Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Ecología austral
versión On-line ISSN 1667-782X
Ecol. austral vol.22 no.1 Córdoba abr. 2012
ARTÍCULO ORIGINAL
The influence of Ditylenchus (Nematoda) galls and shade on the fluctuating asymmetry of Miconia fallax (Melastomataceae)
Estevão Alves-Silva*
Programa de Pós-Graduação em Ecologia e Conservação dos Recursos Naturais; Instituto de Biologia, Universidade Federal de Uberlândia
* Programa de Pós-Graduação em Ecologia e Conservação dos Recursos Naturais; Instituto de Biologia, Universidade Federal de Uberlândia, Rua Ceará, s/n°. Bloco 2D - Campus Umuarama, Cep: 38400902. Email: estevaokienzan@yahoo.com.br
Recibido: 4 de junio de 2011;
Fin de arbitraje: 23 de agosto de 2011;
Revisión recibida: 25 de septiembre de 2011;
Aceptado: 6 de noviembre de 2011
ABSTRACT. Fluctuating asymmetry (FA) can be defined as small, random deviations from bilateral symmetry in structures that are bilaterally symmetrical and can estimate a population's inability to buffer its growth against perturbations of environmental origins. Another important issue about FA is whether biotic factors may also induce stress in organisms. Therefore, in this study I aimed to investigate the influence of both the abiotic (light exposure) and biotic (Ditylenchus sp. galls) factors accounting for increased FA in Miconia fallax leaves. Galls are known as parasites and a major cause of leaf stress. Additionally, since M. fallax is a pioneer plant species, individuals in the shade were supposed to present high levels of FA relative to plants on the edges exposed to direct sunlight. Results showed no concomitant interaction between gall abundance, light exposure and FA. Separate analysis showed that plants in the shade presented the highest level of FA, almost 25% higher than plants on the edges, indicating that plants growing in places with little sunlight were in stressful condition. The presence of galls did not cause alterations in FA relative to leaves without galls. The lack of relationship between galls and leaf FA indicates that M. fallax can tolerate and buffer the presence of these leaf parasites, revealing the high homeostasis ability of this plant species against a biotic stress.
Keywords: Edge effect; Light exposure; Pioneer plant; Cerrado.
RESUMEN. La influencia de las agallas de Ditylenchus (Nematoda) y la sombra en la asimetría fluctuante de Miconia fallax (Melastomataceae): La asimetría fluctuante (AF) se refiere a las pequeñas desviaciones al azar en la simetría bilateral de las estructuras y órganos que son bilateralmente simétricos, y permite estimar la incapacidad de una población cualquiera para corregir su crecimiento de acuerdo con disturbios y tensiones ambientales. Una consideración importante en los estudios de la AF es si los factores bióticos también pueden causar estrés en la planta. Por lo tanto, el objetivo de este estudio fue investigar la influencia conjunta de factores abióticos (exposición solar) y bióticos (presencia de agallas de Ditylenchus sp.) en la asimetría de las hojas de Miconia fallax. Las agallas de las hojas son parásitos, y como M. fallax es una planta pionera, los individuos que se encuentran en la sombra pueden presentar niveles altos de AF en comparación con los de los bordes, expuestos a la luz solar directa. El análisis no mostró ninguna interacción entre la abundancia de agallas, la exposición al sol y AF. El análisis separado reveló que los individuos a la sombra tenían niveles más altos de la AF, aproximadamente 25% más alto que los individuos en los bordes, lo cual muestra que los individuos de M. fallax con poca luz se encuentran en condiciones de estrés. La presencia de agallas no causó ningún cambio o aumento de la AF en comparación con las hojas sin agallas. La falta de relación entre la presencia de agallas y AF indica que los individuos de M. fallax tienen una gran capacidad para mantener la homeostasis, ya que la planta ha tolerado estos parásitos sin cambios significativos en la AF de las hojas.
Palabras clave: Efecto de borde; Exposición a la luz; Planta pionera; Cerrado.
INTRODUCTION
Fluctuating asymmetry (FA) can be defined as small, random deviations from bilateral symmetry in structures that, typically, are bilaterally symmetrical (Cornelissen & Stiling 2005). Asymmetries in bilateral traits are widely recognized to estimate a population's inability to buffer its growth against perturbations of environmental origins (Møller & Swadle 1997).
This occurs because the left and right parts of an individual share the same genome. However, when subjected to external stressing factors, the body parts will suffer small random perturbations of cellular processes, which eventually will accumulate in the developing organs on the left and right sides separately, leading to asymmetries during development (Wilsey et al. 1998; Woods et al. 1998). In this sense FA analysis performs an important function because it can be used to monitor the quality of organisms living under different environments (Kozlov et al. 1996). Moreover FA can be used to anticipate decrements in species fitness (Møller 1997).
