Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Ecología austral
versão On-line ISSN 1667-782X
Ecol. austral vol.22 no.2 Córdoba maio/ago. 2012
COMUNICACIÓN BREVE
Demographic response to the manipulation of adult females in corn mouse populations
Respuesta demográfica a la manipulación de hembras adultas en poblaciones del ratón maicero
María D. Gómez*; Andrea R. Steinmann & José W. Priotto
Departamento de Ciencias Naturales, Universidad Nacional de Río Cuarto, Conicet. Agencia Postal 3; (5800) Río Cuarto, Córdoba, Argentina. mail: dgomez@exa.unrc.edu.ar
Recibido: 28 de julio de 2011; Fin de arbitraje: 22 de octubre;
Revisión recibida: 13 de marzo de 2012;
Aceptado: 20 de abril
ABSTRACT
Variation in population abundance is a consequence of changes in demographic parameters such as survival and recruitment. These demographic parameters can be affected by intraspecific competition, thus adults may play an important role infl uencing the survival and/or reproduction of juvenile individuals in many species of small mammals. We present an analysis of Calomys musculinus populations using capturemark- recapture models in order to evaluate the effect of female removal on their demography. The study was carried out in four enclosures during two different periods: a reference period and a treatment period, with the removal of adult females. Reference period was analyzed to describe population demography without manipulations. In treatment period, two control enclosures maintained both parents remained with their offspring whereas other two enclosures had their adult females removed. Seven monthly trapping sessions were carried out (spring to autumn). We estimated apparent survival, recruitment, population growth rates and recapture probabilities. Models considered these parameters constrained to vary as a function of time, enclosure, sex and/or treatment. During the reference period survival and recruitment showed sex and time effects, survival was higher in females and decreased towards June; recruitment showed a peak in February with a higher number of recruited males; population growth rates peaked in February and decreased towards June. During the treatment period survival showed time effect whereas recruitment showed time and treatment effects. Survival was higher at the beginning of the breeding period and decreased afterwards, and recruitment was higher in control enclosures; population growth rates showed a similar pattern than in reference period. There were not differences in growth rates and abundances between control and experimental enclosures. Under our experimental and methodological conditions, the results would indicate that the absence of females in experimental enclosures was not enough to cause a clear impact on final population size.
Keywords: Adult female removal; Calomys musculinus; Population growth; Recruitment; Survival
RESUMEN
La variación del tamaño de una población obedece a cambios en parámetros demográficos tales como sobrevida y reclutamiento. Estos parámetros demográficos pueden estar afectados por la competencia intraespecífica, de modo que los adultos pueden desempeñar un papel importante en la sobrevida y/o la reproducción de los juveniles en muchas especies de mamíferos pequeños. Estudiamos una población de Calomys musculinus utilizando modelos de captura-marcado-recaptura para evaluar el efecto de la remoción de hembras sobre la demografía de la población. El estudio se realizó en cuatro clausuras durante dos períodos: período de referencia y de tratamiento. El período de referencia permitió describir la demografía poblacional sin manipulación mientras que el período de tratamiento evaluó la remoción de hembras. Se realizaron siete sesiones de trampeo mensuales entre primavera y otoño. Se estimaron la sobrevida aparente, el reclutamiento, la tasa de crecimiento poblacional y la probabilidad de recaptura. Los modelos fueron construidos con dichos parámetros restringidos a variar como función del tiempo, la clausura, el sexo y/o el tratamiento. Durante el período de referencia la sobrevida fue mayor en hembras y disminuyó hacia el mes de junio; el reclutamiento mostró un pico en febrero con un mayor número de machos reclutados; la tasa de crecimiento poblacional mostró un pico en febrero y disminuyó hacia junio. Durante el período de tratamiento la sobrevida fue mayor al comienzo del período reproductivo disminuyendo posteriormente y el reclutamiento fue mayor en los controles que en los tratamientos sin hembras; la tasa de crecimiento mostró un patrón similar al del período de referencia. La remoción de hembras no afectó las tasas de crecimiento ni el tamaño poblacional. Bajo nuestras condiciones experimentales y metodológicas, los resultados indicarían que la ausencia de hembras en las clausuras experimentales no fue sufi ciente para causar un efecto claro en el tamaño final de la población.
