of the Bío Bío basin, central Chile. Leaf litter from exotic pines used in forestry in Patagonia may have deleterious effects on stream functioning if afforestations replace deciduous forests, as they represent a very low quality resource in terms of leaf toughness and nitrogen content (Albariño & Balseiro 2001; Balseiro & Albariño 2006). Finally, temporal changes associated to deciduous riparian species may also affect detritus pathway dynamics by reducing shredder growth; a circumstance likely to happen during winter-early spring after leaf shedding along small streams. We found that K. kuscheli nymphs fed on leaf litter that had been exposed to sunlight grew significantly slower than those fed on leaves incubated under heavy shading (Albariño et al. 2008). As microorganisms growing on leaves have an essential role in shredder nutrition (Suberkropp & Wallace 1992), the reduction in fungal biomass observed in sunlight-incubated leaves was attributed to cause lower shredder growth.
Resource availability influences the flow of energy through food webs, thus affecting biomass and trophic structure of consumer communities (Durant et al. 2005). In contrast to the patterns observed in canopied upper reaches, we found that grazer invertebrates dominated downstream open extensions (Díaz Villanueva 2002). Periphyton in the studied streams are mostly represented by diatoms but, in the areas with higher sunlight irradiance, the chrysophyte Hydrurus foetidus is responsible of a seasonal algal bloom dominating the stream bottoms (Díaz Villanueva & Albariño 1999). Ephemeropteran nymphs are numerically the most abundant clinger-grazers both at canopied and open reaches. Particularly, nymphs of the ubiquitous Meridialaris chiloeensis were reported as scrapers that efficiently reduce periphyton biomass and affect community composition, promoting an assemblage dominated by Nitzschia palea instead of Achnanthidium minutissimum that dominated the grazer-free controls (Díaz Villanueva et al. 2004). In high order streams, snails may codominate the benthic invertebrate community (Díaz Villanueva 2002). The grazing effect of M. chiloeensis is quantitatively similar to the effect of the snail Chilina dombeiana. Experimental results indicated that the individual mayfly effect on chlorophyll-a and ash free dry mass of the biofilm was lower than that of the individual snail. However, considering the spring and autumn abundances of both populations in a natural environment, their grazing impact might be similar or even higher for the mayfly. In addition, the taxonomic composition of the periphyton changed very little when the grazer was the snail (Díaz Villanueva et al. 2004).
Forested upstream reaches receiving enough sunlight are also inhabited by nymphs of the large plecopteran Notoperla archiplatae (Díaz Villanueva 2002). They have been classified as scrapers (Díaz Villanueva & Albariño 1999) being highly dependent on periphyton (Albariño & Díaz Villanueva 2006). The effect of N. archiplatae on periphyton was evaluated in a field experiment. The grazer reduced the abundance of filamentous algae favoring the increase of attached diatoms. In the ungrazed treatments, Melosira varians was the most abundant species; however, in the grazed treatments it was strongly reduced whereas Achnanthidium minutissimum and Gomphonema angustum increased. Besides, under grazing pressure the Chl a/OM ratio was significantly higher, suggesting that N. archiplatae maintained an autotrophic epilithic layer and prevented the development of a senescent deep layer in the periphyton (Díaz Villanueva & Albariño 1999; Albariño & Díaz Villanueva 2006).
Finally, benthic communities hold several species of invertebrate predators. Although large size carnivores (e.g., efemeropteran ameletopsids and plecopteran perlids and eusthenids) are present at a regional scale (Velásquez & Miserendino 2003), the upper sections of Challhuaco, Ñireco and Pescadero streams only hold mid-size predator species (e.g., trichopteran hydrobiosids, dipteran athericids, chironomids, empidids and tipulids) (Albariño 1994; Buria 2008). It is likely that the latter group have an important effect only on small sized prey, particularly affecting the mortality of early instar larvae of detritivores and grazers. Fish predators are known to exert strong control on their invertebrate prey, changing their size, number, biomass, and composition (Gibson et al. 2004; Herbst et al. 2009). Thus, in the absence of fish, benthic communities are likely to be controlled both by abiotic factors and resources abundance. On the contrary, we expect strong top down control on benthic communities in reaches with fish (see below).
SALMONID INTRODUCTIONS IN LAKES AND RIVERS
One common practice in almost all Patagonian lakes was the introduction of salmonids about hundred years ago, at the beginning of XX century. Unfortunately, no plankton assemblage was studied before this practice began, since the oldest plankton samples obtained in these lakes were those of Kuno Thomasson in 1950, about 50 years after salmonids were brought (Thomasson 1963). Some years later, researchers from the INALI studied lake Mascardi (Bonetto et al. 1971; Drago 1974), and then the expeditions leaded by Quirós (Menu Marque & Marinone 1986; Quirós 1988, 1990) extensively studied Patagonian lakes.