The environmental causes which increase FA may include changes in temperature, climate, nutrients and light exposure (Markow 1995; Hódar 2002). Light conditions during plant growth may considerably alter the structure of leaves from individuals growing in the sun relative to those growing in the shade (Møller 1995). For instance, for pioneer plant species sunlight is a very important resource promoting its growth, consequently plants located in the shade or understory would be subjected to a stressful condition (Pearson et al. 2003). This tension may in turn be reflected as elevated levels of leaf FA. This occurs because structures such as leaves, with large functional importance are normally under strong selective pressures (Cowart & Graham 1999).
Plants are good models to monitor morphological traits that may vary along plant distribution range. In face of different environmental stresses, plants show differences in development and in leaf FA. Cornelissen & Stiling (2010) observed that the stress rates suffered by two oak species in Florida depended on whether individuals were growing in center sites (inland) or on the coast. On the coast FA was 20-30% higher, possibly due to nutritional and/or water stresses in this location.
One important consideration about studies assessing leaf asymmetry is whether biological interactions may also induce stress on the plant, resulting in elevated FA levels. Zvereva et al. (1997a) showed that leaf FA in Salix borealis (Fries) Nasarow (Salicaceae) was related to herbivory caused by beetles and Møller (1996) showed that several herbivores were related to leaf FA as well in many other plant species.
Up to 130000 organisms induce plant galls (Espírito-Santo & Fernandes 2007). However except by few studies (Olofsson & Strengbomand 2000; Cornelissen & Stiling 2005), the influence of galls on leaf asymmetry has been neglected as stressors, despite the fact that galls are rigorously parasitic (Weis & Kapelinski 1984), causing severe decrements in plant growth and reproduction (Price et al. 1987). In this sense Zvereva et al. (1997b) in agreement with Møller (1995) suggested that FA is a useful and objective measure of stress levels experienced by plants in studies of insect-plant interactions as FA could be detected before any significant reduction in plant fitness.
To test the hypothesis that galls increase leaf FA, I compared the asymmetries between leaves with and without Ditylenchus sp. (Nematoda) galls in Miconia fallax DC. (Melastomataceae). I also predicted that M. fallax individuals living along the forest edge and exposed to direct sunlight, would present less FA. M. fallax is pioneer plant species and therefore sunlight is an essential environmental resource for its growth. Hence this study permits one to examine whether the concomitant intensity of biotic and abiotic factors caused stress in this plant species. According to Puerta-Piñero et al. (2008) the majority of studies consider only one single stressful condition at a time as the cause of FA. However organisms in natural conditions are under simultaneous stresses, both biotic and abiotic. Thus in this study I aimed to compare the relative strengths of different stressing factors on M. fallax.
METHODS
Fieldwork was carried out in May and April of 2010, in an area of Brazilian savanna vegetation at the Panga Ecological Station (PES: 19º10' S, 48º24' E), located about 30 km from Uberlândia city, in the state of Minas Gerais, Brazil. Cerrado sensu strictu is the main vegetation type in the reserve. This vegetation is dominated by trees and shrubs (0.4-5 m tall) and a fair amount of herbaceous vegetation. The climate in the region presents two well characterized seasons, a dry winter (April to September) and a rainy summer (October to March). For more details about the study area, see Cardoso et al. (2009).
The genus Miconia Ruiz & Pav. (Melastomataceae) is a group of pioneer plants which are very common at the secondary successional vegetation of PES. The genus presents rapid resprouting capacity, higher seed production rate in forest gaps, absence of small seedlings from the understory and strong responses of seedlings to variation in light, which is also a limiting factor for seed germination (Dalling et al. 2001; Pearson et al. 2003). M. fallax is a woody shrub (<2 m) which grows well in cerrado vegetation (Maruyama et al. 2007). At PES the range of M. fallax occurrence is limited to 20 m from the edge limit to the forest core. Some individuals grow well at the borders, receiving sunlight most part of the day, while others are located in understory and covered by the canopy of large trees. M. fallax young leaves possess sessile to short-stalked, non-glandular stellate trichomes which occur on both surfaces, forming a dense cover. On the other hand, the adaxial surface of mature leaves is glabrous and highly reflexive (Milanez & Machado 2011).
Galls are formed by the nematode Ditylenchus sp. (hereafter Ditylenchus) and occur throughout the leaf blade of M. fallax. Galls are round, about 0.14 mm in diameter and grow on the abaxial surface of leaves, where several globes develop and form small protuberances (Figure 1A). This paper will focus on the influence of Ditylenchus galls in M. fallax FA rather than their biology. Substantial information about its life histories can be found in Seixas et al. (2004a) and Seixas et al. (2004b).