Palabras clave: Calomys musculinus; Crecimiento poblacional; Reclutamiento; Remoción de hembras adultas; Sobrevida
INTRODUCTION
Variation in population abundance is a consequence of changes in demographic parameters such as survival, recruitment and immigration (Dobson & Oli 2001; Lima et al. 2001).
Demographic parameters can vary with individual characteristics such as age, sex, weight and reproductive condition, and also as a function of biotic and abiotic environmental variables (Lebreton et al. 1992; Eccard et al. 2002; Crespin & Lima 2006). Intra and interspecific competition can also affect demographic parameters (Gurevitch et al. 1992; Eccard et al. 2002; Crespin & Lima 2006). Regarding intraspecific competition and age structure of a population, adults play an important role influencing the survival and/or reproduction of juvenile individuals in many species of small mammals (Wolff 1992; Pusenius & Viitala 1993; Wolff et al. 2002). In most rodent species, females are generally assumed to have a greater impact on reducing juvenile performance than males due to the fact that females typically compete for exclusive offspring-rearing space (Bond& Wolff 1999; Wolff & Macdonald 2004).
The corn mouse, Calomys musculinus (Muridae: Sigmodontinae), is the dominant rodent species of central Argentina, and it is mainly studied due to its role as reservoir of Junin virus, the etiological agent of the Argentine Hemorragic Fever (AHF) (de Villafañe & Bonaventura 1987; Mills & Childs 1998). Calomys musculinus is a short-live grassland mouse and is found in a variety of habitats including natural pastures, crop-fields, border areas protected by wire fences with little agricultural disturbance, road borders, borders between cultivated fields or pastures and railway banks of pampean agrarian ecosystems (Busch et al. 2000; Sommaro et al. 2010a). Calomys musculinus populations are characterized by seasonal density changes with low density during winter and peaks during late summer or mid autumn (Mills& Childs 1998; Sommaro et al. 2010a). This mouse has a promiscuous mating system and during the breeding period females keep exclusive home ranges and actively defend breeding spaces (territories) irrespective of population density (Steinmann et al. 2009; Sommaro et al. 2010b). Reproductive females of C. musculinus would defend an exclusive area to avoid infanticidal females as an adaptation for pup defense (Coda et al. 2011). Therefore, young females reaching reproductive age will be at risk of competition with resident adult females and should disperse. Calomys musculinus males have home ranges twice as large as those of females with high intra and intersexual home range overlap, and exhibit high level of intrasexual tolerant and amicable behaviours (e.g., sniffing partner, encounter, sharing space) (Steinmann et al. 2005, 2009).
We present a demographic analysis of C. musculinus enclosure populations using capture-mark-recapture models in order to evaluate the effect of adult female removal on the demographic parameters of populations.
METHODS
Experimental setup and field procedures
The study was performed in four 0.25 ha outdoor enclosures (Figure 1) on Espinal Reservation, in the National University of Río Cuarto Campus, Argentina (33° 07' S, 64° 14' W). The study area comprised a natural pasture interspersed with brush and weed species, similar to C. musculinus natural habitats. The four enclosures had similar habitat conditions (i.e., floristic composition and vegetation cover). Enclosures were successful in limiting the entry of competing herbivores and terrestrial predators. Although the natural pasture within each enclosure had a high vegetative cover of about 95% throughout the year, each enclosure was supplied with six artificial shelters built with bricks. Each shelter was enclosed by a concrete circle of 1m diameter and 0.7 m high and covered with iron mesh. On the inner margin of each enclosure, a 1 m wide grass strip was devegetated .