Patagonia fishless ponds have been also stocked with fishes for recreational purposes since early in the 20th century. We have analyzed the changes in plankton (phytoplankton and zooplankton) structure due to these introductions, by comparing lakes with and without fish introduction (Reissig et al. 2006). We sampled 18 Patagonian lakes situated on a latitudinal gradient from 39° to 49° S: 12 fishless, 5 with introduced fishes and one with endemic fish fauna. Results showed differences between lakes with and without fishes: in the presence of fish, zooplankton size spectrum tended to be narrower because of the disappearance of Daphnia and large centropagid copepods. Zooplankton composition changed: centropagid species richness decreased and rotifers dominated. In contrast, we found that coexisting in fishless lakes 3 or 4 centropagid species, differing markedly in body size and exploiting different food niches, besides the presence of large Daphnia. These changes in zooplankton seemed to cascade down to phytoplankton. Fish introduction increased the phytoplankton similarity in lakes even belonging to different basins in a latitudinal gradient. Indeed, cyanobacteria dominated only in lakes with introduced fishes. Probably the elimination of Daphnia favored cyanobacteria proliferation due to nutrient rebalance. As a consequence, water quality decreased and the value of sport fisheries reduced (Reissig et al. 2006).
We also analyzed the effect of introduced salmonids (i.e., rainbow trout Oncorhynchus mykiss) on benthic macroinvertebrate community of low order streams. As was explained above, there is a lack of information before salmonid introduction occurred, thus we can only reconstruct the original food web studying upper stream sections without fishes. For this purpose, we carried out studies in the upper-forested sections of three streams (Challhuaco, Cascada and Pescadero streams) located around 41° S at 1200-1300 m above sea level (Buria et al. 2007; Buria et al. 2009). In the presence of trout, we observed significant shifts in invertebrate body size towards smaller individuals, which also resulted in a reduction of total invertebrate community biomass (Buria et al. 2007). Rainbow trout fed on approximately 40 invertebrate species, though large taxa (Klapopteryx kuscheli, Tipula sp.) and active swimming species (Metamonius anceps and Hyalella curvispina) were positively selected, and either reduced in abundance, or were absent in reaches with trout (Buria et al. 2007; Buria et al. 2009). Functional feeding groups were differentially affected since rainbow trout's diet was mostly composed by shredders and scrapers. Gathering collectors mainly represented by chironomids and oligochaetes increased their number and biomass in trout reaches. Thus, the benthic community structure in trout reaches drastically changed. The reduction of shredders may suggest an indirect effect on Nothofagus leaf litter breakdown and hence, a reduction of FPOM supply to "in situ" and downstream reaches (Buria et al. 2007). In summary, trout selective feeding behavior causes species loss and modifies the food web altering matter and energy pathways.
NEW PERSPECTIVES: STOICHIOMETRY CONSTRAINS, ULTRAVIOLET RADIATION EFFECTS AND CLIMATE CHANGE
North-Patagonian Andean lakes are high light:low nutrient environments and therefore they are good scenarios for studying ultraviolet radiation effect under stoichiometric constraints (Balseiro et al. 2008). In freshwater environments direct biological effect of ultraviolet radiation (UVR) result from absorption of specific wavelengths by macromolecules and alteration of biochemical processes. Indirect effects are related to UVR interaction with water and dissolved organic carbon to form chemically reactive species (ROS: reactive oxygen species) that also affect biochemical processes (see Gonçalves this volume). Zooplankton photoprotection includes mycosporine-like amino acids, photoprotective pigments, production of quenching agents and antioxidant enzymes (Siebeck et al. 1994; Borgeraas & Hessen 2002; Marinone et al. 2006). The relative importance of each mechanism would depend on the different organisms involve. As a first step, we aimed to determine the antioxidant enzyme activities of GST (Glutathion-S-transferase) and CAT (catalase), both involved in protection and repair of damage caused by UVR, in the two dominant zooplankters: the copepod Boeckella gracilipes and the cladoceran Ceriodaphnia dubia. We observed differences in antioxidant enzymes expression between copepods and cladocerans since CAT was significantly higher in C. dubia than in B. gracilipes and GST was similar in both species (Souza et al. 2007). Also we observed that dissolved organic matter (DOC) decreases the exposure by absorption of UVR but simultaneously acts as photosensitizer producing ROS and their successive toxic products in the surface waters. These combined effects would be of particular importance in shallow lakes and would also cause differences in the enzymatic response of zooplankton species (Souza et al. 2007).
As a second step and more recently, we experimentally tested the effect of food quality (C:P ratio) on the response of antioxidant enzymes to UVR in Daphnia commutata (Balseiro et al. 2008) (Figure 6). Algal cultures (Chlamydomonas reinhardtii) at different concentrations of phosphorus and light intensities, gave food resource of different in the C:P ratios. D. commutata grown under these different food qualities resulted in significant differences in individual biomass and protein content of Daphnia. Moreover, we observed stoichiometric constraints imposed limits on enzymatic defenses against UVR oxidative stress. In high C:P treatments, there was significantly lower enzyme activity (GST and CAT) in response to UVR. Low food quality (less P for biosynthesis) may also impose a weaker antioxidant response on the organisms, a response of considerable ecological relevance in transparent Andean lakes which combine high UVR intensities with low seston P:C ratios (Balseiro et al. 2008).
