Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Ecología austral
versión On-line ISSN 1667-782X
Ecol. austral vol.22 no.2 Córdoba mayo/ago. 2012
ARTÍCULO ORIGINAL
Assessment of water quality in temperate-plain streams (Argentina, South America) using a multiple approach
Evaluación de la calidad del agua mediante un enfoque múltiple en arroyos de la llanura templada (Argentina, América del Sur)
Carolina S. Ocon* & Alberto Rodrigues Capítulo
*Instituto de Limnología "Dr. Raúl A. Ringuelet", CCT La Plata, CONICET-UNLP. Av. Calchaquí km 23,5. (1888) Florencio Varela, Pcia. de Buenos Aires, Argentina. carolina@ilpla.edu.ar
Recibido: 12 de septiembre de 2011; Fin de arbitraje: 19 de diciembre de 2011;
Revisión recibida: 1 de febrero de 2012;
Aceptado: 14 de abril de 2012
ABSTRACT
We assessed the water quality in two pampean lotic systems (Argentina), the Juan Blanco and Buñirigo streams, subjected to different land uses (i.e., the UNESCO Biosphere Nature Reserve vs. industry and agriculture, respectively) through measurements of physicochemical data and the structural parameters of the macroinvertebrate assemblages in addition to ecotoxicological analyses. The objective was to identify the degree of ecological impairment in adversely affected areas and the consequent effects on the biota. The results obtained allowed the establishment of different water-quality classes within the study area. According to the indices applied, the downstream zone of the Buñirigo stream was categorized as moderately polluted on the basis of losses of sensitive benthic species or changes in their abundance. Likewise, acute ecotoxicological bioassays demonstrated that the water from this site had lethal effects on Caenis nemoralis (Ephemeroptera: Caenidae) larvae. This sampling point also exhibited relevant physicochemical features, such as high water conductivity and nutrient levels as well as low contents of dissolved oxygen.
Keywords: Pollution; Macroinvertebrates; Physicochemical parameters; Ecotoxicological analyses; Pampean streams
RESUMEN
Se realizó un estudio de la calidad del agua en dos sistemas lóticos, los arroyos Juan Blanco y Buñirigo (Argentina), sujetos a diferente uso del suelo (el primero se ubica dentro de una reserva de Biosfera de la UNESCO, mientras que el segundo en una zona agrícolo-ganadera e industrial). Se analizaron datos físico-químicos, parámetros estructurales del ensamble de macroinvertebrados y ensayos ecotoxicológicos. El objetivo fue identifi car el grado de deterioro en las áreas afectadas por efl uentes y los consiguientes efectos sobre la biota. Los resultados obtenidos permitieron establecer diferentes clases de calidad del agua dentro de la zona de estudio. De acuerdo a los índices aplicados la cuenca baja del arroyo Buñirigo fue caracterizada como moderadamente contaminada sobre la base de la pérdida de especies bentónicas sensibles o cambios en su abundancia. Del mismo modo, los ensayos ecotoxicológicos agudos demostraron que el agua de este sitio tuvo efectos letales sobre larvas de Caenis nemoralis (Ephemeroptera: Caenidae). Este punto de muestreo también evidenció características físico-químicas desfavorables para la biota, como alta conductividad y niveles de nutrientes, así como bajo contenido de oxígeno.
Palabras clave: Polución; Macroinvertebrados; Parámetros físico-químicos; Análisis ecotoxicológicos; Arroyos pampeanos
INTRODUCTION
The existence of sources of freshwater and their locations has historically been a determining condition for the establishment of human settlements worldwide. This association results in the modification of natural systems through increases in organic matter, the discharge of industrial effluents, changes in the architecture of the natural channels, and the introduction of exotic species, among other deleterious effects. Within this scenario of environmental impact stemming from human interventions, the various hydrologic networks show differing types and degrees of deterioration in acceptable water quality and biotic integrity sensu Karr & Dudley (1981), who defined these criteria as the capability of supporting and maintaining a balanced, integrated, and adaptive community of organisms having a species composition, diversity, and functional organization comparable to that of the pristine habitat.
For water-quality assessment originally proposed individual analytical methods were later combined in order to carry out more comprehensive studies, such as that of Chapman & Long (1983). According to these authors, the evaluation of pollution impact should combine chemical, ecological (e.g., macroinvertebrate-community structure) and ecotoxicological analyses. The combination of these strategies allows to quantify the effect of a pollutant on the biota along with an identification of the resulting impact on the latter occurring within the localized area. This methodology, initially proposed for marine environments, was subsequently applied to freshwater systems (Canfield 1994; Forbes & Callow 2004; Winger et al. 2005). Chapman (1990) concluded that the combination of potential cause-and-effect measurements in the laboratory constitutes a thorough and powerful tool for predicting the extent and significance of pollution-induced environmental degradation. Biological systems (such as the zoobenthic community) have proven to be useful as indicators because they more precisely reflect the bioavailability and toxicity of contaminants than do strict chemical analyses. Furthermore, resident organisms reflect the totality of the environmental conditions over time and can indicate the impact of various types of anthropic effects (Leslie et al. 1999). Thus, according to these authors, once a given type of contamination is identified and quantified, the structure of the benthic-macroinvertebrate community provides a simultaneous indication of both the bioavailability and the potential deleterious effects of that pollution. Toxicity tests are useful means of evaluating the existence of a cause-and-effect relationship between toxic agents and the extant organisms so as to provide fundamental information directly at the biological level for the assessment and interpretation of water quality (Gerhardt 1999). Feeding strategies, taken into account in such functional analyses, can play a key role in biomonitoring and conservation (Charvet et al. 1998; Príncipe et al. 2010).
