Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Ecología austral
versión On-line ISSN 1667-782X
Ecol. austral vol.20 no.3 Córdoba sep./dic. 2010
TRABAJOS ORIGINALES
Spatial-temporal patterns of functional feeding groups in mountain streams of Córdoba, Argentina
Romina E Príncipe*, Cristina M Gualdoni, Ana M Oberto, Graciela B Raffaini & María C Corigliano
* Universidad Nacional de Río Cuarto, Departamento de Ciencias Naturales, Río Cuarto, Pcia. de Córdoba, Argentina.
Universidad Nacional de Río Cuarto, Departamento de Ciencias Naturales, AP Nº 3, (X5804BYA) Río Cuarto, Pcia. de Córdoba, Argentina.
Emal: principe.romina@gmail.com.
Recibido: 18 de mayo de 2010;
Fin de arbitraje: 11 de julio de 2010;
Revisión recibida: 26 de agosto de 2010;
Aceptado: 30 de agosto de 2010
ABSTRACT. Trophic structure of benthic communities is influenced by the availability of food resources which indeed may be conditioned by stream size, shading and substrate. This study aims to analyze the distribution of macroinvertebrate Functional Feeding Groups in different habitats of mountain streams (Córdoba, Argentina) and to assess the environmental variables conditioning this distribution at the habitat level. Four streams were sampled in two hydrological periods (high and low discharge) and three benthic samples were taken in riffles and runs of coarse and fine substrate. Gathering collectors were dominant in most of the habitats, streams and periods except in riffles during the low water period in which filtering collectors dominated. At the habitat level, current velocity, substrate, abundance of macroalgae and twigs and leaves were the most important variables explaining functional feeding group distribution. Functional feeding group abundances varied in relation to the stream, the hydrological period and the habitat. The dominance of collectors demonstrates the importance of the role of this functional group and that fine detritus is the main food resource in these lotic ecosystems. The phenology and life history of the species, and the amount and type of organic matter retained in each habitat may explain the observed spatial-temporal patterns.
Keywords: Aquatic macroinvertebrates; Benthos; Habitat; Functional organization; Lotic ecosystem.
RESUMEN. Patrones de variación espacio-temporal de grupos funcionales alimentarios en arroyos serranos de Córdoba, Argentina: La estructura trófica de las comunidades bentónicas está condicionada por la disponibilidad de recursos alimenticios, los cuales a su vez varían en función del tamaño del arroyo, la cobertura y el tipo de sustrato. El objetivo de este trabajo fue analizar la distribución de los grupos funcionales alimentarios de macroinvertebrados en diferentes hábitats de arroyos serranos de la Provincia de Córdoba (Argentina) y examinar las variables ambientales que explican esta distribución a escala de hábitat. Se consideraron cuatro arroyos en dos períodos hidrológicos (alto y bajo caudal) en los cuales se recolectaron tres muestras de bentos en rabiones y correderas de sustrato fino y grueso. Los colectores de depósito fueron dominantes en la mayoría de los hábitats, arroyos y períodos hidrológicos, excepto en los rabiones durante el periodo de bajo caudal en el cual predominaron los colectores filtradores. Las variables más importantes que explicaron la distribución de los grupos funcionales a escala de hábitat fueron la velocidad de la corriente, el tipo de sustrato y la abundancia de macroalgas y de ramas y hojas. La abundancia de los grupos funcionales varió en función de los arroyos, de los distintos hábitats y de los periodos hidrológicos. La dominancia de los colectores demuestra la importancia del rol de este grupo funcional y que el detrito fino sería el recurso alimenticio principal en estos ecosistemas lóticos. La fenología y la forma de vida de las especies y la cantidad y calidad de materia orgánica retenida en cada hábitat explicarían el patrón espacio-temporal de variación de los grupos funcionales alimentarios.
Palabras clave: Macroinvertebrados acuáticos; Bentos; Hábitat; Organización funcional; Ecosistema lótico.
INTRODUCTION
The assessment of the functional organization of aquatic communities is an important tool in stream ecology since the ability to view a faunal assemblage as a collection of functional groups provides valuable insight into which food resources are prevalent, and allows one to observe how different groups of organisms respond to environmental variables (Allan & Castillo 2007).The term "functional feeding group" was first used by Cummins (1974), who stated that it was necessary the identification of functional groups of organisms, at least partially independent of taxonomic determinations, in order to address important process-oriented ecosystem questions. The functional group concept is concerned with how a resource or any other ecological component is processed by different species to provide a specific ecosystem service or function (Blondel 2003).
The feeding roles of invertebrates in lotic ecosystems are categorized according to food sources and mechanisms of food acquisition, which in turn are related to morphological and behavioral adaptations of the consumer (Allan & Castillo 2007). This grouping reflects both convergent and parallel evolution leading to functionally similar organisms. The functional feeding groups reflect the four most important food resources found in streams: periphyton, coarse particulate organic matter, fine particulate organic matter, and animal prey. Shredders feed on coarse particulate organic matter, collectors feed on fine particulate organic matter either from the water column or the streambed, scrapers ingest periphyton, and predators consume prey (Cummins & Klug 1979; Merrit & Cummins 1996; Merritt & Cummins 2006).