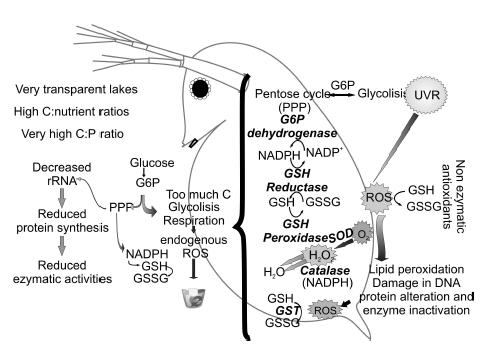
Figure 6. Diagram showing the antioxidant defense mechanisms implied in the UVR-generated oxidative stress and the interaction with metabolic pathways.
References: UVR can induce oxidative stress condition as a result of increasing in reactive oxygen species (ROS). ROS are either free radicals (O2-), or peroxides (H2O2) or molecules containing oxygen atoms forming free radicals. Endogenous ROS are generated in aerobic respiration and others catabolic pathways (electron transport), exogenous ROS by stress factors as UVR. Cellular defenses against ROS include non enzymatic compounds as glutathione (GSH) and enzymatic defenses as superoxide dismutase (SOD), catalase (CAT), glutathione S- transferase (GST), glutathione peroxidase (GSH peroxidase), and glutathione reductase (GSH reductase). These processes are energetically demanding, and imply metabolites of crucial anabolic pathways as phosphate pentoses pathway (PPP).
More recently, we observed that calanoid copepods may also have stoichiometric constraints that affect the UVR enzymatic response. In addition, copepod life history involves distinct feeding modes in relation with the development (nauplii and copepodites) so we aimed to identify stoichiometric constraints in the development of copepods in relation to ontogenetic changes in their diet (Laspoumaderes unpublished data). Global changes including increase in carbon dioxide and increment in UVR effect will impact the nutrient ratios and this would strength different constraints for species and for the whole trophic food web.
Consequences of global change are being studied also in stream environments. Global warming is altering pluvial regimes and ultimately, stream discharges. In northwestern Patagonia, climate is predicted to become dryer and small perennial streams may suffer drought in the dry season (summer), becoming intermittent streams. This will impose deep changes to the biota and the physico-chemical and biological processes that characterize those aquatic systems (Dahm et al. 2003; Dewson et al. 2007). In this context, we are comparing ecosystem processes and the functional and taxonomical structure of benthic invertebrate communities of perennial and intermittent headwater streams (Mariluán unpublished data).
Exotic plant species invading stream surroundings are expected to affect instream ecology. Albariño and Balseiro (2002) reported differences in leaf litter processing of native (N. pumilio) and exotic (P. ponderosa) leaves, and the negative effect on the stream invertebrates. Now we are investigating the effect of Salix fragilis, that has settled along riparian habitats displacing native shrubs and trees (e.g., species of the genus Berberis, Discaria, Escallonia, Lomatia, Maytenus, Ribes), on stream invertebrate communities and on organic matter processing.
Wetlands are also very sensitive environments to anthropogenic changes. Our previous results indicated that in shallow lakes and ponds dissolved organic carbon (DOC) has a particular importance interacting with light climate (Pérez et al. 2002), bacteria activity (Bastidas Navarro et al. 2009) and oxidative stress (Souza et al. 2007). At the same time, macrophytes play a crucial role in zooplankton behavior (Trochine et al. 2009) and are very important as DOC producers [Bastidas Navarro et al. 2009; Bastidas Navarro & Modenutti (in press)]. Thus, plant species introduction would impact the trophic dynamics of the systems, through driving changes in the C:nutrient ratios. Based on this idea we are analyzing the consequences of colonization by different macrophytes and the effect of the input of elements (C, N and P) on the whole ecosystem (Cuassolo unpublished data).
Direct anthropogenic impacts interacting with large atmospheric perturbations, ultraviolet radiation and increasing nutrient inputs are phenomena that simultaneously would affect aquatic organisms inhabiting these ecosystems. All these impacts should be considered when making resource management decisions. Thus, we consider that further studies analyzing multiple interactive factors will contribute certainly to the maintenance of the functional biodiversity of these particular ecosystems.
ACKNOWLEDGEMENTS
We would like to acknowledge the following Institutions that have made possible these studies on Andeanpatagonian food webs: Agencia Nacional de Promoción Científica y Tecnológica - ANPCyT (PICT 2007-01256, PICT 2007-01258, PICT 2007-01747), Universidad Nacional del Comahue (B141), CONICET (PIP 6507, PIP 112-200801-01702). We are very grateful to Cooperative Programs: CONICET-CNR (Argentina-Italia), AECI (Spain) and SECyT-GRISES (Argentina-Portugal) for supporting of international collaborative research.