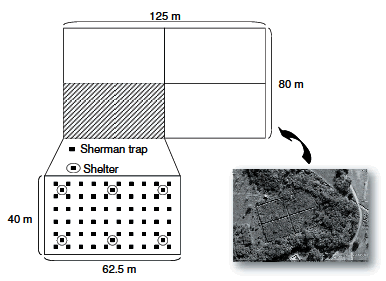
Figure 1. Enclosures located in the Espinal Reservation (UNRC) campus. Enclosure details, with traps location and artificial shelters.
Figura 1. Clausuras ubicadas en la Reserva de Espinal (UNRC). Detalle de una clausura, con la ubicación de las trampas y los refugios artificiales.
The study had two periods: 1) a reference period (from November 2002 to June 2003) and 2) a treatment period, with the removal of adult females (from October 2004 to June 2005). The first study was performed in order to describe population demography without population manipulations. To obtain the initial population for each enclosure C. musculinus individuals were translocated from an area located 30 km far from our study place and mated in the laboratory. In November - December 2002 (reference period), 24 adult females and 24 adult males were mated whereas 16 adult females and 16 adult males were mated in October - November 2004 (treatment period). This difference was due to the fact that a lower number of individuals were captured in treatment period. Both sex and birth date of the offspring of the first litter were recorded. Adults and their offspring were ear-tagged for permanent identifi cation. In reference period, the initial population in each enclosure was made up by six families (the mate with their offspring). In treatment period, the initial population in each enclosure was made up by four families. In both periods, each family was carried to the enclosures and located in an artifi cial shelter. After three days the shelters were opened and the animals dispersed into the enclosures. In treatment period, treatments were assigned randomly to each enclosure, in two enclosures (Experimental I and II) only the adult males remained with their offspring. The other two enclosures served as controls (Control I and II) where both parents remained with their offspring.
Each enclosure had a grid of 6x10 Sherman-live traps placed at 6 m intervals. Thirty-two additional traps were placed in the devegetated edge. Traps were baited with a mixture of peanut butter and cow fat and were checked each morning. For each captured individual sex and body measurements (weight and length of body and tail) were recorded. All new individuals were marked with numbered ear-tags and released at their site of capture.
Trapping sessions were taken from January to June in reference period and from December to June in treatment period. Monthly CMR trapping sessions were conducted for 8 consecutive days. In order to detect animals that were not able to settle in the habitat area of the plot, animals that were trapped two consecutive times in the devegetated edge in the same trapping session were removed from the population since we assumed that they were not able to settle within the enclosures. During the study, only five and 10 animals in reference period and treatment period respectively were removed because they did not settle in the habitat area of the plot. In reference period, one of the enclosures had to be discarded from the analysis because a high trap mortality registered in January could have confounded the results obtained. This trap mortality could be in relation to the fact that the traps were checked just at midday in this enclosure due to operational problems.
Analytical procedures
Apparent survival (ϕ) and seniority (γ) probabilities variation was modelled following the CMR methodology using program MARK (White & Burnham 1999). To assess the goodness-of-fit of the models we used program U-CARE (Choquet et al. 2003). In reference period several models were constructed with the two parameters constrained to vary as a function of time (t), sex (s) and enclosure (e). Models were ranked according to Akaike's Information Criterion, corrected for small sample size (AICc) (Burnham & Anderson 1998). Model comparison was based on the differences in AICc values (ΔAICc), so when ΔAICc values were greater than two units, the model with the lowest AICc could be considered a statistically better description of the process that generated the data. We started by modelling survival and recapture probabilities as a function of time, sex and enclosure in reference period (with interactions and additive effects among them). Seniority was also constrained according to time, sex and enclosure in reference period (with interactions and additive effects among them). Seniority probability was estimated by a method developed by Pradel (1996). Seniority probabilities are used to estimate recruitment component (1-γ) of population growth rate (Nichols et al. 2000). The best resulting models obtained from the analyses of survival and seniority were selected and included in the analysis of treatment effect in treatment period to test if these parameters were best modelled by treatment.