Feeding strategies, incorporated in functional analysis, can play an important role in biomonitoring (Charvet et al. 1998) and the trophic structure of a stream can be indirectly evaluated on the basis of functional feeding groups (Paunovic et al. 2006). Functional feeding classification of aquatic organisms enhances the knowledge of trophic dynamics in streams by simplifying the benthic community into trophic groups. In addition, this approach provides a further perspective that can be combined with other community attributes to ensure better understanding of the match between habitat and aquatic fauna (Townsend et al. 1997).
Macroinvertebrates derive their nutrition from a spatially and temporally variable system since streams and rivers are characterized by seasonal, local, and streamorder differences in inputs, production, and storage of food resources (Cummins & Klug 1979). The distribution of the functional groups may be determined by changes in food availability which indeed is influenced by stream size, shading and substrate (Allan & Castillo 2007). At the habitat level, the functional feeding group distribution may be determined by food retention capacity of the different stream habitats which varies mainly according to substrate type and current velocity.
Callisto et al. (2001) pointed out that the use of functional trophic groups and the colonization of characteristic habitats constitute a useful tool for conservation. Intact biological assemblages with a diverse mix of species are expected to carry out various ecosystem functions including primary production, organic matter decomposition, nutrient cycling, and secondary production. As species are lost from ecosystems due to human activities, the extent to which system function and resilience depend on the number and characteristics of species present becomes an issue of considerable concern (Covich et al. 2004).
Considering the importance of the functional feeding group approach in biomonitoring and conservation, the assessment of the functional organization of macroinvertebrate community turns out to be essential. Although distribution patterns of macroinvertebrate assemblages have already been evaluated in stream habitats of the central region of Argentina (Príncipe et al. 2007, 2008), information in relation to the functional organization of the community at the habitat level is still scarce (Corigliano & Malpassi 1998). This study aims to analyze the distribution of functional feeding groups in different habitats of mountain streams of central Argentina and to assess the environmental variables conditioning this distribution at the habitat level. Taking into account that functional feeding groups greatly depend on food resources and that food is conditioned, among other factors, by shading and substrate of the streams (Alan & Castillo 2007), we expect to find differences in the abundance of the functional feeding groups in relation to habitat type. As the study streams are low order streams but they are not canopied, we expect to find a less proportion of shredders compared to the proportion hypothesized by the River Continuum Concept (Vannote et al. 1980). However, the abundance of shredders will be higher in riffles since these habitats are more heterogeneous and retain more quantities of coarse organic matter.Additionally, we expect to find in general a dominance of gathering collectors since many studies in the Neotropical region have reported the prevalence of this functional group (Palmer et al. 1993; Tomanova et al. 2006). Seasonal variation is also expected and the main variables explaining the distribution not only at the temporal scale but also at the habitat level would then be current velocity, substrate type and organic matter.
METHODS
The study was carried out in four streams of Chocancharava River and Ctalamochita River upper sub-basins, Córdoba, Argentina. These rivers are the main tributaries of Carcarañá River and belong to Río de la Plata River basin. The Carcarañá River system is submitted to a highly dynamic hydrology, with short and intense floods in specific periods of the year (Cantero et al. 1998). The rainy season starts in October and ends in April with a maximum of nearly 750 mm in this period, whereas minimum precipitation (150 mm approximately) occurs between April and September (Capitanelli 1979). Maximum temperature reaches 34 ºC in summer (December-March) and decreases up to -5 ºC in winter (June-September). Vegetation of the study area, which is only partially shaded, changes in relation to the altitudinal gradient and its distribution is modified by human activities (Cabido et al. 2003).Location and environmental characteristics of sampling sites are shown in Table 1.
Table 1. Location and environmental characterization of the study sites in tributaries of the Carcarañá River.
Tabla 1. Ubicación y caracterización ambiental de los sitios de estudio en los tributarios del río Carcarañá.

Sampling was carried out during high (March 2003) and low water period (July 2003). All streams were visited twice in each period since temporal replication is required to detect seasonal differences in abundance (Underwood 1994). Three different habitats were sampled in each site and sampling date: riffles, coarse substrate runs, and fine substrate runs. Three replicate Surber samples (0.09 m2, 300 µm mesh size) were taken in each of these habitat units following a stratified random sampling design. A total of 144 benthic samples were collected (2 hydrological periods, 4 streams, 3 habitat units and 3 replicates).
Substrate composition and flow type were visually assessed (Gordon et al. 1994) in each habitat unit and assigned to a category according to Thomson et al. (2001). The proportional abundance of macrophytes, macroalgae, twigs and leaves, and detritus were also evaluated. Current velocity (m/s) and depth (m) were measured with a Global Flow Probe FP101-FP201 for each sample (three times in each habitat unit). Conductivity (µS/cm), pH, temperature (ºC) and turbidity (UTM) were measured with portable sensors on each sampling occasion. Invertebrates were preserved in 4% formaldehyde solution. At the laboratory, organisms were sorted, identified to the lowest possible taxonomic level, counted and kept in 70% ethanol. After identification, macroinvertebrates were assigned to a functional feeding group (gathering collector, filtering collector, scraper, shredder, predator or generalist) using available references (Berg 1995; Lopreto & Tell 1995; Merrit & Cummins 1996, 2006; Callisto et al. 2001; Tomanova et al. 2006; Allan & Castillo 2007). Abundance of each functional feeding group was calculated as number of individuals/m2.