According to Greve et al. (1998, 1999), the monitoring of species representative of undisturbed environments as alarm mechanisms is a relevant approach. Likewise, Van der Geest (1998), Karouna- Renier & Zehr (2003), and Gerhardt et al. (2004) argued against the use of foreign species as a tool in water-quality evaluation. The species of Ephemeroptera have been extensively employed in ecotoxicological studies as a result of their key metabolic role within the aquatic environment (Khatami et al. 1998; Goodyear & McNeill 1999; Malmqvist & Hoffsten 1999). Although these taxa are considered exquisitely sensitive to environmental changes and pollution, only a few studies have been made on the streams in the pampean temperate plains (Ocon& Rodrigues Capítulo 2004). Within this region, different types of contamination (chemical, organic, and combinations of both) affect both the streams and rivers (Rodrigues Capítulo et al. 2001; Graça et al. 2002; Jergentz et al. 2004). Only a few lotic systems can be considered as pristine within this area of Argentina because cattle raising and agricultural activities are widely disseminated therein, and diverse industries and urban centers with different degrees of development are also present.
The objective of this study was to identify the degree of ecological impairment in a stream of an agricultural landscape that is also adversely affected by industrial effluents in the downstream zone. To assess the possible effects on the biota, we examined the physicochemical conditions, investigated the structural parameters of the macroinvertebrate assemblages and functional feeding groups (FFGs), and performed ecotoxicological analyses. We then compared these results with those obtained in a reference stream.
MATERIALS AND METHODS
Study area and sampling design
The study sites are located in Magdalena, Buenos Aires Province, Argentina (Río de la Plata basin). Pampean grassland is the typical vegetation type containing grasses and other herbaceous species (Cabrera 1971). The predominant anthropic activities are extensive agriculture (22% of the area) and widespread cattle raising (70% of the area). The remaining land is devoted to urban settlements and a few industries (Alperin et al. 2003). Nevertheless, the Juan Blanco was chosen as a reference site for the study area because a sizeable sector of this stream is located within the UNESCO Biosphere Nature Reserve (Ocon& Rodrigues Capítulo 2004). In contrast, waterways nearby with similar geologic and morphometric characteristics, such as the Buñirigo stream, show significant ecologic deterioration. The mid- and downstream sections of this latter lotic system receive the combined effluents from a foodproduction plant (instant coffee, chocolate milk, cereal flours, and instant mashed potatoes) and a tannery, whereas the headwaters only receive diffuse loadings of organic matter and pesticides from extensive farming activity. We selected three undisturbed sites and one site affected by anthropic activities. The Buñirigo-stream sites were selected in order to compare the ecologic states of both upstream (B1: 35º09'37" S - 57º40'24" W) and downstream (B2: 35º03'47" S - 57º33'12" W) from the industrial disturbance. The Juan Blanco stream sites (the headwaters JB1: 35º14'11" S - 57º31'16" W and the downstream region JB2: 35º08'32" S - 57º26'24" W) were selected because of their similar geomorphological characteristics but different chemical water qualities compared to the site of disturbance (B2), according to Bauer et al. (2002).
Physicochemical analyses
Physicochemical parameters were registered seasonally (March=autumn, June=winter, September=spring and December=summer) from each sampling site over a 2-year period (4/year during 2005-2006) on the same date as the biological data were taken.
The sediment samples were removed only once in December 2006 (on the last sampling date) with an Ekman grab (100 cm2) for analysis of heavy metals, Pb, Cr, Cu, Zn (atomic-absorption spectrophotometry; EPA 3500), Cd (atomicabsorption spectrophotometry; EPA 3005 A-7130), and Hg (GVF 7471 A) with detection limits at: Pb, 2 μg/g; Cr, 2, μg/g; Cu, 1.0 μg/g; Zn, 4 ng/g; Cd, 0.5, μg/g; and Hg, 0.1 μg/g. The dissolved oxygen (DO), water conductivity (μS/cm), pH, and temperature (ºC) were measured in the field with portable instruments. In the laboratory, the biochemical oxygen demand (BOD5: 5-day incubation in the dark), chemical oxygen demand (COD: oxidation with K2Cr207), NH4 +-N, NO2 --N, NO3 --N (spectrophotometry at 635 nm), soluble reactive phosphorus (SRP: spectrophotometry at 885 nm), and ions (CO3 -2, HCO3 -, SO4 -2, Cl-, Ca+2, Mg+2, Na+, K+) were all measured according to APHA (1998). The percent organic matter (OM%) in the sediments was calculated by weight loss after ignition at 500 °C for 4 h from a sample (50 g fresh weight) removed with an Ekman grab, while granulometric analysis was performed following Depetris (1995) from 100 g of sediment removed from the same samples.
Benthic macroinvertebrates
At each sampling site 3 replicates of benthic macroinvertebrates were removed with an Ekman grab (100 cm2); and in order to characterize the nonbenthic macroinvertebrates, a set of complementary qualitative samples were taken with sieves (250 μm) from the macrophytes present. A total of 96 benthic and 64 qualitative samples were analyzed. The samples were fixed in situ with formaldehyde (5% [v/v]) and the organisms identified and counted under a stereomicroscope through the use of the taxonomic keys of Lopretto & Tell (1995) and Fernández & Domínguez (2001). The determinations were made down to different taxonomic levels. In order to characterize the macroinvertebrate assemblages the IBPamp biotic index (Rodrigues Capítulo et al. 2001) was calculated. The FFG approach used in this study focusses on the morphologic features of the invertebrates and the behavioral mechanisms by which they acquire their food resources after Merritt & Cummins (1996). For this purpose we used the complete list of macroinvertebrates and their densities obtained at each site and on each sampling date.