Figure 1. Abaxial side of Miconia fallax leaves. (A) Globose galls occur along the leaf blade. (B) Ungalled leaf with the indication of the way that measurements were taken in order to assess fluctuating asymmetry. RW: right width; LW: left width.
Figura 1. Lado abaxial de las hojas de Miconia fallax. (A) agallas globosas se producen al largo de la hoja. (B) hoja sin agallas con la indicación de cómo las medidas fueron hechas con el fin de examinar la asimetría fluctuante. RW: ancho de la derecha; LW: ancho de la izquierda.
A total of 35 M. fallax individuals were tagged along an area of approximately 1.5 ha within PES. I chose only one block, which corresponded to the area where M. fallax was most abundant (about 50 individuals per hectare). Individuals were located along the edge and within a range of 20 m from the forest edge. After 30 m distant from the edge no individual was found.
From each tree I collected 20 leaves, totaling 700 leaves. To assess leaf FA, widths of all leaves were measured on both the right (RW) and left sides (LW), from the leaf edge to the midrib, at the middle point of the leaf, which corresponds to its widest part (Figure 1B). In order to measure the leaves with precision I placed them under a section of transparent glass and photographed them with a digital camera. These photos were then transferred to a computer and measurements were made with the Image Tool software (sensu Cornelissen & Stilling 2005).
To test the accuracy of the measurements, a subsample of 300 leaves was remeasured and compared with the original RW and LW measurements. The second measurements were made in order to test the reliability of the first. FA represents small deviations of perfect symmetry so previous analyses are required in order to examine the degree of measurement error (Woods et al. 1998). The repeatability of measurements indicates whether leaf sides and their asymmetries were measured with sufficient precision to allow for the use of subsequent analyses.
A two factor analysis of variance (ANOVA) was used to determine whether the between-sides variation was significantly larger than the measurement error. The variables used were the individuals of M. fallax and the sides of leaves (RW and LW). FA dependence on leaf size was tested through linear regression with the absolute difference of the right minus left measurements and leaf length (Woods et al. 1998).
According to Palmer & Strobeck (1986) it is necessary to discriminate FA from other kinds of asymmetry. Thus, I checked whether the kind of bilateral asymmetry found among M. fallax leaves could be considered true FA. Directional asymmetry (significant differences in size between leaf sides) was checked by testing that the average RW-LW value did not differ from zero (invariably P>0.05, one sample Student's t-test). To check for anti-symmetry (significant differences on the RW-LW distribution from the normal curve), the right minus left values were tested with the Kolmogorov-Smirnov normality test (sensu Møller 1995). Finally, once the tests for directional asymmetry and anti-symmetry were not statistically significant, I could evaluate FA, calculated as the mean difference between the right and the left side [i.e., FA = [(∑ |(RW-LW)|/n] (Palmer & Strobeck 1986)]. Absolute FA is measured in milimeters in this syudy.
All leaves collected had the number of Ditylenchus galls counted and the result is presented as mean±1 standard deviation. I also examined the side of the leaf in which galls were more abundant and the comparison was made with the Wilcoxon test. The number of galled leaves per tree is displayed as mean±1 standard deviation. The Wilcoxon test was used to compare the number of galled and ungalled leaves per tree.
In order to examine the effects of the sun and shade on leaf FA, M. fallax, individuals were separated in two groups according to plant location. The group "sun" was composed of trees on the edge and subjected to direct (vertical and lateral) sunlight most part of the day. The group "shade" was composed of trees in understory, about five to 20 m away from the edge and receiving low lateral and no vertical light during the day (adapted from Puerta-Piñero et al. 2008).
To examine whether gall abundance and tree location were concomitantly responsible for leaf FA, I conducted a general linear model (GLM) test. FA values were considered as the dependent variable. Gall abundance per leaf and plant location were assumed as independent variables. In addition I performed another GLM to check whether leaf width was related to gall abundance and plant location. The continuous variables (FA, gall abundance and leaf width) were log+1 transformed to fit normal distributions.
To check for a possible alteration in leaf FA according to gall abundance I performed a Pearson correlation test. This test was also used to check for alterations in leaf width according to the number of Ditylenchus galls per leaf. In these correlations only leaves with Dytilenchus were used.
To analyze M. fallax between-tree variation of gall abundance I ranked the mean number of galls per tree as follows: rank was equal to "1" if the mean number of galls in a tree ranged from 0 to 0.99; 2=1-1.99; 3=2-2.99; 4=3-3.99; 5=4-4.99; 6=5-5.99; 6=7-7.99; 7=8-8.99; 8=9-9.99; 10=more than 10 galls per leaf within a tree. Between-tree variation was checked with the Kruskal-Wallis test. This same test was used to analyze M. fallax between-tree variation of FA levels. Data ranking was chosen as it provides a better visualization of data in this analysis.