A Canonical Correspondence Analysis (ter Braak 1986) was performed to analyze the distribution of the functional feeding groups in the different habitat units and their relationships with environmental variables characterizing these habitats. Abundance data were log10 (Y+1) transformed and a restricted Monte Carlo permutation test was performed (199 permutations) for determining the significance of eigenvalues derived from the canonical correspondence analysis. Restricted permutations favoured the null model (completely random permutations) because benthic samples were collected in a special spatial structure (sampling scheme). Under this permutation scheme, only samples collected in the same stream and during the same hydrological period were permuted. The correspondence analysis was carried out using the statistical package CANOCO version 4.02 (ter Braak & Smilauer 1998).
Samples were also classified by discriminant analysis to confirm the results of the canonical correspondence analysis in determining the differences in functional feeding groups at the habitat level. DA is useful in order to build a predictive model of group membership based on observed characteristics of each sample. DA generates discriminant functions based on linear combinations of the predictor variables that provide the best discrimination between the groups. Additionally, DA provide valuable information since allow to obtain a list of the most significant predictors, a quantification of the change expressed by the corresponding discriminant functions and a classification matrix that checks the goodness of such discriminant functions (Wunderlin et al. 2001). In this study, predictor variables were FFG abundances and the grouping variable was habitat type (riffles, coarse substrate runs and fine substrate runs).
Variation of functional feeding group abundances were assessed at different spatial and temporal scales (among streams, among habitats, among samples within habitats, between hydrological periods, between dates within periods) using a model of ANOVA with two nested random factors and three crossed fixed factors. Abundance of scrapers, shredders and generalists were log10(Y+1) transformed and abundance of predators and filtering collectors were ![]() transformed achieving thereby the assumptions of normality and homoscedasticity.
transformed achieving thereby the assumptions of normality and homoscedasticity.
RESULTS
During the high water period, the average temperature was 18 ºC considering the 4 streams; pH ranged from 7.5 to 8, turbidity values ranged from 0.6 UTM to 1.7 UTM and the average conductivity was 110.9 µS/cm with the maximum value being registered in Río de los Sauces (172.6 µS/cm) and the minimum in El Talita (59.3 µS/cm). In the low water period, the temperature ranged from 8.6 ºC in El Talita to 12.7 ºC in Piedras Blancas, the average pH value was 8, turbidity ranged from 0.8 UTM in Rio de los Sauces to 2.6 UTM in Piedras Blancas; and conductivity presented the maximum value in Río de los Sauces (202.9 µS/cm) and the minimum in El Talita (72.9 µS/cm).
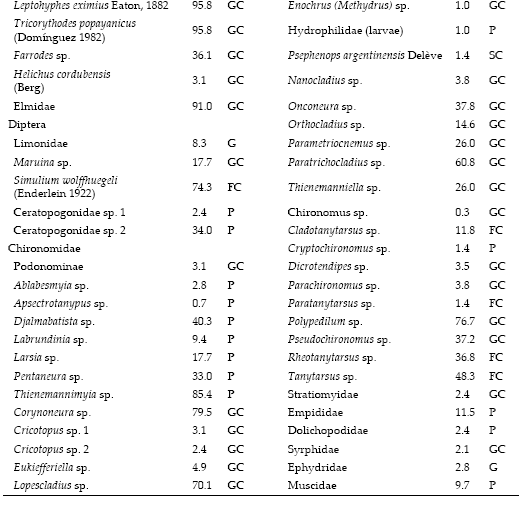
A total of 97 macroinvertebrate taxa were collected in this study (see supplementary information in www.ecologiaaustral.com.ar ), 37 were assigned as gathering collectors, 30 predators, 16 scrapers, 8 filtering collectors, and 3 were assigned as shredders. Nematoda, Limonidae and Ephydridae were assigned as generalists since available literature state that these taxa feed on a great variety of food and present different mechanisms of food acquisition (Lopreto & Tell 1995; Merrit & Cummins 1996).
Gathering collectors were the dominant functional feeding group in most of the habitats, streams and hydrological periods, but in riffles of Río de los Sauces, the proportional abundance of this group decreased to 5% during the low water period where filtering collectors predominated (Figure 1). Scrapers presented the highest proportional abundance (almost 40%) in Las Cañitas and Río de los Sauces during the high water period. The highest proportional abundance of predators (28%) occurred in Las Cañitas during the low water period, mainly in fine substrate runs. Shredders presented a proportional abundance <5% in all cases and generalist <1%.

Figure 1. Proportional abundance of the functional feeding groups in riffles (r), coarse substrate runs (cr) and fine substrate runs (fr) of the studied streams (El Talita, Las Cañitas, Río de los Sauces, Piedras Blancas) during the low (Lw) and high water period (Hw).
Figura 1. Abundancia proporcional de los Grupos Funcionales Alimentarios en rabiones (r), correderas de sustrato grueso (cr) y correderas de sustrato fino (fr) de los arroyos estudiados (El Talita, Las Cañitas, Río de los Sauces, Piedras Blancas) durante los períodos de bajo (Lw) y alto caudal (Hw).