Ecotoxicological bioassays
Laboratory bioassays with preimaginal stages of Caenis nemoralis (Ephemeroptera: Caenidae) from the JB2 site were carried out. Larvae were obtained by means of a sieve (mesh size=250 μm). In the laboratory, the organisms were sorted and placed into plastic aquaria with water and substratum from the sampling site for two weeks for acclimatization. The sediments of the lotic systems studied are fine with high organic matter content; this material in turn acts as a trap for certain chemicals, thereby decreasing their bioavailability. Because of this characteristic and the habits of the selected organisms (epibenthic or associated with vegetation), the test was made with water rather than sediment. Larvae were placed in plastic receptacles (500 mL) filled with 250 mL of each water type and each receptacle contained 10 individuals in quadruplicate receptacles (i.e., a total of 40 organisms per treatment condition). Reconstituted water (USEPA 1985), moderately hard (pH, 7.4-7.8; conductivity, <300 μS/cm), was used as a control because of its similarity to the water of the local environments. Water from the industrial effluents (B2 site) and water from an undisturbed site (JB2) were used as the test substances. We assume that under natural conditions of no disturbance the water from both stream systems should give similar results. The assays were acute (lasting for 10 days) and static (without water renewal). The green algae Chlorella sp. (Chlorophyta) were isolated and purified from samples obtained at the reference site (JB2) to eliminate other species (Mosto Cascallar & Tell 1995) and were then added to each receptacle (concentration 5 x 106 cells/mL) at time zero to avoid larval mutual cannibalism. Laboratory conditions were constant in temperature (20±2 ºC) and employed a natural photoperiod. Dissolved oxygen, pH, and conductivity were measured daily. Live and dead larvae were counted each day and the dead larvae removed. The mortality rate was calculated at the final time point.
Statistical analyses
We used two-way ANOVA analyses, followed post-hoc by the Holm-Sidak tests (significance level 0.05), when corresponded, to assess differences in macroinvertebrate attributes (e.g., biotic indices) between sites and to test the effect of seasonality, while the nonparametric Kruskal Wallis test was applied to assess differences in the FFGs between sites (Chapman 1996; Magurran 2004). The principal-components analyses (PCAs) were performed to examine the ordination of the sampling sites based on the physicochemical parameters. This indirect-ordination analysis was preferable because the length of the gradients was <2 units of the standard deviation (Ter Braak& Smilauer 2002). All variables (except the pH) were log-transformed (ln[n+1]) before the analysis in order to reduce the effects of scale variation.
With the laboratory bioassays, differences in the larval cumulative mortality between the treatments were analyzed at the final time point by a one-way ANOVA followed post-hoc by the Tukey test, when corresponded. All statistical analyses were performed via the Statistica 8 and Sigma Stat 3.1 software packages.
RESULTS
Physicochemical analyses
All the studied sites had mud and silt as dominant granulometric fractions, whereas sand and gravel were generally absent, though consolidated carbonates (caliche) were occasionally observed. As expected, the higher mean values of conductivity, COD , NH4+, and heavy metals (except for Hg) along with lower DO values were observed at the Buñirigo downstream site (B2) (Table 1). The heavymetal concentrations there, however, were lower than the reference values (Manassero et al. 1998) for the general pampean area. The maximum value for the OM% in the sediments, nearly 25%, was observed at that same Site B2; where the effluents from the food-production plant were entering.
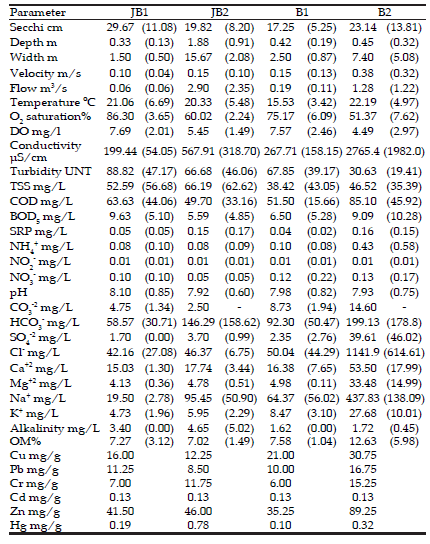
Table 1. Mean values (+SD) for each physicochemical and morphometric parameter measured in the study sites during the assessment period. JB1: Juan Blanco upstream, JB2: Juan Blanco downstream, B1: Buñirigo upstream, B2: Buñirigo downstream.
Tabla 1. Valores promedio (+DS) para cada parámetro físico-químico y morfométrico registrado en los sitios de muestreo durante el período de estudio. JB1: Juan Blanco nacientes, JB2: Juan Blanco zona cercana a la desembocadura, B1: Buñirigo nacientes, B2: Buñirigo zona cercana a la desembocadura.
The PCA showed that factor 1 accounted for 37.02% of variation and corresponded to a water-quality gradient, while factor 2 accounted for 16.36% of variation and corresponded to the geomorphologic variation (Figure 1, Table 2). Those variables showing collinearity were removed from the analysis (width, flow, SO4 -2, Cl-, Na+, K+ and toxicity). The sites having high DO and turbidity values (JB1 and B1) were placed in the upper-right quadrant. In JB2, the stream depth has the greatest influence among the parameters (the lower-right quadrant). Points corresponding to the Buñirigo downstream site (B2, the left sector of the plot) indicated higher values for conductivity and ions. This site also exhibited increases in nutrient levels and the COD values.