REFERENCES
ALBARIÑO, RJ. 1994. Análisis de la fracción macrozoobentónica en un cuerpo lótico andino. Tesina de Licenciatura Centro Regional Universitario Bariloche, Universidad Nacional del Comahue. [ Links ]
ALBARIÑO, RJ. 1999. Dinámica del procesamiento de la materia orgánica particulada gruesa por parte de macroinvertebrados en arroyos andinos. Tesis Doctoral: Centro Regional Universitario Bariloche, Universidad Nacional del Comahue. [ Links ]
ALBARIÑO, RJ & EG BALSEIRO. 1998. Larval size and leaf conditioning in the breakdown of Nothofagus pumilio leaves by Klapopteryx kuscheli (Insecta Plecoptera) in a South Andean Stream. Int. Rev. Hydrobiol., 83:397-404. [ Links ]
ALBARIÑO, RJ & EG BALSEIRO. 2001. Food quality, larval consumption, and growth of Klapopteryx kuscheli (Plecoptera : Austroperlidae) from a south Andes stream. Freshwater Biol., 16:517-526. [ Links ]
ALBARIÑO, RJ & EG BALSEIRO. 2002. Leaf litter breakdown in Patagonian streams: native versus exotic trees and the effect of invertebrate size. Aquat. Conserv.: Mar. Freshw. Ecosyst., 12:181-192. [ Links ]
ALBARIÑO, RJ & V DÍAZ VILLANUEVA. 2006. Feeding ecology of two plecopterans in low order Andean-Patagonian streams. Int. Rev. Hydrobiol., 91:122-135. [ Links ]
ALBARIÑO, RJ; V DÍAZ VILLANUEVA & L BURIA. (in press). Leaf litter dynamics in a forested small Andean catchment, northern Patagonia, Argentina. Pp. 183-211 in: Oyarzún, C, NEC Verhoest, P Boeckx and R Godoy (eds.). Ecological advances on Chilean temperate rainforests. Academia Press, Gent. [ Links ]
ALBARIÑO, RJ; V DÍAZ VILLANUEVA & C CANHOTO. 2008. The effect of sunlight on leaf litter quality reduces growth of the shredder Klapopteryx kuscheli. Freshwater Biol., 53:1881-1889. [ Links ]
ALBARIÑO, RJ & AC VALVERDE. 1998. Hábito alimentario del estado larval de Parasericostoma cristatum (Trichoptera: Sericostomatidae). Rev. Soc. Entomol. Argent., 57:131-135. [ Links ]
ALLAN, DJ & MM CASTILLO. 2007. Stream ecology: structure and function of running waters. Springer. [ Links ]
ANESIO, AM; LJ TRANVIK & W GRANELI. 1999. Production of inorganic carbon from aquatic macrophytes by solar radiation. Ecology, 80:1852-1859. [ Links ]
BALSEIRO, E & R ALBARIÑO. 2006. C-N Mismatch in the Leaf Litter-Shredder Relationship of an Andean Patagonian Stream Detritivore. J. N. Am. Benthol. Soc., 25:607-615. [ Links ]
BALSEIRO, E; B MODENUTTI; C QUEIMALIÑOS & M REISSIG.2007. Daphnia distribution in Andean Patagonian lakes: effect of low food quality and fish predation. Aquat. Ecol., 41:599-609. [ Links ]
BALSEIRO, E; MS SOUZA; B MODENUTTI & M REISSIG. 2008. Living in transparent lakes: Low food P:C ratio decreases antioxidant response to ultraviolet radiation in Daphnia. Limnol. Oceanogr., 53:2383-2390. [ Links ]
BALSEIRO, EG. 1992. The role of pelagic water mites in the control of cladoceran population in a temperate lake of the southern Andes. J. Plankton Res., 14:1267-1277. [ Links ]
BALSEIRO, EG & BE MODENUTTI. 1990. Zooplankton dynamics of Lake Escondido (Río Negro, Argentina), with special reference to a population of Boeckella gracilipes (Copepoda, Calanoida). Int. Rev. Gesamt. Hydrobiol., 75:475-491. [ Links ]
BALSEIRO, EG; BE MODENUTTI & CP QUEIMALIÑOS. 1997. Nutrient recycling and shifts in N:P ratio by different zooplankton structures in a South Andes lake. J. Plankton Res., 19:805-817. [ Links ]
BALSEIRO, EG; BE MODENUTTI & CP QUEIMALIÑOS. 2001. Feeding of Boeckella gracilipes (Copepoda, Calanoida) on ciliates and phytoflagellates in an ultraoligotrophic Andean lake. J. Plankton Res., 23:849-857. [ Links ]
BALSEIRO, EG; CP QUEIMALIÑOS & BE MODENUTTI. 2004. Grazing impact on autotrophic picoplankton in two south andean lakes (Patagonia, Argentina) with different light:nutrient ratios. Rev. Chil. Hist. Nat., 77:73-85. [ Links ]
BALSEIRO, EG & M VEGA. 1994. Vulnerability of Daphnia middendorffiana to Parabroteas sarsi predation: the role of the tail spine. J. Plankton Res., 16:783-793. [ Links ]
BASTIDAS-NAVARRO, M & B MODENUTTI. 2007. Effect of macrophytes and food resources on the horizontal distribution of testate amoebae and rotifers in an Andean-Patagonian lake. Rev. Chil. Hist. Nat., 80:345-362. [ Links ]