The canonical correspondence analysis allowed the assessment of the functional feeding groups distribution in the different habitat units and of its relationship with the environmental variables that define the fluvial habitats (Figure 2). The first four axes of the ordination explained 30.3% of species data and 99.8% of species-environment relation (Eigenvalues, Axis 1: 0.021, Axis 2: 0.013, Axis 3: 0.006, Axis 4: 0.001). The restricted Monte Carlo permutation test showed that all axes were significant (F=4.02, P=0.005), showing a good relationship between functional feeding group distribution and measured environmental variables. The proportional abundance of sand and bedrock, the current velocity and the flow type were the main variables associated to axis 1 (Figure 2) whereas the abundance of boulder and cobble, and the presence of macroalgae and twigs and leaves were the most important variables explaining the ordination pattern along the axis 2. Filtering collectors and shredders were mainly associated to riffles which were characterized by coarse substrate high current velocity and turbulent flow. Predators, scrapers and gathering collectors presented a similar distribution, and in the ordination they were mainly related to fine substrate runs. Generalists, located in the lower left quadrant of the plot, were mainly related to runs.

Figure 2. Canonical Correspondence Analysis of benthic samples (A) and functional feeding groups (B) from riffles (1), coarse substrate runs (2) and fine substrate runs (3) of the studied streams (El Talita: circles, Las Cañitas: squares, Río de los Sauces: triangles, Piedras Blancas: diamonds) during the high (open symbols) and the low water period (close symbols). Floating, Submerged and Emergent refers to types of macrophytes. Crosses in B refer to functional feeding groups.
Figura 2. Análisis de Correspondencias Canónicas de las muestras de bentos (A) y los grupos funcionales alimentarios (B) de rabiones (1), correderas de sustrato grueso (2) y correderas de sustrato fino (3) de los arroyos estudiados (El Talita: círculos, Las Cañitas: cuadrados, Río de los Sauces: triángulos, Piedras Blancas: rombos) durante los periodos de alto y bajo caudal (símbolos blancos y negros respectivamente). Flotante, Sumergido y Emergente se refiere a los tipos de macrófitas. Las cruces en B representan los grupos funcionales alimentarios.
Discriminant analysis enabled quite good differentiation of habitat types mainly between riffles and runs (Figure 3). Axis 1 of the discriminant analysis explained 93.5% of the variation between groups. The most important predictors for differentiating the habitats were the abundance of filterers, shredders and scrapers (Table 2) which were more abundant in riffle habitats. Sixty-five (65) of 144 samples were misclassified with 54.9 % of correct classification (Table 3). Since the discriminant analysis did not distinguish runs of different substrate, we also carried out the analysis considering only two habitats (riffles and runs) in the grouping variable. In this case, 79.1% of the samples were correctly classified and the discriminant function coefficients of the predictor variables were: filtering collectors 0.62, shredders and scrapers 0.46, gathering collectors -0.77, predators -0.23 and generalists -0.01.
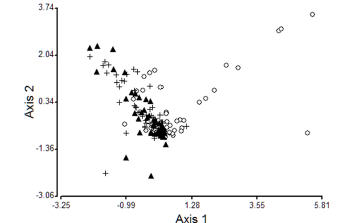
Figure 3. Classification of benthic samples from riffles (white circles), coarse substrate runs (black triangles) and fine substrate runs (crosses) by discriminant analysis.
Figura 3. Clasificación de las muestras de bentos de rabiones (círculos blancos), correderas de sustrato grueso (triángulos negros) y correderas de sustrato fino (cruces) por análisis discriminante.
Table 2. Predictor variables of the discriminant analysis with the corresponding discriminant function coefficients.
Tabla 2. Variables predictivas del análisis discriminante con los coeficientes de la función discriminante correspondientes.

Table 3. Cross-classification matrix for discriminant analysis of spatial variation in the different habitats of the study streams.
Tabla 3. Matriz de clasificación cruzada para el análisis discriminante de la variación espacial en los diferentes hábitats de los arroyos bajo estudio.

The abundance of gathering collectors, scrapers, predators and filtering collectors depended on the joint effect of stream, period and habitat (Table 4).The abundance of shredders was influenced by the joint effect of stream and habitat, and the abundance of generalists showed dependence of the single effect of the stream. Three functional feeding groups (gathering collectors, scrapers and predators) presented differences in the abundance among samples within the same habitat (spatial scale) and also three groups (predators, filtering collectors and generalists) showed differences at the temporal scale of dates within periods.
Table 4. Results of nested ANOVAs showing the variation of functional feeding group abundances at different spatial (stream, habitat and sample) and temporal scales (hydrological period and date). The influence of the interaction terms on abundances was also evaluated.
Tabla 4. Resultados de ANOVAs anidados mostrando la variación de la abundancia de los grupos funcionales alimentarios a diferentes escalas espaciales (arroyo, hábitat, repetición) y temporales (periodo hidrológico y fecha). Se presenta además la influencia de las interacciones sobre la abundancia.