This study is not experimental, thus the plant location (sun/shade) and the number of galls were not manipulated, but rather examined in field conditions. All statistical procedures were made in accordance with Zar (1984) using the softwares Systat 10.2. and GraphPad Prism 5.0.
RESULTS
Among the 700 leaves collected from M. fallax, 17 of them presented large variations in RW-LW values due to herbivory or unknown biological/physical causes and were discarded in subsequent analyses or otherwise, could bias the results. The measurement error of leaves was insignificant, as indicated by the side versus individual effect on two-way Anova (F=7.17; df=1.299; P<0.0001). One sample Student's t test revealed no differences in the mean of RW-LW measurements, consequently directional asymmetry was discarded (t=0.017; df=682; P>0.05). Antisymmetry was rejected according to the non significant Kolmogorov-Smirnov normality test (P>0.05). Thus in this study FA was confirmed in M. fallax. Additionally, no relation was observed between leaf length and the absolute FA [RW-LW] (r²=-0.0006; df=1.298; P>0.05). Therefore the measurements were considered reliable. It means that FA is not dependent on leaf size and thus can be assessed unambiguously in subsequent tests.
Ditylenchus galls were present in 63% of leaves (n=453). The number of galled leaves per tree varied from 7-18 and the Wilcoxon test revealed statistical significant differences between the number of galled and ungalled leaves (T=19; P<0.0001) (Figure 2). The number of galls per leaf varied from 0-29. No difference was found between the abundance of galls and leaf sides (galls right side 1.91±3.17; left side 1.96±3.28) (T=38025; P>0.05).

Figure 2. Mean and standard deviation of number of M. fallax galled and ungalled leaves.
Figura 2. Media y desviación estándar del número de hojas de M. fallax con y sin agallas.
Among the 35 M. fallax individuals sampled, 20 of them were located in the shade and 15 were found in the sun. The GLM analysis did not show interaction between leaf FA, the number of galls and plant location (F=0.3262; df=1.679; P>0.05), so the independent variables were examined separately. The test revealed an important finding that Dytilenchus galls were not responsible for elevated levels of FA in M. fallax leaves. This result is interesting since the FA was 5% higher in leaves with galls, however it was not statistically significant (F=0.8952; df=1.679; P>0.05).
On the other hand plant location accounted for a highly statistical significant difference and was the main cause of increased FA in M. fallax. Individuals in the shade presented the highest level of FA, almost 25% higher than plants in the sun, indicating that plants growing in places with low sunlight were in stressful condition (F=16.3584; df=1.679; P<0.0001) (Figure 3).
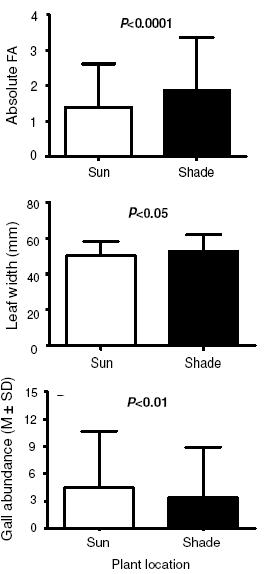
Figure 3. Leaf FA, width and gall abundance in M. fallax individuals located in the sun and in the shade.
Figura 3. AF de las hojas, ancho y agallas de individuos de M. fallax situados en sitios con sol y sombra.
The other GLM test did not show any interaction between gall abundance, leaf width and plant location (F=3.57; df=1.679; P>0.05). Leaves with Ditylenchus galls were in average 4% wider than leaves without galls (52.22±8.89 and 50.39±8.68 cm, respectively) and this difference was significant (F=3.769; df=1.679; P<0.05) (Figure 3). Plant location was also responsible for differences in leaf width. M. fallax individuals in the shade had their leaves wider than those on the edge exposed to sunlight, and this difference was significant (F=14.53; df=1.679; P<0.001). In respect to Ditylenchus galls, the two GLM tests showed that galls do increase leaf width but do not increase leaf FA at the extent of causing differences in leaf right or left widths.
Pearson correlation test revealed that neither leaf FA or leaf width were influenced by the number of Ditylenchus galls per leaf (r=0.02; P>0.05 and r=0.05; P>0.05 respectively). Leaves with elevated abundance of Ditylenchus were not more asymmetrical or wider than leaves with few nematodes.
The variation between trees was markedly significant for gall abundance (H=140.56; P<0.0001). The ranks established for the mean number of galls per tree showed differences depending on the FA values for each tree (H=17.92; P<0.05), however it was revealed that trees with higher gall abundance did not present elevated levels of FA (Figure 4).

Figure 4. Mean and standard deviation of FA levels between trees according to the number of Ditylenchus galls. There was no relationship between these two variables, that is, trees with high gall abundance did not present high FA levels. Ranks of the mean number of galls: 1=0-0.99; 2=1-1.99; 3=2-2.99; 4=3-3.99; 5=4-4.99; 6=5-5.99; 6=7-7.99; 7=8-8.99; 8=9-9.99; 10=more than 10 galls per leaf within a tree. Thirty five M. fallax individuals were analyzed.