Population growth rate (λ) was modelled using Pradel (1996) survival and lambda model in program MARK. The best model for survival and recapture probabilities in each period was used to model population growth. To minimize the number of parameters to be estimated in λ analyses, we analysed sex and enclosure (in reference period) and sex and treatment (in treatment period) effects by examining both combined and additive contributions of each effect to time dependent population growth rates. Population densities were estimated using the program CAPTURE (White et al. 1982) incorporated as an independent module into program MARK. The estimations were performed for each enclosure in reference period and for control and experimental enclosures in treatment period.
RESULTS
The goodness of fit test for reference period data showed there was not either transient (individuals never recaptured) (N(0,1) statistic for transients=-0.956; P=0.830) or trap dependency (N(0,1) signed statistics for trap dependency=-1.163; P=0.245) effects. By contrast, in treatment period there was no transient effect (N(0,1) statistic for transients=-0.115; P=0.546), but significant trap dependency (trap happiness; N (0,1) signed statistics for trap dependency=-4.705; P<0.000) was registered. Hence we used QAIC for model selection. QAICc is a measure that provides a compromise between bias and precision when the global model does not fit the data and incorporates a variation inflation factor c (Anderson et al. 1994; Telfer et al. 2002). We estimated c from the goodness of fit chi-square statistic and its degrees of freedom (Anderson et al. 1994). Models with differences in QAICc of <2 were considered similar in their ability to describe the data (Burnham & Anderson 1998).
Survival probabilities decreased towards the end of breeding (April) and the beginning of the non-breeding periods (May-June) (Figure 2). Females generally had higher survival probabilities but the inverse was observed in April- May. There were three approximating models with similar support (ΔAIC<2) for describing survival and recapture probabilities in reference period (Table 1). We did not expect enclosure effect in survival since the three enclosures had similar foundational populations and habitat conditions (vegetation cover, weather) and trapping sessions were carried out at the same time. The low number of replicates could cause the inclusion of the models {(e+s+t), p(.),(e+s+t), p(s)} as two of the best models. For this reason, we selected the model with sex and time effects for survival and constant recapture probabilities {(s*t), p(.)}, which was biologically plausible, for analysing survival in this period. We included the most biologically plausible and parsimonious model identified for survival and recapture in reference period and tested for treatment, sex and/or time variations in these parameters in treatment period. From the candidate model set, there were three models with similar support for describing survival (Table 1). We selected the simplest of the best approximating models, thus survival showed a time effect whereas recapture was constant {(t), p(.)}. Apparent survival probabilities had similar time pattern than in reference period but without sex effect, with decreasing values towards the end of the reproductive period (April) and the beginning of non-reproductive period (May-June) (Figure 2).
Table 1. Statistical best models of reference and treatment (removal of adult females) periods are denoted according to each model-specific variation in the probabilities of survival (ϕ) and recapture (p)); (*) and (+) symbols mean combined effects and additive effects respectively. Only models with ΔAICc or ΔQAICc≤2 are shown. AICc and QAICc: measure of each modelfit; K: number of estimable parameters.
Tabla 1. Mejores modelos estadísticos de los períodos de referencia y tratamiento (remoción de hembras adultas), denotados de acuerdo a cada variación específica en las probabilidades de sobrevida (ϕ) y recaptura (p); los símbolos (*) y (+) significan efectos combinados y aditivos respectivamente. Sólo se muestran modelos con ΔAICc o ΔQAICc≤2. AICc y QAICc: medida de ajuste de cada modelo; K: número de parámetros estimables.