BASTIDAS NAVARRO, M. 2009. Dinámica de microcomunidades en ambientes acuáticos con alta concentración de materia orgánica en relación con factores de cambio global: el incremento de la RUV. Tesis Doctoral: Centro Regional Universitario Bariloche, Universidad Nacional del Comahue. [ Links ]
BASTIDAS NAVARRO, M; E BALSEIRO & B MODENUTTI. 2009. Effect of UVR on Lake Water and Macrophyte Leachates in Shallow Andean-Patagonian Lakes: Bacterial Response to Changes in Optical Features. Photochem. Photobiol., 85:332-340. [ Links ]
BASTIDAS NAVARRO, M & B MODENUTTI. (in press). UVR induce optical changes and phosphorous release of lake water and macrophyte leachates in shallow Andean lakes. J. Limnol., 69:112-119 [ Links ]
BASTIDAS NAVARRO, M; B MODENUTTI; C CALLIERI; R BERTONI & E BALSEIRO. 2009. Balance between primary and bacterial production in North Patagonian shallow lakes. Aquat. Ecol., 43:867-878 [ Links ]
BASTIDAS NAVARRO, MA & V DÍAZ VILLANUEVA. 2004. Distribución espacial de las diatomeas en el fitoplancton de un lago somero andino-patagónico. Bol. Soc. Arg. Bot., 39:33-40. [ Links ]
BAYLY, IA. 1992. The non-marine Centropagidae. SPB Academic Publishing. [ Links ]
BERTILSSON, S & JB JONES. 2003. Supply of Dissolved Organic Matter to aquatic Ecosystem: autochthonous sources. Pp. 3-24 in: Findlay, SEG and RL Sinsabaugh (eds.). Aquatic Ecosystems: Interactivity of Dissolved Organic Matter. Academic Press. [ Links ]
BERTONI, R; C CALLIERI; E BALSEIRO & B MODENUTTI. 2008. Susceptibility of bacterioplankton to nutrient enrichment of oligotrophic and ultraoligotrophic lake waters. J. Limnol., 67:120-127. [ Links ]
BONETTO, AA; W DIONI & P DEPETRIS. 1971. Informe preliminar sobre las investigaciones linológicas de la cuenca del Río Manso y Lago Mascardi (Río Negro - Patagonia). Fundación Bariloche. [ Links ]
BORGERAAS, J & DO HESSEN. 2002. Variations of antioxidant enzymes in Daphnia species and populations as related to ambient UV exposure. Hydrobiologia, 477:15-30. [ Links ]
BROOKS, JL & SL DODSON. 1965. Predation, body size, and composition of plankton. Science, 150:28-35. [ Links ]
BURIA, L. 2008. Efecto de la depredación en la estructuración comunitaria del zoobentos en ambientes lóticos norpatagónicos. Tesis Doctoral: Centro Regional Universitario Bariloche, Universidad Nacional del Comahue. [ Links ]
BURIA, L; R ALBARIÑO; V DÍAZ VILLANUEVA; B MODENUTTI & E BALSEIRO. 2007. Impact of exotic rainbow trout on the benthic macroinvertebrate community from Andean-Patagonian headwater streams. Fundamental and Applied Limnology, 168:145-154. [ Links ]
BURIA, LM; RJ ALBARINO; BE MODENUTTI & EG BALSEIRO. 2009. Temporal variations in the diet of the exotic rainbow trout (Oncorhynchus mykiss) in an Andean-Patagonian canopied stream. Rev. Chil. Hist. Nat., 82:3-15. [ Links ]
CALLIERI, C. 1996. Extinction coefficient of red, green and blue light and its influence on picocyanobacterial types in lakes at different trophic levels. Mem.Ist.Ital.Idrobiol., 54:135-142. [ Links ]
CALLIERI, C; B MODENUTTI; C QUEIMALIÑOS; R BERTONI & E BALSEIRO. 2007. Production and biomass of picophytoplankton and larger autotrophs in Andean ultraoligotrophic lakes: differences in light harvesting efficiency in deep layers. Aquat. Ecol., 41:511-523. [ Links ]
CARPENTER, SR; JF KITCHELL & JR HODGSON. 1985. Cascading trophic interactions and lake productivity. Bioscience, 35:634-638. [ Links ]
CORNO, G; B MODENUTTI; C CALLIERI; E BALSEIRO; R BERTONI; ET AL. 2009. Bacterial diversity and morphology in deep ultraoligotrophic Andean lakes: the role of UVR on vertical distribution. Limnol. Oceanogr., 54:1098-1112. [ Links ]
DAHM, CN; MA BAKER; DI MOORE & JR THIBAULT. 2003. Coupled biogeochemical and hydrological responses of streams and rivers to drought. Freshwater Biol., 48:1219-1231. [ Links ]
DENWARD, CMT & LJ TRANVIK. 1998. Effects of solar radiation on aquatic macrophyte litter decomposition. Oikos, 82:51-58. [ Links ]
DEWSON, ZS; ABW JAMES & RG DEATH. 2007. Invertebrate community responses to experimentally reduced discharge in small streams of different water quality. J. N. Am. Benthol. Soc., 26:754-766. [ Links ]