Figura 4. Media y desviación estándar de las variaciones en los niveles de AF entre los individuos de M. fallax de acuerdo con las agallas de Ditylenchus. No se encontró relación entre estas dos variables, es decir, las plantas con gran abundancia de agallas no presentaron altos niveles de AF. Rangos de la media de agallas: a 1=0-0.99; 2=1-1.99; 3=2-2.99; 4=3-3.99; 5=4-4.99; 6=5-5.99; 6=7-7.99; 7=8-8.99; 8=9-9.99; 10= más de 10 agallas por hoja en una planta. Se analizaron 35 individuos de M. fallax.
In sum, the results showed so far that leaves with galls were not more asymmetric than leaves without galls and that higher gall abundance did not inflict higher levels of FA. Thus it seems unlikely that galls were a stressing factor for M. fallax and the levels of FA observed cannot be attributed to gall presence. Therefore the other variable measured, the plant location, was supposed to account for most of the FA variation evidenced in M. fallax.
M. fallax individuals located in the sun presented more Ditylenchus galls per leaf than plants in the shade (U=49620; P<0.01) (Figure 3). This result only reinforces the fact that the effects of galls on M. fallax FA were too subtle and not responsible for elevated stress rates. If leaf FA was caused by gall presence, plants on the edge would present the highest FA levels. That is the reason why the GLM tests did not show interaction between the number of galls and the plant location, since FA was caused in the most part by plant location and not by galls.
DISCUSSION
Several species of parasitic nematodes are recognized to cause a negative impact on their host plants (Hussey 1989). In this sense it would be expected to find high levels of FA in M. fallax leaves supporting Ditylenchus galls. Although galled leaves were larger, no relation was observed between FA and the presence of galls, given that leaves bearing Ditylenchus galls presented similar stress rates to ungalled ones.
According to Møller & Pomiankowski (1993) morphological traits subjected to stress for many generations are expected to show low variability in FA. When these traits are important for survival, the population may be facing stabilizing selection in which departures from the optimum phenotype are minimized. This happens because large modifications in traits which are important for growth and development may influence negatively the population survival and fitness (Lomônaco & Germanos 2001).
Another hypothesis that could explain the lack of gall effect in M. fallax FA is the plastic potential of the species. Phenotypic plasticity (PP) is the ability of an organism to alter its physiology or morphology as a response to an unstable and heterogeneous environment, in order to maintain its vigor (Debat & David 2001). As shown by Lomônaco & Germanos (2001) biotic factors are also involved in the mechanisms underlying PP. In this sense, morphological alterations in M. fallax provoked by galls may have been buffered to maintain the species vigor.
In one study involving M. prasina (Sw.) DC, the presence of Ditylenchus galls was found not to affect the plant vigor, such as plant height, number of shoots and leaves (Santos et al. 2009). The authors agreed that M. prasina could tolerate the nematode impact by compensation, where damaged and undamaged plants had the same fitness.
Even though PP and compensatory effects may be important for a species, when the environment condition is too harsh the population may be incapable of maintaining its homeostasis or buffer its growth against these conditions. This stress is then reflected as high FA. FA levels among a population living in different environments or in a continuum may be indicative of which condition is more stressful.
The main cause of FA in M. fallax was plant location in that individuals in the shade presented the highest levels of stress. Since M. fallax is a pioneer plant species, high FA levels were expected in individuals in the shade. Shade alters the growth, vigor, physiology, metabolism and productivity of several plant species and is a known form of stress (Lee et al. 1990; Gordon et al. 1994). In regard to pioneer plants, shade may also alter their occurrence, establishment and reproduction (Dalling & Hubbell 2002; Pearson et al. 2003) and some studies have revealed that the lack of sunlight also influences shade tolerant plants. For instance Puerta-Piñero et al. (2008) showed that light significantly affected leaf FA of the mediterranean oak Quercus pyrenaica Willd. (Fagaceae) in that FA was higher in the shade than in the sun. Furthermore, shade-treated plants showed the worst seedling performance in all cases. The larger leaves of M. fallax individuals in the shade may be a compensation for the lack of light, where leaf area increases in order to capture more light for photosynthesis (Farnsworth et al. 1996; Van Hinsberg & Van Tiendere 1997; Lentz & Cipollini Jr 1998).