The highest density of gathering collectors was found in runs of Río de los Sauces during the low water period (43360 individuals/m2) and the lowest was registered in riffles of the same stream and period (2222 individuals/m2). However, the abundance of this functional feeding group was high in most of the sampling occasions becoming thus, the most ubiquitous group. Scrapers presented the maximum density in riffles of Río de los Sauces during the high water period (26388 individuals/m2) and the minimum during the low water period in the same habitat of this stream (1451 individuals/m2). The highest abundance of shredders was found in riffles of Piedras Blancas and Río de los Sauces (711 individuals/m2 and 395 individuals/m2, respectively) and the highest density of predators was observed in fine substrate runs of Las Cañitas stream during the low water period (16060 individuals/m2). Filtering collectors presented the maximum density in riffles of Río de los Sauces during the low water period (38764 individuals/m2) and the highest abundance of generalists was found in Piedras Blancas stream (52 individuals/m2).
DISCUSSION
Ecological functions can be described by a variety of biological traits that reflect the adaptation of species to environmental conditions (Townsend & Hildrew 1994). This adaptation can be explored by the assessment of feeding strategies which are used as a unified measure to compare communities with different taxonomic composition (Statzner et al. 2001). In this study we found that functional feeding group abundances differed among habitat units. Gathering collectors were dominant in all cases except in riffles of Río de los Sauces during the low water period in which filtering collectors dominated. This group, mainly represented by Simulium wollfhuegeli, showed the highest density in riffles and appeared related to high current velocity and turbulent flow. Scrapers, mainly represented by caddisflies of the Hydroptilidae family, were more abundant in habitats of coarse substrate with large amount of macroalgae and predators were more abundant in runs of fine substrate.
In general, scrappers and filterers have been found mainly associated to riffle habitats, whereas predators have been more abundant in habitats characterized by fine substrate and smooth flow (Merritt & Cummins 1996; Schmera & Eros 2004). Streams with stable substrate, high current velocity and high-quality organic seston concentrations often allocate massive standing stocks of filterfeeding hydropsychids and/or black flies (Wallace & Webster 1996). Filterer densities that are higher than those of other functional groups are possible because filterers use the kinetic energy of the current to exploit food produced in upstream habitats. As a consequence, filterers expend less energy in search of food; then the stream segment in which they occur can support a higher biomass per unit area (Cudney & Wallace 1980). On the other hand, our results showed that scrappers were not only associated to riffle habitats but also to the presence of macroalgae, which constitute the main food resource of Hydroptilidae caddisflies (Wiggins 1996). Additionally, the highest densities of predators found in fine substrate habitats correspond to Hydrachnidia and the Tanypodinae Thienmannimyia sp.It has already been reported that fine substrate is preferred by this Chironomidae (Fittkau & Roback 1983; Principe et al. 2008) whereas aquatic Acari can exploit a wide variety of instream habitats. However, Chironomidae larvae constitute the main food resource of Hydrachnidia (Rosso de Ferradás & Fernández 2009); therefore, the increase in the density of both taxa in fine substrate runs could be linked by a trophic relationship.
In this study, shredders were more abundant in riffles which agree with the results obtained in previous research (Velásquez & Miserendino 2003b; Schmera & Eros 2004). This result might be explained taking into account the differences in the accumulation and retention of coarse particulate organic matter among the habitat units. According to Brussock & Brown (1991) coarse particulate organic matter accumulates on riffles due to the roughness and the heterogeneity that characterize these habitats. Therefore, the amount of particulate organic matter in streams depends not only of the riparian vegetation (Graça 2001) but also of the instream habitat features (Arscott et al. 2003; Subramanian & Sivaramakrishnan 2005).
The discriminant analysis showed that filterers and shredders were more associated to riffles whereas collectors and predators were more related to runs, similarly to the results obtained by the canonical correspondence analysis. However, the analysis failed in distinguishing runs of different substrate since a high proportion of samples were misclassified (45.2%). However, we found that 79.1% of the samples were correctly classified when considering only two habitats in the grouping variable (riffles and runs). This result may suggest that the discrimination of runs with different substrate may not be necessary since the predictive value of functional feeding groups is higher when habitat units are considered at a higher scale. As a consequence, it could be important to take into account this finding when analyzing the cost-benefit of sampling effort for the design of sampling procedures, since this may imply a considerable reduction of the effort employed to sample habitats units in the field work.
According to the River Continuum Concept (Vannote et al. 1980) low order streams, similar to those considered in this study, should present a relatively high density of shredders, of about 30% of proportional abundance. However, we found no more than 5% of proportional abundance of shredders in this study. This finding should be interpreted considering the framework in which this concept was developed. In its original postulation the concept considered a river system with headwater streams (order 1-3) flowing through forested regions with headwaters heavily shaded and abundant leaf litter input from the riparian forest leading to a relatively high density of shredders. On the contrary, headwaters of the river system considered in this study are located in natural pasturelands. In these pasture streams the input of organic matter from the riparian vegetation is noticeably less significant than in forested streams, then proportional abundance of shredders diminish. Moreover, as the studied streams are not canopied, they should have more autochthonous production similar to the production expected for middle order streams in forested river systems, according to the river continuum concept.