DÍAZ VILLANUEVA, V. 2002. Efecto del pastoreo en la estructura y dinámica de la comunidad de algas epilíticas en un arroyo andino. Tesis Doctoral: Fac. Cs. Ex. y Naturales, UBA. [ Links ]
DÍAZ VILLANUEVA, V & R ALBARIÑO. 1999. Feeding habit of Notoperla archiplatae (Plecoptera) larvae in a North Patagonia Andean stream, Argentina. Hydrobiologia, 412:43-52. [ Links ]
DÍAZ VILLANUEVA, V; R ALBARIÑO & B MODENUTTI. 2004. Grazing impact of two aquatic invertebrates on periphyton from an Andean-Patagonian stream. Arch. Hydrobiol., 159:455-471. [ Links ]
DÍAZ VILLANUEVA, V & C TROCHINE. 2005. The role of microorganisms in the diet of Verger cf. limnophilus (Trichoptera: Limnephilidae) larvae in a Patagonian Andean temporary pond. Wetlands, 25:473-479. [ Links ]
DIÉGUEZ, M & E BALSEIRO. 1998. Colony size in Conochilus hippocrepis: defensive adaptation to predator size. Hidrobiología, 387:421-425. [ Links ]
DIEHL, P; MJ MAZZARINO; F FUNES; S FONTENLA; M GOBBI; ET AL. 2003. Nutrient conservation strategies in native Andean-Patagonian forests. J. Vegetation Science, 14:63-70. [ Links ]
DIEHL, S. 2002. Phytoplankton, light, and nutrients in a gradient of mixing depths: Theory. Ecology, 83:386-398. [ Links ]
DRAGO, E. 1974. Estructura térmica de Lago Mascardi (pcia de Río Negro, Argentina). Physis (B), 33:207-216. [ Links ]
DURANT, JM; DØ HJERMANN; T ANKER-NILSSEN; G BEAUGRAND; A MYSTERUD; ET AL. 2005. Timing andabundance as key mechanisms affecting trophic interactions in variable environments. Ecol. Lett., 8:952-958. [ Links ]
ELSER, J & D HESSEN. 2005. Biosimplicity via stoichiometry: the evolution of food-web structure and processes. In Belgrano, A; UM Scharler; J Dunne & RE Ulanowicz (eds.). Aquatic Food Webs, An ecosystem approach. Oxford University Press. [ Links ]
ELSER, JJ; K HAYAKAWA & J URABE. 2001. Nutrient limitation reduces food quality for zooplankton: Daphnia response to seston phosphorus enrichment. Ecology, 82:898-903. [ Links ]
GIBSON, CA; RE RATAJCZAK JR & GD GROSSMAN. 2004. Patch based predation in a southern Appalachian stream. Oikos, 106:158-166. [ Links ]
GRAÇA, MAS; CGMO CRESSA; MJ FEIO; KA CALLIES & C BARIOS. 2001. Food quality, feeding preferences, survival and growth of shredders from temperate and tropical streams. Freshwater Biol., 46:947-957. [ Links ]
HAIRSTON, NG & NG HAIRSTON. 1993. Cause-effect relationships in energy flow, trophic structure, and interspecific interactions. Am. Nat., 142:379-411. [ Links ]
HAIRSTON, NG; FE SMITH & LB SLOBODKIN. 1960. Community structure, population control, and competition. Am. Nat., 94:421-425. [ Links ]
HERBST, DB; EL SILLDORFF & SD COOPER. 2009. The influence of introduced trout on the benthic communities of paired headwater streams in the Sierra Nevada of California. Freshwater Biol., 54:1324-1342. [ Links ]
HESSEN, DO; PJ FAEROVIG & T ANDERSEN. 2002. Light, nutrients, and P:C ratios in algae: Grazer performance related to food quality and quantity. Ecology, 83:1886-1898. [ Links ]
HRBACEK, J. 1962. Species composition and the amount of zooplankton in relation to the fish stock. Rozpr. Cesk. Akad. Ved. Rada. Mat. Prir., 72:1-116. [ Links ]
HUISMAN, J; J SHARPLES; JM STROOM; PM VISSER; WEA KARDINAAL; ET AL. 2004. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology, 85:2960-2970. [ Links ]
IRIONDO, MH. 1989. Quaternary lakes of Argentina. Palaeogeogr., Palaeoclimatol., Palaeoecol., 70:81-88. [ Links ]
KIRK, JTO. 1996. Light and photsynthesis in aquatic ecosystems, 2nd ed. Cambridge University Press. [ Links ]
MARINONE, MC; S MENU MARQUE; D AÑÓN SUÁREZ; MDC DIÉGUEZ; P PÉREZ; ET AL. 2006. UV radiation as a potential driving force for zooplankton community structure in Patagonian lakes. Photochem. Photobiol., 82:962-971. [ Links ]
MENU MARQUE, SA & MC MARINONE. 1986. El zooplancton de seis lagos del Chubut (Argentina) y sus probables relaciones con la ictiofauna y algunos factores ambientales. Pp. 90-114 in: Vila, I & E Fagetti (eds.). Trabajos presentados al taller internacional sobre ecología y manejo de peces en lagos y embalses. Santiago de Chile, 5-10 / nov. 1984. FAO. [ Links ]
MODENUTTI, BE. 1993. Summer population of Hexarthra bulgarica in a high altitude lake of South Andes. Hydrobiologia, 259:33-37. [ Links ]