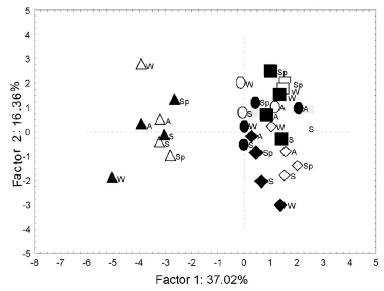
Figure 1. Ordination of sites according to Factors 1 and 2 of PCA on physicochemical and morphologic parameters measured at four sites on the Buñirigo and Juan Blanco streams for two years. Circles: B1 site; triangles: B2 site; squares: JB1 site; diamond: JB2 site. Open symbols: first year; filled symbols: second year. A, autumn; W, winter; Sp, spring; S, summer.
Figura 1. Ordenamiento de los sitios de muestreo de acuerdo a los Factores 1 y 2 del ACP basado en los parámetros físico-químicos y morfológicos medidos en los cuatro sitios de los arroyos Buñirigo y Juan Blanco durante dos años. Círculos: sitio B1; triángulos: sitio B2; cuadrados: sitio JB1; rombos: sitio JB2. Símbolos abiertos: primer año; Símbolos llenos: segundo año. A, otoño; W, invierno; Sp, primavera; S, verano.
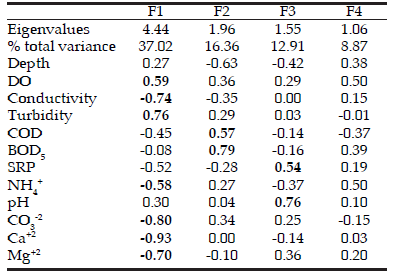
Table 2. PCA eigenvalues and factor loadings of physicochemical and morphometric variables for the four principal factors resulting from multivariate analysis. The fi rst two factors accounted for more than 60% of the variance in the mentioned parameters for the Buñirigo and Juan Blanco systems.
Tabla 2. Autovalores y factor loadings obtenidos del ACP de 24 variables físico-químicas y morfométricas para los cuatro principales factores resultantes del análisis multivariado. Los primeros dos factores representaron más del 60% de la varianza
Benthic macroinvertebrates
The lowest number of taxa was registered in the Buñirigo downstream site (B2) (Table 3). The highest values were observed at the Juan Blanco downstream site (JB2); where sensitive species such as Palaemonetes argentinus, Macrobrachium borrellii, Callibaetis guttatus, Callibaetis willineri, Callibaetis radiatus, Campsurus major, Elmidae sp. 1, Cyrnellus sp., Verger bruchinus, and Leptoceridae sp. 1 were registered. The differences in the taxa between the sites or among the four seasons were not, however, statistically significant (two-way ANOVA, F=0.74; P=0.53).
Table 3. Mean values of density (+SD) of the more representative taxonomic groups recorded in the Juan Blanco and Buñirigo streams, Buenos Aires province, Argentina, during the study period. Abbreviations for study sites in heading of Table 1.
Table 3. Valores promedio de densidad (+DS) de los grupos taxonómicos más representativos registrados en los arroyos Juan Blanco y Buñirigo, provincia de Buenos Aires, Argentina, durante el período de estudio. Abreviaturas para los sitios estudiados en epígrafe de Tabla 1.
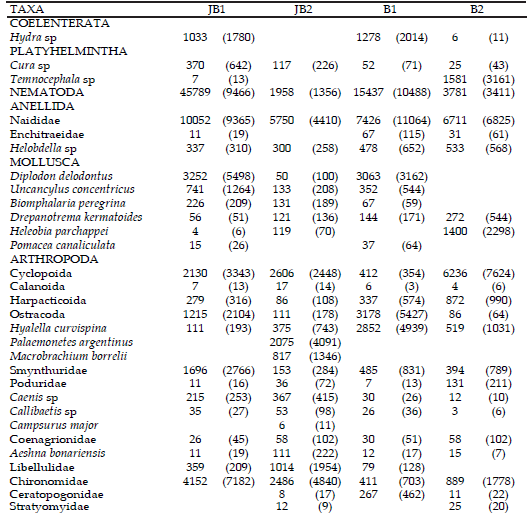
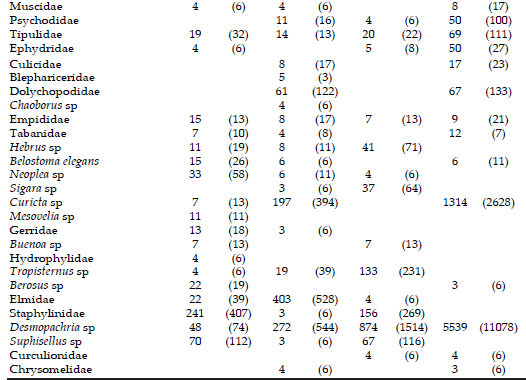

Nematoda was the dominant group at headwaters of both stream systems (Figure 2). The crustaceans Ostracoda and Amphipoda had the highest densities at B1, whereas other crustaceans were more representative at the additional sites. The insects were prevalent at JB2; where Ephemeroptera, Trichoptera, and Coleoptera were notable among those registered. The Acari were the most abundant taxon at the downstream site of the Buñirigo (B2) possibly related to the decrease of water quality there.

Figure 2. Relative abundances of macroinvertebrates at each sampling site during the 2-year study period, Buenos Aires province, Argentina.
Figura 2. Abundancia relativa de macroinvertebrados en cada sitio de muestreo durante los dos años de estudio, en la provincia de Buenos Aires, Argentina.