As we found here, several studies have also reported the dominance of gathering collectors and variations in the abundances of the functional feeding group among the different habitat units (Nessimian & Sanseverino 1998; Callisto et al. 2001; Velásquez & Miserendino 2003a,b; Fenoglio et al. 2004; Subramanian & Sivaramakrishnan 2005). In streams of the Neotropical region it has been observed a common affinity of many taxa by fine detritus, demonstrating the importance of this food resource and of the gathering collector role in these freshwater ecosystems (Palmer et al. 1993; Tomanova et al. 2006). The studied streams are submitted to seasonal hydraulic disturbances and to cycles of several rainy years alternated with dry years. Discharge can increase manyfold in few time (scouring floods) especially during the high water period. According to the habitat templet concept (Townsend & Hildrew 1994), macroinvertebrates inhabiting these environments should develop specific adaptations that increase their probability of survival and reproduction. Feeding strategies of scrapers, predators and shredders involve a higher mobility (active searching for food) or the visit to unstable substrates (shredders in leaf litter). As a consequence, these organisms have higher exposure to the current and then higher risk to drift. In streams submitted to seasonal disturbances the feeding strategy of gatherers has been selected since the organisms that belong to this functional feeding group have less exposure to catastrophic floods and then they are able to face the disturbance (Lamouroux et al. 2004).
Considering the high exposure to the current of scrapers, predators and shredders, it would has been expected that the highest densities of these functional feeding groups occur during the low water period but this pattern was not observed in our study. Nested ANOVAs showed a close relationship between temporal and spatial scales since significant interactions were found. Then, the same temporal pattern of variation in functional feeding groups abundances may not be expected in all streams and habitats. Boyero & Bosch (2002) and Schmera & Eros (2004) obtained similar results in studies about macroinvertebrate drift and caddisfly assemblages, respectively. This demonstrates that multifactor effects must be considered when trying to evaluate the spatial and temporal patterns of benthic stream communities. In addition, our results showed significant differences between dates within the same period and among samples within the same habitat in some cases. These variations among replicates and the fact that multiple factors interact to determine distribution patterns are related to the heterogeneity that characterized lotic ecosystems (Palmer & Poff 1997).
In summary, in this study we found that the functional organization of the benthic macroinvertebrate community shows different patterns of variation at the spatial and temporal scales. The substantial dominance of gathering collectors demonstrates the importance of the role of this functional group and that fine detritus is the main food resource in these lotic ecosystems. At the habitat level, current velocity, substrate and organic matter would be the most important variables explaining functional feeding group distribution. The phenology and life history of the species, and the amount and type of organic matter retained in each habitat may explain the spatial-temporal patterns. The highly flexible life histories and mobility that seem to characterize many neotropical stream taxa, may well influence their flexibility in obtaining food resources (Covich 1988). As macroinvertebrates seem to have a quite variable feeding behaviour future research on feeding habits and plasticity may allow more precise results in relation to the spatial-temporal variations in the functional organization of benthic communities. The elucidation of functional patterns of benthic communities is of great ecological importance since, in combination with other monitoring procedures, the functional approach is clearly superior to the commonly used biomonitoring procedures (Charvet et al. 1998), as those successfully used in the assessment of river integrity by the application of metric indexes (Barbour et al. 1999; Tripole & Corigliano 2005; Boccolini et al. 2005). Hence, this approach may allows a more accurate assessment of water quality and ecological integrity which indeed could makes possible the application of more appropriate conservation and restoration strategies in the regional lotic ecosystems.
SUPPLEMENTARY INFORMATION
Aquatic macroinvertebrates collected in the study streams and the Functional Feeding Group (FFG) assigned to each taxon: Gathering collector (GC), Filtering collector (FC), Scraper (SC), Shredder (SH), Predator (P), Generalist (G). %F: Frequency of occurrence (n=144).
Lista de macroinvertebrados acuáticos colectados en los arroyos de estudio y Grupos Funcionales Alimentarios (FFG) asignados a cada taxón: Colector de depósito (GC), Colector filtrador (FC), Raspador (SC), Desmenuzador (SH), Depredador (P), Generalista (G). %F: Frecuencia de ocurrencia (n=144).

ACKNOWLEDGEMENTS
The authors wish to thank C. Duarte, E. Medeot and M. Boccolini for their assistance in the collection of field data and in the laboratory work. This research received financial support from Secretaría de Ciencia y Técnica, Universidad Nacional de Río Cuarto. The first author was supported by a fellowship from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina). The authors are also grateful to anonymous reviewers for the comments that improve the early version of the manuscript.