MODENUTTI, BE. 1997. Distribución de los ciliados planctónicos Ophrydium naumanni y Stentor araucanus en lagos oligotróficos andinos. Rev. Soc. Mex. Hist. Nat., 47:79-83. [ Links ]
MODENUTTI, BE & E BALSEIRO. 1994. Zooplankton size spectrum in four lakes of the Patagonian Plateau. Limnologica, 24:51-56. [ Links ]
MODENUTTI, BE & EG BALSEIRO. 2002. Mixotrophic ciliates in an Andean lake: dependence on light and prey of an Ophrydium naumanni population. Freshwater Biol., 47:121-128. [ Links ]
MODENUTTI, BE; EG BALSEIRO & PM CERVELLINI. 1993. Effect of selective feeding of Galaxias maculatus (Salmoniforme, Galaxiidae) on zooplankton of a South Andes lake. Aquat. Sci., 55:65-75. [ Links ]
MODENUTTI, BE; EG BALSEIRO & R MOELLER. 1998b. Vertical distribution and resistance to ultraviolet radiation of a planktonic ciliate Stentor araucanus. Verh. Internat. Verein. Limnol., 26:1636-1640. [ Links ]
MODENUTTI, BE; EG BALSEIRO & CP QUEIMALIÑOS. 2000. Ciliate community structure in two South Andean lakes: the effect of lake water on Ophrydium naumanni distribution. Aquat. Microb. Ecol., 21:299-307. [ Links ]
MODENUTTI, BE; EG BALSEIRO; C CALLIERI & R BERTONI. 2008. Light versus food supply as factors modulating niche partitioning in two pelagic mixotrophic ciliates. Limnol. Oceanogr., 53:446-455. [ Links ]
MODENUTTI, BE; C QUEIMALIÑOS; E BALSEIRO & M REISSIG. 2003. Impact of different zooplankton structures on the microbial food web of a South Andean oligotrophic lake. Acta Oecologica-International Journal of Ecology, 24:S289-S298. [ Links ]
MODENUTTI, BE; EG BALSEIRO; C CALLIERI; R BERTONI & CP QUEIMALIÑOS. 2005. Effect of UV-B and different PAR intensities on the primary production of the mixotrophic planktonic ciliate Stentor araucanus. Limnol. Oceanogr., 50:864-871. [ Links ]
MODENUTTI, BE; E BALSEIRO; C CALLIERI ; C QUEIMALIÑOS & R BERTONI. 2004. Increase in photosynthetic efficiency as a strategy of planktonic organisms exploiting deep lake layers. Freshwater Biol., 49:160-169. [ Links ]
MODENUTTI, BE; EG BALSEIRO; CP QUEIMALIÑOS; D AÑÓN SUÁREZ; MC DIÉGUEZ: ET AL. 1998a. Structure and dynamics of food webs in Andean lakes. Lakes Reservoirs: Res. Manage., 3:179-186. [ Links ]
MORIN, PJ. 1999. Community Ecology. Blackwell Scientific. [ Links ]
MORRIS, DP; H ZAGARESE; CE WILLIAMSON; EG BALSEIRO; BR HARGREAVES; ET AL. 1995. The attenuation of solar UV radiation in lakes and the role of dissolved organic carbon. Limnol. Oceanogr., 40:1381-1391. [ Links ]
NAIMAN, RJ; H DÉCAMPS & ME MCCLAIN. 2005. Riparia: ecology, conservation and management of streamside communities. Elsevier Academic Press. [ Links ]
OSBURN, CL; HE ZAGARESE; DP MORRIS; BR HARGREAVES & WE CRAVERO. 2001. Calculation of spectral weighting functions for the solar photobleaching of chromophoric dissolved organic matter in temperate lakes. Limnol. Oceanogr., 46:1455-1467. [ Links ]
PARUELO, JM; A BELTRÁN; E JOBBÁGY; O SALA & R GOLLUSCIO. 1998. The climate of Patagonia: general patterns and controls on biotic processes. Ecol. Austral, 8:85-101. [ Links ]
PÉREZ, G; C QUEIMALIÑOS; E BALSEIRO & B MODENUTTI. 2007. Phytoplankton absorption spectra along the water column in deep North Patagonian Andean lakes (Argentina): Limnology of Temperate South America. Limnologica, 37:3-16. [ Links ]
PÉREZ, GL; CP QUEIMALIÑOS & BE MODENUTTI. 2002. Light climate and plankton in the deep chlorophyll maxima in North Patagonian Andean lakes. J. Plankton Res., 24:591-599. [ Links ]
PÉREZ, MT & R SOMMARUGA. 2007. Interactive effects of solar radiation and dissolved organic matter on bacterial activity and community structure. Environ. Microbiol., 9:2200-2210. [ Links ]
PETERSEN, RC & KW CUMMINS. 1974. Leaf processing in a woodland stream. Freshwater Biol., 4: 343-368. [ Links ]
QUIRÓS, R. 1988. Relationship between air temperature, depth, nutrient and chlorophyll in 103 Argentinian lakes. Verh. Internat. Verein. Limnol., 23:647-658. [ Links ]
QUIRÓS, R. 1990. Predictors of relative fish biomass in lakes and reservoirs of Argentina. Can. J. Fish. Aquat. Sci., 47:928-939. [ Links ]
QUIRÓS, R. 1997. Fish effects on trophic relationships in the pelagic zone of lakes. Hydrobiologia, 361:101-111. [ Links ]