As shown above M. fallax individuals located in the shade presented the highest levels of stress. However Ditylenchus galls were more abundant on plants at the borders, exposed to direct sunlight. By means of comparison, Fernandes & Price (1992) studied the influence of xeric and mesic habitats in galling insects and noted that galling populations were significantly larger in xeric habitats compared to mesic habitats for six out of eight species studied (Tscharntke 1989). According to the authors this pattern is widespread in nature, since gall formers may experience high levels of parasitism and fungal diseases in mesic habitats. This is presumably the reason why Ditylenchus galls were more abundant on the edge, given that plant-parasitic nematodes are commonly favored by higher temperatures that allow for a rapid increase in their populations and are less susceptible to fungus diseases and moisture as well (Crow & Dunn 2005).
In this work I considered only two factors accounting for the stress in M. fallax (i.e., the number of galls and plant location). The choice of the variables which accounts for the most stress in a given organism is not arbitrary, but a result of field observations and the knowledge of the life history of that organism.
For M. fallax, shade was considered as a stressing factor because this is a pioneer plant species. Additionally galls are parasitic herbivores and thus were supposed to inflict a high degree of stress on leaves, which would be reflected as high FA levels. Other possible biotic and abiotic effects were disregarded as I had no evidence that they could play a role in M. fallax asymmetry. In respect to biotic effects no other herbivores than Ditylenchus were sampled.
It is possible that not only the sun, but also other abiotic factors on the edge might be affecting M. fallax, such as wind, soil content differences and low moisture. However these variables are often highly correlated and intermingled on the edges (see Matlack 1994; Gehlhausen et al. 2000; Harper et al. 2005), so their relative roles could not be assessed without ambiguity. Therefore individual analyses about these effects on leaf FA would be redundant.
The influence of biotic factors on leaf FA has received little attention, and the few studies have so far showed contrasting results. Cornelissen & Stiling (2005) concluded that insect miners did not inflict FA on two oak species. On the other hand Møller (1995) demonstrated that leaves with mines of the curculionid beetle Rhynchaenus rufus (Schrank) (Curculionidae) were significantly more asymmetrical than the nearest neighbouring leaf without a mine. In both cases it seems that the relationship between foliar asymmetry and herbivory is causal, since increased levels of asymmetry caused by environmental/experimental treatments gave rise to an increase in the abundance of leaf miners.. Usually stressed plants present elevated levels of free nitrogen and low levels of secondary defense compounds, thus benefiting insects through bottom-up forces (White 1984).
Alternatively another question that remains is whether herbivores themselves directly cause high stress levels on plants. Cuevas-Reyes et al. (2011) found a high positive relationship between leaf herbivory and FA in Heliocarpus pallidus Rose (Tiliaceae) but they were unable to state whether previously stressed leaves were more susceptible to herbivores or if FA was a result of leaf area loss. In contrast Cornelissen & Stiling (2011) found a causal relationship between FA and gall formers. Nevertheless, the relation was weak and marginally significant.
In this study it is unclear whether Ditylenchus infects previously asymmetric leaves. However, I have a reason to believe that gall induction is random. If infection occurred in previously asymmetric leaves, then gall development would maximize leaf asymmetries and there would be a difference between the FA for galled and ungalled leaves.
To sum up this study showed that shade and galls did not cause simultaneous stress in M. fallax. Plant location was the main cause of stress since individuals in the shade had the highest FA values. This study showed that Ditylenchus galls were not at all a stressing factor on the plant, as indicated by similar FA in leaves with and without galls. Data regarding the association between FA and herbivory is still scarce and long term studies are necessary to uncover the mechanism underlying this relationship.
ACKNOWLEDGMENTS
Dr. K. Del-Claro, Dr. C. Lomônaco and Dr. Tatiana Cornelissen for academical support; Dr. JC Santos for gall identification; Msc. V. Gonçalves and C. Belchior for statistical assistance; KCFL Souto, PKM Mendonça and A Bächtold for comments; P Palhares for Spanish review; JD Bagnall for English review; Capes (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and Fapemig (Fundação de Amparo à Pesquisa do Estado de Minas Gerais) for funding.