REFERENCES
1. ALLAN, JD & MM CASTILLO. 2007. Stream ecology. Structure and function of running waters. Springer, Dordrecht. [ Links ]
2. ARSCOTT, DB; B KELLER; K TOCKNER & JB WARD. 2003. Habitat structure and Trichoptera diversity in two headwater flood plains, N.E. Italy. Int. Rev. Hydrobiol., 88:255-273. [ Links ]
3. BARBOUR, MT; J GERRITSEN; BD SNYDER & JB STRIBLING. 1999. Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish. Second Edition. EPA 841-B-99-002. U.S. EPA, Office of Water, Washington, D.C. [ Links ]
4. BERG, MB. 1995. Larval food and feeding behaviour. Pp. 136-168 in: Armitage, PD ; PS Cranston & LCV Pinder (eds.). The Chironomidae. The biology and ecology of non-biting midges. Chapman & Hall. London. [ Links ]
5. BLONDEL, J. 2003. Guilds or functional groups: does it matter? Oikos, 100:223-231. [ Links ]
6. BOCCOLINI, MF; AM OBERTO & MC CORIGLIANO. 2005. Calidad ambiental en un río urbano de llanura. Biol. Acuática, 22:59-69. [ Links ]
7. BOYERO, L & J BOSCH. 2002. Spatial and temporal variation of macroinvertebrate drift in two neotropical streams. Biotropica, 34:567-574. [ Links ]
8. BRUSSOCK, PP & AV BROWN. 1991. Riffle-pool geomorphology disrupts longitudinal patterns of stream benthos. Hydrobiologia, 220:99-108. [ Links ]
9. CABIDO, D; M CABIDO; SM GARRE; JA GORGAS; R MIATELLO; ET AL. 2003. Regiones Naturales de la Provincia de Córdoba. Serie C. Publicaciones Técnicas. Agencia Córdoba, Dirección de Ambiente, Córdoba, Argentina. [ Links ]
10. CALLISTO, M; P MORENO & FAR BARBOSA. 2001. Habitat diversity and benthic functional trophic groups at Serra do Cipó, Southeast Brazil. Rev. Bras. Biol., 61:259-266. [ Links ]
11. CANTERO, GA; MP CANTU; JM CISNEROS; JJ CANTERO; M BLARASIN; ET AL. 1998. Las tierras y aguas del sur de Córdoba: propuestas para un manejo sustentable. Universidad Nacional de Río Cuarto, Río Cuarto, Córdoba, Argentina. [ Links ]
12. CAPITANELLI, RG. 1979. Clima. Pp. 456-138 in: JV Vázquez; RA Miatello & ME Roqué (eds.). Geografía Física de la Provincia de Córdoba. Editorial Boldt. Buenos Aires, Argentina. [ Links ]
13. CHARVET, S; A KOSMALA & B STATZNER. 1998. Biomonitoring through biological traits of benthic macro invertebrates: perspectives for a general tool in stream management. Arch. Hydrobiol., 142:415-432. [ Links ]
14. CORIGLIANO, MC & R MALPASSI. 1998. Food web structure in riffles and marginal pools of a mountain stream. Verh. Internat. Verein. Limnol., 26:996-1001. [ Links ]
15. COVICH, AP. 1988. Geographical and historical comparisons of neotropical streams: biotic diversity and detrital processing in highly variable habitats. J. North Am. Bethol. Soc., 7:361-386. [ Links ]
16. COVICH, AP; MC AUSTEN; F BÄRLOCHER; E CHAUVET; BJ CARDINALE; ET AL. 2004. The role of biodiversity in the functioning of freshwater and marine benthic ecosystems. BioScience, 54:767-775. [ Links ]
17. CUDNEY, MD & JB WALLACE. 1980. Life cycles, microdistribution and production dynamics of six species of net-spinning caddisflies in a large southeastern (USA) river. Holarctic Ecology, 3:169-82. [ Links ]
18. CUMMINS, KW. 1974. Structure and function of stream ecosystem. BioScience, 24:631-641. [ Links ]
19. CUMMINS, KW & MJ KLUG. 1979. Feeding ecology of stream invertebrates. Annu. Rev. Ecol. Syst., 10:147-172. [ Links ]
20. FENOGLIO, S; T BO & M CUCCO. 2004. Small-scale macroinvertebrate distribution in a riffle of a neotropical rainforest stream (Río Bartola, Nicaragua). Caribb. J. Sci., 40:253-257. [ Links ]
21. FITTKAU, EJ & SS ROBACK. 1983. The larvae of Tanypodinae (Diptera: Chironomidae) of the Holartic region - Keys and diagnoses. Pp. 33-110 in: Wiederholm, T (ed.). Chironomidae of the Holartic Region: keys and diagnoses. Part 1. Larvae. Ent. Scand. Suppl., 19:1-457. [ Links ]
22. GORDON, ND; TA MCMAHON & BL FINLAYSON. 1994. Stream hydrology, an introduction for ecologists. Wiley & Sons, New York. [ Links ]
23. GRAÇA, MAS. 2001. The role of invertebrates on leaf litter decomposition in streams - a review. Int. Rev. Hydrobiol., 86:383-393. [ Links ]
24. LAMOUROUX, N; S DOLÉDEC & S GAYRAUD. 2004. Biological traits of stream macroinvertebrate communities: effects of microhabitat, reach and basin filters. J. North Am. Benthol. Soc., 23:449-466. [ Links ]
25. LOPRETTO, EC & G TELL (eds.). 1995. Ecosistemas de aguas continentales. Metodologías para su estudio. Tomos II y III. Ediciones Sur. La Plata, Argentina. [ Links ]
26. MERRITT, RW & KW CUMMINS (eds.). 1996. An Introduction to the Aquatic Insects of North America. 3º Edition. Kendall/Hunt. Dubuque. [ Links ]
27. MERRITT, RW & KW CUMMINS. 2006. Trophic relationships of macroinvertebrates. Pp. 585-601 in: Hauer, FR & GA Lamberti (eds.). Methods in Stream Ecology. 2º Edition, Academic Press. San Diego. [ Links ]