With respect to the FFGs, the gathering collectors were the most representative group in the Juan Blanco and Buñirigo headwaters (JB1 and B1), consistent with a high availability of food there (in the form of detritus). At Site B2 this FFG was second in prevalence and consisted of Nematoda, Oligochaeta, several Crustacea, and certain insects such as some of the Diptera. The predators were the most highly represented at Site B2, where the mites (Acari), Cyclopoida, and certain Coleoptera also reached high densities. These taxa have been considered tolerant on the basis of biotic indices. The shredders were observed principally at the downstream sites with their highest density corresponding to a prevalence of Hyalella curvispina, followed by other crustaceans and Ephydridae. Only in the headwaters of the both streams were the filtering-collectors well represented consistent with the higher DO at those sites. At these headwater sites, the Bivalvia were the principal filterers, whereas at Site JB2 certain Trichoptera were also recorded. The scrapers were registered principally at Site B2, where higher densities of Gastropoda (e.g., H. parchappei) were observed. The nonparametric statistical analyses (Kruskal-Wallis) indicated, however, that the differences in representation among the FFGs between the four sites were not significant (P>0.05).
The highest values for the IBPamp biotic index were recorded in the downstream zone of the Juan Blanco Stream (JB2). As expected, given the background of the environmental disturbances present, lower values for this index were recorded at the Buñirigo downstream site (Table 4). The observed differences between the two downstream sites were statistically significant (two-way ANOVA test F=13.5; P=0.001), while the posthoc Holm-Sidak test (significance-threshold level=0.05) indicated significant differences (P<0.001) between B2 and the other sites. We found no significant differences either between the three remaining sites or among the four seasons at any given site.
Table 4. Mean values (±SD) of biotic index, total density, FFGs and FFG ratios for the sampling sites on the Juan Blanco and Buñirigo streams during the study period. IBPamp: Pampean biotic index. p: predators. f-c: fi lteringcollectors. g-c: gathering-collectors. s-g: scrapers-grazers. sh: shredders. Total coll: fi ltering + gathering collectors. Abbreviations for study sites in heading of Table 1.
Tabla 4. Valores promedio (±DS) de del índice biótico, densidad total, GFA y relaciones entre GFA para los sitios de muestreo en los arroyos Juan Blanco y Buñirigo durante el período de estudio. IBPamp: Índice Biótico Pampeano. p: depredadores. f-c: colectores-filtradores. g-c: colectores-recolectores. s-g: raspadores. sh: fragmentadores. Total coll: colectores-filtradores + colectores-recolectores. Abreviaturas para los sitios estudiados en epígrafe de Tabla 1

Ecotoxicological bioassays
We did not observe larval mortality in C. nemoralis exposed to control (reconstituted water) or the reference-site water (JB2), but in the water subjected to the anthropic effluents (B2) larval mortality was registered starting at Day 4. By the end of the bioassay 40% of the exposed individual organisms had died (Figure 3). The one-way ANOVA test indicated significant differences between the applied treatments vs. the control (F=7.129; P<0.01). The observed differences were tested for the Control-B2 (P=0.009) and JB2-B2 (P=0.002) treatments, by means of the posthoc Tukey test.

Figure 3. Cumulative mortality of larvae of C. nemoralis exposed to reconstituted water (control), water of the reference stream (Juan Blanco downstream, JB2) and water containing the effl uent (Buñirigo, downstream from the effl uent, B2) for 10 days. SD is shown for each day. Mortality was zero for the control and reference water throughout the experiment.
Figura 3. Mortalidad acumulativa de larvas de C. nemoralis expuestas a agua reconstituida (control), agua del sitio de referencia (Juan Blanco cuenca baja, JB2) y agua del efl uente (B2) durante 10 días. Se muestra el DS para cada día. La mortalidad fue cero para el control y el agua del sitio de referencia a lo largo del experimento.
DISCUSSION
The downstream zone of the Buñirigo stream (B2) was classified as moderately polluted, as a result of the biotic-index values and the bioassay results obtained there. Accordingly, at this site, we registered higher values of conductivity, COD, nutrients, and heavy metals along with lower DO concentrations. Nevertheless, the concentrations of those heavy metals were lower than the reference values for this area according to Manassero et al. (1998). Likewise, the bioassays performed with the effluent water enabled a confirmation of the deleterious effects of that input into the stream and a demonstration of the contaminated water's lethality to the species C. nemoralis. A general toxicity and an alteration of the biotic communities, but not an industrial pollution, was confirmed because the analyses of pollutants (such as metals) proved to be under the maximum levels permitted by legislation. As a consequence, we are forced to conclude, as did Chapman (1996), that these effects are most likely caused by contaminants not measured by these specific analyses.
In Kenya, Ndaruga et al. (2004) reported high levels of total dissolved solids and turbidity attributable to a point pollution from a tannery factory, whose discharges were also suspected to contain high amounts of organic matter that were drastically reducing the DO concentration downstream. In our study the total dissolved solids and turbidity were higher for the nonpolluted sites. The organic matter content values were elevated at Site B2 (with a corresponding reduction in DO), whereas at the rest of the sites this parameter was similar to the levels reported in other pampean lotic systems not affected by factories (Ocon & Rodrigues Capítulo 2004). At this Site B2, however, the organic pollution may result from a spillover from the food industry (principally featuring coffee-processing residues), not simply from the tannery effluents. The community changes observed at Site B2 with respect to the reference sites were probably related to the above-mentioned altered parameters. This attribution would agree with Jonsson et al. (2002) that concluded that organic pollution as a primary deleterious effect has globally altered many stream communities from their original species composition. Hering et al. (2006) and Friberg et al. (2009), however, considered that organic pollution as such was not likely to stress macroinvertebrate communities in lowland systems directly, whereas reduced oxygen levels appeared to be extremely critical. The conclusion would be, then, that macroinvertebrates are presumably less strongly affected by organic pollution "per se" than by the oxygen depletion that results from such contamination.