REFERENCES
1. CARDOSO, E; MIC MORENO; EM BRUNA & HL VASCONCELOS. 2009 Mudanças fitofisionômicas no cerrado: 18 anos de sucessão ecológica na Estação Ecológica do Panga, Uberlândia - MG. Caminhos da Geografia 10:254-268. [ Links ]
2. CORNELISSEN, T & P STILING. 2005. Perfect is best: low leaf fluctuating asymmetry reduces herbivory by leaf miners. Oecologia 142:46-56. [ Links ]
3. CORNELISSEN, T & P STILING. 2010. Small variations over large scales: fluctuating asymmetry over the range of two oak species. Int. J. Plant Sci. 171:303-309. [ Links ]
4. CORNELISSEN, T & P STILING. 2011. Similar responses of insect herbivores to leaf fluctuating asymmetry. Arthropod-Plant Inte. 5:59-69. [ Links ]
5. COWART, NM & JH GRAHAM. 1999. Within and among-individual variation in fluctuating asymmetry of leaves in the fig (Ficus carica L.). Int. J. Plant Sci. 160:116-121. [ Links ]
6. CROW, WT & RA DUNN. 2005. Introduction to plant nematology. Fact Sheet ENY-016 (NG006). Florida Nematode Management Guide from the Department of Entomology and Nematology, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. [ Links ]
7. CUEVAS-REYES, P; K OYAMA; A GONZÁLEZ-RODRÍGUEZ; GW FERNANDES & L MENDOZA-CUENCA. 2011. Contrasting herbivory patterns and leaf fluctuating asymmetry in Heliocarpus pallidus between different habitat types within a Mexican tropical dry forest. J. Trop. Ecol. 27:383-391. [ Links ]
8. DALLING, JW; K WINTER; JD NASON; SP HUBBELL; DA MURAWSKI; ET AL. 2001. The unusual life history of Alseis blackiana: a shade-persistent pioneer tree? Ecology 82:933-945. [ Links ]
9. DALLING, JW & SP HUBBELL. 2002. Seed size, growth rate and gap microsite conditions as determinants of recruitment success for pioneer species. J. Ecol. 90:557-568. [ Links ]
10. DEBAT, V & P DAVID. 2001. Mapping phenotypes: canalization, plasticity and developmental stability. Trends Ecol. Evol. 16:555-561. [ Links ]
11. ESPÍRITO-SANTO, MM & GW FERNANDES. 2007. How many species of gall-inducing insects are there on Earth, and where are they? Ann. Entomol. Soc. Am. 100:95-99. [ Links ]
12. FARNSWORTH, EJ & ME AARON. 1996. Sun-shade adaptability of the red mangrove, Rhizophora mangle (Rhizophoraceae): changes through ontogeny at several levels of biological organization. Am. J. Bot. 83:1131-1143. [ Links ]
13. FERNANDES, GW & PW PRICE. 1992. The adaptative significance of insect gall distribution: survivorship of species in xeric and mesic habitats. Oecologia 90:14-20. [ Links ]
14. GEHLHAUSEN, SM; MW SCHWARTZ & CK AUGSPURGER. 2000. Vegetation and microclimatic edge effects in two mixed-mesophytic forest fragments. Plant Ecol. 147:21-35. [ Links ]
15. GORDON, DM; KA GREY; SC CHASE & CJ SIMPSON. 1994. Changes to the structure and productivity of a Posidonia sinuosa meadow during and after imposed shading. Aquat. Bot. 47:265-275. [ Links ]
16. HARPER, KA; SE MACDONALD; PJ BURTON; J CHEN; KD BROSOFSKE; ET AL. 2005. Edge influence on forest structure and composition in fragmented landscapes. Conserv. Biol. 19:768-782. [ Links ]
17. HÓDAR, JA. 2002. Leaf fluctuating asymmetry of Holm oak in response to drought under contrasting climatic conditions. J. Arid. Environ. 52:233-243. [ Links ]
18. HUSSEY, RS. 1989. Disease-inducing secretions of plant-parasitic nematodes. Annu. Rev. Phytopathol. 27:123-141. [ Links ]
19. KOZLOV, MV; BJ WILSEY; J KORICHEVA & E HAUKIOJA. 1996. Fluctuating asymmetry of Birch leaves increases under pollution impact. J. Appl. Ecol. 33:1489-1495. [ Links ]
20. LEE, DW; RA BONE; SL TARSIS & D STORCH. 1990. Correlates of leaf optical properties in tropical forest sun and extreme-shade plants. Am. J. Bot. 77:370-380. [ Links ]
21. LENTZ, KA & DF CIPOLLINI JR. 1998. Effect of light and simulated herbivory on growth of endangered northeastern bulrush, Scirpus ancistrochaetus Schuyler. Plant Ecol. 139:125-131. [ Links ]
22. LOMÔNACO, C & E GERMANOS. 2001. Variações fenotípicas em Musca domestica L. (Diptera: Muscidae) em resposta à competição larval por alimento. Neotrop. Entomol. 30:223-231. [ Links ]
23. MARKOW, TA. 1995. Evolutionary ecology and developmental instability. Annu. Rev. Entomol. 40:105-120. [ Links ]