REISSIG, M; B MODENUTTI; E BALSEIRO & C QUEIMALIÑOS. 2004. The Role of the Predaceous Copepod Parabroteas Sarsi in the Pelagic Food Web of a Large Deep Andean Lake. Hidrobiología, 524:67-77. [ Links ]
REISSIG, M; C TROCHINE; C QUEIMALIÑOS; E BALSEIRO & B MODENUTTI. 2006. Impact of fish introduction on planktonic food webs in lakes of the Patagonian Plateau. Biol. Conserv., 132:437-447. [ Links ]
SHAPIRO, J. 1990. Biomanipulation: the next phase, making it stable. Hydrobiologia, 200/201:13-28. [ Links ]
SIEBECK, O; T VAIL; CE WILLIAMSON; R VETTER; D HESSEN; ET AL. 1994. Impact of UV-B radiation on zooplankton and fish in pelagic freshwater ecosystems. Arch. Hydrobiol. Beih. Ergebn. Limnol., 43:101-104. [ Links ]
SOUZA, MS; BE MODENUTTI & EG BALSEIRO. 2007. Antioxidant defences in planktonic crustaceans exposed to different underwater light irradiances in Andean lakes. Water, Air, Soil Pollut., 183:49-57. [ Links ]
STERNER, RW & JJ ELSER. 2002. Ecological stoichiometry. The biology of elements from molecules to the biosphere. Princeton University Press. [ Links ]
STERNER, RW; JJ ELSER; EJ FEE; SJ GUILDFORD & TH CHRZANOWSKI. 1997. The light:nutrient ratio in lakes: The balance of energy and materials affects ecosystem structure and process. Am. Nat., 150:663-684. [ Links ]
STOMP, M; J HUISMAN; L VOROS; FR PICK; M LAAMANEN; ET AL. 2007. Colourful coexistence of red and green picocyanobacteria in lakes and seas. Ecol. Lett., 10:290-298. [ Links ]
STRAILE, D. 2005. Food webs in lakes - seasonal dynamics and the impact of climate variability. Pp. 41-50 in: Belgrano, A; U Scharler; J Dunne & B Ulanowicz (eds.). Aquatic Food Webs: an Ecosystem Approach. Oxford University Press. [ Links ]
SUBERKROPP, K & E CHAUVET. 1995. Regulation of leaf breakdown by fungi in streams: Influences of water chemistry. Ecology, 76:1433-1445. [ Links ]
SUBERKROPP, K & JB WALLACE. 1992. Aquatic hyphomycetes in insecticide-treated and untreated streams. J. N. Am. Benthol. Soc., 11:165-171. [ Links ]
THOMASSON, K. 1963. Araucanian Lakes. Acta Phytogeograp. Suec., 47:1-139. [ Links ]
TROCHINE, C; E BALSEIRO & B MODENUTTI. 2008. Zooplankton of Fishless Ponds of Northern Patagonia: Insights into Predation Effects of Mesostoma ehrenbergii. Int. Rev. Hydrobiol., 93:312-327. [ Links ]
TROCHINE, C; B MODENUTTI & E BALSEIRO. 2005. When prey mating increases predation risk: The relationship between the flatworm Mesostoma ehrenbergii and the copepod Boeckella gracilis. Arch. Hydrobiol., 163:555-569. [ Links ]
TROCHINE, C; B MODENUTTI & E BALSEIRO. 2006. Influence of spatial heterogeneity on predation by the flatworm Mesostoma ehrenbergii (Focke) on calanoid and cyclopoid copepods. J. Plankton Res.,28:267-274. [ Links ]
TROCHINE, C; BE MODENUTTI & EG BALSEIRO. 2009. Chemical signals and habitat selection by three zooplankters in Andean Patagonian ponds. Freshwater Biol., 54:480-494. [ Links ]
URABE, J; M KYLE; W MAKINO; T YOSHIDA; T ANDERSEN & JJ ELSER. 2002. Reduced light increases herbivore production due to stoichiometric effects of light/nutrient balance. Ecology, 83:619-627. [ Links ]
VALDOVINOS, C. 2001. Procesamiento de detritus ripariano por macroinvertebrados bentónicos en un estero boscoso de Chile central. Rev. Chil. Hist. Nat., 74:445-453. [ Links ]
VELÁSQUEZ, SM & ML MISERENDINO. 2003. Habitat type and macroinvertebrate assemblages in low order patagonian streams. Arch. Hydrobiol., 158:461-483. [ Links ]
VILLAFAÑE, VE; AGJ BUMA; P BOELEN & EW HELBLING. 2004. Solar UVR-induced DNA damage and inhibition of photosynthesis in phytoplankton from Andean lakes of Argentina. Arch. Hydrobiol., 161:245-266. [ Links ]
VÖRÖS, L; C CALLIERI; KV BALOGH & R BERTONI. 1998. Freshwater picocyanobacteria along a trophic gradient and light quality range. Hydrobiologia, 370:117-125. [ Links ]
WOELFL, S & W GELLER. 2002. Chlorella-bearing ciliates dominate in an oligotrophic North Patagonian lake (Lake Pirehueico, Chile): abundance, biomass and symbiotic photosynthesis. Freshwater Biol., 47:231-242. [ Links ]
ZAGARESE, HE; M DÍAZ; F PEDROZO; M FERRARO; W CRAVERO; ET AL. 2001. Photodegradation of natural organic matter exposed to fluctuating levels of solar radiation. J. Photochem. Photobiol. B: Biol., 61:35-45. [ Links ]