28. NESSIMIAN, JL & A SANSEVERINO. 1998. Trophic functional characterization of chironomidae larvae (Diptera: Chironomidae) in a first order stream at the mountain region of Rio de Janeiro State, Brazil. Verh. Internat. Verein. Limnol., 26: 2115-2119. [ Links ]
29. PALMER, MA & NL POFF. 1997. The influence of environmental heterogeneity on patterns and processes in streams. J. North Am. Benthol. Soc., 16:169-173. [ Links ]
30. PALMER, C; J O'KEEFFE; A PALMER; T DUNNE & S RADLOFF. 1993. Macroinvertebrate functional feeding groups in the middle and lower reaches of the Buffalo River eastern Cape, South Africa. I. Dietary variability. Freshwat. Biol., 29:441-453. [ Links ]
31. PAUNOVIC, M; D JAKOVCEV-TODOROVIC; V SIMIC; B STOJANOVIC & A PETROVIC. 2006. Trophic relations between macroinvertebrates in the Vlasina river (Serbia). Archives of Biological Sciences, 58:105-114. [ Links ]
32. PRINCIPE, RE; GB RAFFAINI; CM GUALDONI; AM OBERTO & MC CORIGLIANO. 2007. Do hydraulic units define macroinvertebrate assemblages in mountain streams of central Argentina?. Limnologica, 37: 323-336. [ Links ]
33. PRINCIPE, RE; MF BOCCOLINI & MC CORIGLIANO. 2008. Structure and spatial-temporal dynamics of Chironomidae fauna (Diptera) in upland and lowland fluvial habitats of the Chocancharava River basin (Argentina). Int. Rev. Hydrobiol., 93: 342-357. [ Links ]
34. ROSSO DE FERRADÁS, B & HR FERNÁNDEZ. 2009. Acari, Parasitengona, Hydrachnidia. Pp. 497-549 in: Domínguez, E & HR Fernández (eds.). Macroinvertebrados bentónicos sudamericanos. Sistemática y Biología. Fundación Miguel Lillo, Tucumán, Argentina. [ Links ]
35. SCHMERA, D & T EROS. 2004. Effect of riverbed morphology, stream order and season on the structural and functional attributes of caddisfly assemblages. Ann. Limnol. - Int. J. Lim., 40:193- 200. [ Links ]
36. STATZNER, B; AG HILDREW & VH RESH. 2001. Species traits and environmental constraints: entomological research and the history of ecological theory. Annu. Rev. Entomol., 46:291- 316. [ Links ]
37. SUBRAMANIAN, KA & KG SIVARAMAKRISHNAN. 2005. Habitat and microhabitat distribution of stream insect communities of the Western Ghats. Current Science, 89:976-986. [ Links ]
38. TER BRAAK, CJF. 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology, 67: 1167-1179. [ Links ]
39. TER BRAAK, CJF & P SMILAUER. 1998. CANOCO Reference Manual and User's Guide to Canoco for Windows: Software for Canonical Community Ordination (version 4). Microcomputer Power. Ithaca. [ Links ]
40. THOMSON, JR; MP TAYLOR; KA FRYIRS & GJ BRIERLEY. 2001. A geomorphological framework for river characterization and habitat assessment. Aquat. Conserv. Mar. Freshw. Ecosyst., 11:373-389. [ Links ]
41. TOMANOVA, S; E GOITIA & J HELEŠIC. 2006. Trophic levels and functional feeding groups of macroinvertebrates in neotropical streams. Hydrobiologia, 556:251-264. [ Links ]
42. TOWNSEND, CR & AG HILDREW. 1994. Species traits in relation to a habitat templet for river system. Freshwat. Biol., 31:265-276. [ Links ]
43. TOWNSEND, CR; S DOLEDEC & MR SCARSBROOK. 1997. Species traits in relation to temporal and spatial heterogeneity in streams: a test of habitat templet theory. Freshwat. Biol., 37:367-387. [ Links ]
44. TRIPOLE, ES & MC CORIGLIANO. 2005. Acid stress evaluation using multimetric indices in the Carolina stream (San Luis-Argentina). Acta Limnol. Bras., 17:101-114. [ Links ]
45. UNDERWOOD, AJ. 1994. Spatial and temporal problems with monitoring. Pp. 182-204 in: Calow, P & G Petts (eds.). The Handbook of River. Blackwell. Oxford. [ Links ]
46. VANNOTE, RL; GW MINSHALL; KW CUMMINS; JR SEDELL & CE CUSHING. 1980. The river continuum concept. Can. J. Fish. Aquat. Sci., 37:130-137. [ Links ]
47. VELÁSQUEZ, SM & ML MISERENDINO. 2003a. Análisis de la materia orgánica alóctona y organización funcional de macroinvertebrados en relación con el tipo de hábitat en ríos de montaña de Patagonia. Ecología Austral, 13:67-82. [ Links ]
48. VELÁSQUEZ, SM & ML MISERENDINO. 2003b. Habitat type and macroinvertebrate assemblages in low order Patagonian streams. Arch. Hydrobiol., 158: 461-483. [ Links ]
49. WALLACE, JB & JR WEBSTER. 1996. The role of macroinvertebrates in stream ecosystem function. Annu. Rev. Entomol., 41:115-139. [ Links ]
50. WIGGINS, GB. 1996. Larvae of the North American caddisfly genera. 2º Edition. University of Toronto Press. Toronto. [ Links ]
51. WUNDERLIN, DA; MP DÍAZ; MV AMÉ; SF PESCE, AC HUED; ET AL. 2001. Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. A case study: Suquía River Basin (Córdoba, Argentina). Wat. Res., 35: 2881-2894. [ Links ]