Biotic indices have been successfully used to evaluate organic pollution in different countries (Prenda & Gallardo-Mayenco 1996; Timm et al. 2001; Brabec et al. 2004; Dahl et al. 2004 among others). In this study the decrease in the IBPamp-biotic-index values was always related to declining numbers and abundances of sensitive species, such as Cyrnellus sp., V. bruchinus, Leptoceridae sp. 1 (Trichoptera), Callibaetis spp., C. major (Ephemeroptera), Elmidae sp. 1 (Coleoptera), D. delodontus (Bivalvia), and Palaemonidae (Decapoda). Our observations are consistent with those of Long et al. (2003), who found that gradients in chemical concentrations and the degree of response in laboratory toxicity tests were likewise accompanied by losses in the numbers and abundances of sensitive benthic species. That decrease, in turn, led to a decline in the number of total species and the abundance of dominant species. Strieder et al. (2006), in a study of the temperate streams of Brazil, reported that sensitive taxa (such as certain Crustacea, Odonata, Ephemeroptera and Hemiptera) were associated with higher water quality; while the more tolerant taxa (Chironomidae, Hirudinea and Oligochaeta) were registered in association with contaminant-containing effluents. Other authors such as Brabec et al. (2004) used multivariate analysis for the detection of the response of macroinvertebrate communities to gradients of organic pollution in stream habitats and found relationships between the parameters indicative of bad water quality and tolerant species. Based on the site ordinations obtained by the multivariate analysis (PCA) applied in this work, we were able to establish that the more sensitive organisms were located at sites where favorable conditions prevailed, such as high oxygen concentration along with lower values of those parameters related to contamination (e.g., high conductivity, high nutrient levels). Similarly, taxa such as the Acari and H. parchappei were tolerant to such unfavorable conditions. This observation agrees with the findings of several authors who have argued that either variations in natural conditions or the input of substances from external sources can influence the nature and abundance of the resident organisms (Greve et al. 1998; Van der Geest et al. 1999; Leslie et al. 1999; Courtney & Clements 2001).
At all four sites studied the gathering collectors constituted the dominant FFG. In streams of the Neotropical Region a common affinity of many taxa for fine detritus has been observed, thus demonstrating the significance of this food resource and a prominence of the role of the gathering collectors in these freshwater ecosystems (Tomanova et al. 2006; Príncipe et al. 2010). According to Barbour et al. (1996), trophic dynamics are reflected by FFGs whose profile provides information on the feeding strategies employed in the benthic assemblage. These authors have established that specialized feeders, such as the scrapers and the shredders, are the more sensitive organisms and are thought to be well represented in healthy streams. This FFG profile, however, was not observed among our sampling sites because the percentage of shredders was not diminished in the unfavourable environments (i.e., this FFG behaved as a tolerant species). The ratio of the shredders to the gathering plus filtering collectors was not an accurate descriptor for our study area because, according to Merritt & Cummins (1996), this parameter is lower than 0.25 in polluted environments (cf. Table 5). Generalists, such as the collectors and the filterers, have a broader range of acceptable food materials. No significant differences in terms of feeding strategies between the different sites were registered, probably because of the wide availability of resources, mainly the detritus. The main change observed was a high percentage of predators at the site affected by the effluents (i.e., B2), in agreement with the conclusions of Kerans & Karr (1994), although the specific composition of the FFGs varied, with considerably more resistant taxa being present at Site B2, such as Helobdella sp., Cyclopoida, the Acari and Desmopachria sp.
According to Chapman (2007), data on resident macroinvertebrate communities garnered "in situ" are given greater weight than the less realistic laboratory toxicity tests. Nevertheless, these latter measurements are more reliable in making predictions since data on resident communities in the field reflect only situations that have already occurred, not those that may occur in the future. Besser et al. (1996) established that laboratory bioassays with Hyalella azteca and Chironomus tentans produced a better discrimination among sites with differing degrees of contamination than did the in-situ characterization of benthic communities in the Trenton Channel of the Detroit River (USA), which biotic structures in that locale were dominated by oligochaetes. In our particular study case, both of those applied methods yielded concordant results, and C. nemoralis was furthermore demonstrated to be a sensitive and informative species for biomonitoring.
Chapman (2007) likewise asseverated that an adequate evaluation of the effects of chemical contaminants (and other environment-stressing influences) on a given ecological habitat required a combination of comprehensive and complementary assessments. Reynoldson & Zarull (1989) established that the combined analysis of physical, chemical, and biological data can be used to connect cause and effect between environmental contaminants and benthic communities.
Our results have demonstrated that the coordinate methodological approach applied here constitutes a powerful tool for assessing the ecologic status of temperate-plains streams since [consistent with the previous findings of Winger et al. (2005)] such a tactic furthermore provides critical information for the development of appropriate steps in environmental management.
ACKNOWLEDGEMENTS:
We are grateful to Carlos Roldán for his field assistance; to Jorge Donadelli for water analyses in the laboratory; and to Dr. Donald Haggerty, a retired career investigator and native English speaker, for editing the final version of the manuscript. We sincerely appreciate the reviews and valuable comments of Dr. Laura Miserendino, two anonymous reviewers and the Editor. This work was supported by CONICET PIP 5305 and La Plata University N/469. ILPLA Scientific Contribution N° 878 of the Institute of Limnology "Dr. Raúl A. Ringuelet" (CONICET La Plata-UNLP).