24. MARUYAMA, PK; E ALVES-SILVA & C MELO. 2007. Oferta qualitativa e quantitativa de frutos em espécies ornitocóricas do gênero Miconia (Melastomataceae). Rev. Bras. Bioc. 5:672-674. [ Links ]
25. MATLACK, GR. 1994. Vegetation dynamics of the forest edge - trends in space and successional time. J. Ecol. 82:113-123. [ Links ]
26. MILANEZ, CRD & SR MACHADO. 2011. SEM studies on the leaf indumentum of six Melastomataceae species from Brazilian Cerrado. Rodriguésia 62:203-212. [ Links ]
27. MØLLER, AP & A POMIANKOWSKI. 1993. Fluctuating asymmetry and sexual selection. Genetica 89:267-279. [ Links ]
28. MØLLER, AP. 1995. Leaf-mining insects and fluctuating asymmetry in elm Ulmus glabra leaves. J. Anim. Ecol. 64:697-707. [ Links ]
29. MØLLER, AP. 1996. Parasitism and developmental instability of hosts: a review. Oikos 77:189-196. [ Links ]
30. MØLLER, AP. 1997. Developmental stability and fitness: a review. Am. Nat. 149:916-932. [ Links ]
31. MØLLER, AP & JP SWADDLE. 1997. Asymmetry, Developmental Stability and Evolution. Oxford University Press. [ Links ]
32. OLOFSSON, J & J STRENGBOM. 2000. Response of galling invertebrates on Salix lanata to reindeer herbivory. Oikos 91:493-498. [ Links ]
33. PALMER, AR & C STROBECK. 1986. Fluctuating asymmetry: measurement, analysis, patterns. Annu. Rev. Ecol. Syst. 17:391-421. [ Links ]
34. PEARSON, TRH; D BURSLEM; RE GOERIZ & JW DALLING. 2003. Interactions of gap size and herbivory on establishment, growth and survival of three species of neotropical pioneer trees. J. Ecol. 91:785-796. [ Links ]
35. PRICE, WP; WF FERNANDES & G WARING. 1987. Adaptative nature of insect galls. Environ. Entomol. 16:15-24. [ Links ]
36. PUERTA-PIÑERO, C; JM GÓMEZ & JA HÓDAR. 2008. Shade and herbivory induce fluctuating asymmetry in a mediterranean oak. Int. J. Plant Sci. 169:631-635. [ Links ]
37. SANTOS, JC; CVV MAGALHÃES; CIR SANTOS; JE CARES & JS ALMEIDA-CORTEZ. 2009. Impact of nematode-induced galls on Miconia prasina (Melastomataceae) traits in Atlantic forest of northeastern Brazil. Pp. 1-4. in: Anais do III Congresso Latino Americano de Ecologia. São Lourenço, MG, Brazil. Sociedade de Ecologia do Brasil. [ Links ]
38. SEIXAS, CDS; RW BARRETO; LG FREITAS; LA MAYA & FT MONTEIRO. 2004a. Ditylenchus drepanocercus (Nematoda), a potential biological control agent for Miconia calvescens (Melastomataceae): host-specificity and epidemiology. Biol. Control. 31:29-37. [ Links ]
39. SEIXAS, CDS; RW BARRETO; LG FREITAS; FT MONTEIRO & RDL OLIVEIRA. 2004b. Ditylenchus drepanocercus rediscovered in the neotropics causing angular leaf spots on Miconia calvescens. J. Nematol. 36:481-486. [ Links ]
40. TSCHARNTKE, T. 1989. Attack by a stem-boring moth increases susceptibility of Phragmites australis to gall-making by a midge: mechanisms and effects on midge population dynamics. Oikos 54:93-100. [ Links ]
41. VAN HINSBERG, A & P VAN TIENDEREN. 1997. Variation in growth form in relation to spectral light quality (red/far-red ratio) in Plantago lanceolata L. in sun and shade populations Oecologia 111:452-459. [ Links ]
42. WEIS, AE & A KAPELINSKI. 1984. Manipulation of host plant development by the gall-midge Rhabdophaga strobiloides. Ecol. Entomol. 9:457-465. [ Links ]
43. WHITE, TCR. 1984. The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia 63:90-105. [ Links ]
44. WILSEY, BJ; E HAUKIOJA; J KORICHEVA & M SULKINOJA. 1998. Leaf fluctuating asymmetry increases with hybridization and elevation in tree-line birches. Ecology 79:2092-2099. [ Links ]
45. WOODS, RE; MJ HERCUS & AA HOFFMANN. 1998. Estimating the heritability of fluctuating asymmetry in field Drosophila. Evolution 52:816-824. [ Links ]
46. ZAR, JH. 1984. Biostatistical analysis. 2nd edn. Englewood Cliffs: Prentice-Hall. [ Links ]
47. ZVEREVA, EL; MV KOZLOV; P NIEMELÄ & E HAUKIOJA. 1997a. Delayed induced resistance and increase in leaf fluctuating asymmetry as responses of Salix borealis to insect herbivory. Oecologia 109:368-373. [ Links ]
48. ZVEREVA, EL; MV KOZLOV & E HAUKIOJA. 1997b. Stress responses of Salix borealis to pollution and defoliation. J. Appl. Ecol. 34:1387-1396. [ Links ]