REFERENCES
1. ALPERÍN, MI; VG BORGES & R SARANDÓN. 2002. Caracterización espacial de los tipos de cobertura de suelo usando técnicas geoestadísticas a partir de información satelital. Revista de la Facultad de Agronomía 105:40-51. [ Links ]
2. APHA. 1998. Standard methods for the examination of water. 20th Ed. American Public Health Association. Washington, D.C. [ Links ]
3. BARBOUR, MT; J GERRITSEN; GE GRIFFITH; R FRYDENBORG; E MCCARRON; ET AL. 1996. A framework for biological criteria for Florida streams using benthicmacroinverte brates. J. N. Am. Benthol. Soc. 15:185-211. [ Links ]
4. BAUER, DE; J DONADELLI; N GÓMEZ; M LICURSI; CS OCON; ET AL. 2002. Ecological status of the Pampean plain streams and rivers (Argentina). Verhand. Internat. Verein theoret. Limnol. 28:259-262. [ Links ]
5. BESSER, J; J GIESY; J KUBITZ; D VERBRUGGE; T COON; ET AL. 1996. Assessment of sediment quality in dredged and undredged areas of the Trenton Channel of the Detroit River, Michigan USA, using the Sediment Quality Triad. J. Great Lakes Res. 22:683-696. [ Links ]
6. BRABEC, K; S ZAHRÁDKOVÁ; D NMEJCOVÁ; P PAIL; J KOKE; ET AL. 2004. Assessment of organic pollution effect considering differences between lotic and lentic stream habitats. Hydrobiologia 516:331-346. [ Links ]
7. CABRERA, A. 1971. Fitogeografia de America Latina. Rev. Soc. Arg. Bot. 14:1-42. [ Links ]
8. CANFIELD, T; N KEMBLE; W BRUMBAUGH; F DWYER; C INGERSOLL; ET AL. 1994. Use of benthic invertebrate community structure and the sediment quality triad to evaluate metal-contaminated sediment in the Upper Clark Fork River, Montana. Environ. Toxicol. Chem. 13: 1999-2012. [ Links ]
9. CHAPMAN, P. 1990. The Sediment Quality Triad approach to determining pollution-induced degradation. Sci. Tot. Environ. 97-98:815-825. [ Links ]
10. CHAPMAN, P. 1996. Presentation and interpretation of Sediment Quality Triad data. Ecotoxicology 5:327-339. [ Links ]
11. CHAPMAN, P. 2007. Determining when contamination is pollution - Weight of evidence determinations for sediments and effl uents. Environ. Internat. 33:492-501. [ Links ]
12. CHAPMAN, P & E LONG. 1983. The use of bioassays as part of a comprehensive approach to marine pollution assessment. Mar. Pollut. Bull. 14:81-84. [ Links ]
13. COURTNEY, L & W CLEMENTS. 2001. Sensitivity to acidic pH in benthic invertebrate assemblages with different histories of exposure to metals. JNABS 19:112-127. [ Links ]
14. DEPETRIS, J. 1995. Los sedimentos fluviales y lacustres: granulometría y contenido de materia orgánica. Pp. 67-78 in: Lopretto, E & G Tell (eds.). Ecosistemas de aguas continentales, metodologías para su estudio. Ediciones Sur, La Plata. [ Links ]
15. FERNÁNDEZ, H & E DOMÍNGUEZ. 2001. Guía para la determinación de los artrópodos bentónicos sudamericanos. EudeT. Serie: Investigaciones de la UNT. Pp. 282. [ Links ]
16. FORBES, V & P CALLOW. 2004. Systematic approach to weight of evidence in sediment quality assessment: Challenges and opportunities. Aquat. Ecosyst. Health& Manag. 7:339-350. [ Links ]
17. FRIBERG, N; J SKRIVER; S LARSEN; M PEDERSEN & A BUFFAGNI. 2009. Stream macroinvertebrate occurrence along gradients in organic pollution and eutrophication. Fresh. Biol. doi:10.1111/j.1365-2427.2008.02164.x. [ Links ]
18. GERHARDT, A (ED.). 1999. Biomonitoring of Polluted Water. Reviews on Actual Topics. Environmental Research Forum 9, TransTech Publ., Zürich, Switzerland. [ Links ]
19. GERHARDT, A; L JANSSENS DE BISTHOVEN & A SOARES. 2004. Macroinvertebrate response to acid mine drainage: community metrics and on-line behavioural toxicity bioassay. Environ. Pollut. 130:263-274. [ Links ]
20. GOODYEAR, K & S MC NEILL. 1999. Bioaccumulation of heavy metals by aquatic macroinvertebrates of different feeding guilds: a review. Sci. Tot. Environ. 229:1-19. [ Links ]
21. GRAÇA, M; A RODRIGUES CAPÍTULO; CS OCON & N GÓMEZ. 2002. In situ assays for pollution assessment in pampean (Argentina) streams. Wat. Res. 36:4033-4040. [ Links ]
22. GREVE, G; H VAN DER GEEST; S STUIJFZAND; S ENGELS & M KRAAK. 1998. Development of ecotoxicity test using laboratory reared larvae of the riverine caddisflies Hydropsyche angustipennis and Cyrnus trimaculatus. Proc. Experimental & Applied Entomology N.E.V. Amsterdam 9:205-210. [ Links ]
23. GREVE, G; H VAN DER GEEST; S STUIJFZAND; A KURECK& M KRAAK. 1999. Development and validation of an ecotoxicity test using fi eld collected eggs of the riverine mayfly Ephoron virgo. Proc. Experimental & Applied Entomology N.E.V. Amsterdam 10:105-110. [ Links ]
24. HERING, D; R JOHNSON; S KRAMM; S SCHMUTZ; K SZOSZKIEWICZ; ET AL. 2006. Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fi sh: a comparative metric-based analysis of organism response to stress. Fresh. Biol. 51:1757-1785. [ Links ]
25. JERGENTZ, S; P PESSACQ; H MUGNI; C BONETTO & R SCHULZ. 2004. Linking in situ bioassays and population dynamics of macroinvertebrates to assess agricultural contamination in streams of the Argentine pampa. Ecotox. Environ. Safety 59:133-141. [ Links ]
26. JONSSON, M; O DANGLES; B MALMQVIST & F GUEROLD. 2002. Simulating species loss following perturbation: assessing the effects on process rates. Proc. Royal Soc. London B 269:1047-1052. [ Links ]
27. KAROUNA-RENIER, N & J ZEHR. 2003. Short-term exposures to chronically toxic copper concentrations induce HSP 70 proteins in midge larvae (Chironomus tentans). Sci. Tot. Environ. 312:267-272. [ Links ]
28. KARR, J & D DUDLEY. 1981. Ecological perspectives on water quality. Environ. Manag. 5:55-68. [ Links ]
29. KERANS, BL & JR KARR. 1994. A benthic index of biotic integrity (B-IBI) for rivers of the Tennessee Valley. Ecol. Appl. 4:768-785. [ Links ]
30. LESLIE, H; T PAVLUK; A BIJ DE VAATE & M KRAAK. 1999. Triad assessment of the impact of Chromium contamination on benthic macroinvertebrates in the Chusovaya river (Urals, Russia). Arch. Environ. Contam. Toxicol. 37:182- 189. [ Links ]
31. LONG, E; M DUTCH; S AASEN; K WELCH; M HAMEEDI. 2003. Chemical Contamination, Acute Toxicity in Laboratory Tests, and Benthic Impacts in Sediments of Puget Sound: A summary of results of the joint 1997-1999 Ecology/NOAA survey. Washington State Deptartment of Ecology, Publication Nº 03-03-049 and NOAA Technical Memorandum NOS NCCOS CCMA Nº 163. Washington State Department of Ecology, Olympia, WA. [ Links ]
32. LOPRETTO, E & G TELL (EDS.). 1995. Ecosistemas de aguas continentales, metodologías para su estudio. Ediciones Sur, La Plata. [ Links ]
33. MAGURRAN, A. 2004. Measuring biological diversity. Blakwell Science Ltd., United Kingdom. [ Links ]
34. MALMQVIST, B & P HOFFSTEN. 1999. Influence of drainage from old mine deposits on benthic macroinvertebrates communities in central Swedish streams. Wat. Res. 33: 2415-2423. [ Links ]
35. MERRITT, R & KW CUMMINS. 1996. Trophic relations of macroinvertebrates. Pp. 453-474 in Merritt, R & KW Cummins (eds.). An introduction to the aquatic insects of North America. 3rd edition. Kendall/Hunt, Dubuque, Iowa. [ Links ]
36. MOSTO CASCALLAR, P & G TELL. 1995. Cultivo y aislamiento de algas. Pp. 297-312 in: Lopretto, E & G Tell (eds.). Ecosistemas de aguas continentales, metodologías para su estudio. Ediciones Sur, La Plata. [ Links ]
37. NDARUGA, A; G NDIRITU; N GICHUKI & W WAMICHA. 2004. Impact of water quality on macroinvertebrate assemblages along a tropical stream in Kenya. Afr. J. Ecol. 42:208-216. [ Links ]
38. OCON, C & A RODRIGUES CAPÍTULO. 2004. Presence and abundance of Ephemeroptera and other sensitive invertebrates in relation with habitat conditions in pampean streams (Buenos Aires, Argentina). Arch. Hydrobiol. 159:473-487. [ Links ]
39. PRENDA, J & A GALLARDO-MAYENCO. 1996. Self-purification, temporal variability and the macroinvertebrate community in small lowland Mediterranean streams receiving crude domestic sewage effluents. Arch. Hydrobiol. 136:159-170. [ Links ]
40. REYNOLDSON, T & M ZARULL. 1989. The biological assessment of contaminated sediments: the Detroit River example. Hydrobiologia 188-189:463-476. [ Links ]
41. RODRIGUES CAPÍTULO, A; M TANGORRA & C OCON. 2001. Use of benthic macroinvertebrates to assess the ecological status of pampean streams in Argentina. Aquat. Ecol. 35:109-119. [ Links ]
42. STRIEDER, M; L RONCHI; C STENERT; R THORMANN SCHERER& U NEISS. 2006. Medidas biológicas e índices de qualidade da água de uma microbacia com poluição urbana e de curtumes no Sul do Brasil. Acta Biologica Leopondensia 28:17-24. [ Links ]
43. TER BRAAK, C & P SMILAUER. 2002. CANOCO reference manual and CanoDraw for Windows user's guide: software for canonical community ordination (version 4.5). Microcomputer Power, Ithaca, NY, USA. [ Links ]
44. TIMM, H; M IVASK & T MÖLS. 2001. Response of macroinvertebrates and water quality to long-term decrease in organic pollution in some Estonian streams during 1990-1998. Hydrobiologia 464:153-164. [ Links ]
45. VAN DER GEEST, H; G GREVE; B SCHEPER; E DE HAAS; S STUIJFZAND; ET AL. 1998. Key factors limiting the distribution of sensitive aquatic insects species: effects of copper and diazinon on larvae of the caddisfly Hydropsyche angustipennis (Trichoptera). Proc. of the 9th International Symposium on Trichoptera. Pp. 117-122. [ Links ]
46. WINGER, P; P LASIER & K BOGENREIDER. 2005. Combined use of rapid bioassessment protocols and sediment quality triad to assess stream quality. Environ. Monitor. Assess. 100:267-295. [ Links ]