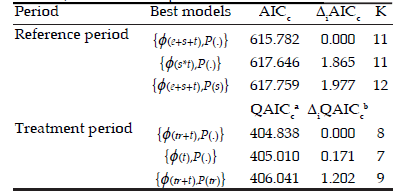

Figure 2. Demographic parameters of C. musculinus in reference period (model used is indicated in each case): survival probabilities {ϕ(s*t), p(.)}, recruitment probabilities {γ(tr+t), p(.)}, population growth rates {ϕ(s*t), p(.), λ(e*t)}, and treatment period: survival probabilities {(tr+t), p(.)}, recruitment probabilities {ϕγ(tr*t), p(.)}, population growth rate {ϕ(s*t), p(.), λ(t)}.
Figura 2. Parámetros demográficos de C. musculinus en el período de referencia y en el período de tratamiento (remoción de hembras). Ve modelos estadísticos de cada parámetro en la leyenda en inglés.
There were three approximating models with similar support (ΔAIC<2) for describing seniority in reference period (Table 2). The most parsimonious model indicated an additive effect of sex and time on seniority probabilities and constant recapture {γ(s+t), p(.)}. Thus, when we analyzed recruitment (1-γ), the peak of recruited individuals was observed at the end of summer (February- March) and there were more males recruited in all trapping sessions (Figure 2). We included the most parsimonious model identified for seniority and tested for treatment, sex and/or time variations in this parameter in treatment period. There were two approximating models with similar support (ΔQAIC<2) and the same number of parameters for describing seniority probabilities (Table 2). In the model with the lower QAICc {γ(tr+t), p(.)}, control enclosures had higher recruitment probabilities than experimental enclosures in all time intervals (Figure 2). The other model {γ(s+t), p(.)} showed more recruited males than females in all months.
Table 2. Statistical best models of reference and treatment (removal of adult females) periods are denoted according to each model-specific variation in the probabilities of seniority (γ) and recapture (p); (*) and (+) symbols mean combined effects and additive effects respectively. Only models with ΔAICc or ΔQAICc≤2 are shown. AICc and QAICc: measure of each model fit; K: number of estimable parameters.
Tabla 2. Mejores modelos estadísticos de los períodos de Referencia y Tratamiento (remoción de hembras adultas) denotados de acuerdo a cada variación modelo-específica en las probabilidades de permanencia (γ) y recaptura (p); los símbolos (*) y (+) significan efectos combinados y aditivos respectivamente. Sólo se muestran modelos con ΔAICc o ΔQAICc≤2. AICc y QAICc: medida de ajuste de cada modelo; K: número de parámetros estimables.

We used the model with sex and time effects in survival and constant recapture {(s*t), p(.)}, which was biologically plausible, for analysing population growth rates in reference period. The best approximating model for describing population growth in this period included the interaction between enclosure and time (Table 3). The pattern was similar among the three enclosures, the highest population growth rates were observed at the end of summer (February-March) in the three enclosures, but enclosure 1 showed a lower value than the other two (Figure 2). Afterwards, population growth started to decrease to values near or lower than 1 (Figure 2). We used the most parsimonious model identified for survival and recapture probabilities {(t), p(.)}, and tested for treatment effect in population growth rates in treatment period. There were three models with similar support (Table 3), but the most parsimonious one did not show treatment effect but only time effect on population growth. The observed pattern was similar to reference period, with the highest population rate at the end of summer (Figure 2).
Table 3. Statistical best models of reference and treatment (removal of adult females) periods are denoted according to each model-specific variation in the probabilities of survival (ϕ), population growth rate (λ) and recapture (p); (*) and (+) symbols mean combined effects and additive effects respectively. Only models with ΔAICc or ΔQAICc≤2 are shown. AICc and QAICc: measure of each model fit; K: number of estimable parameters.
Tabla 3. Mejores modelos estadísticos de los períodos de referencia y tratamiento (remoción de hembras adultas) denotados de acuerdo a cada variación modelo-específica en las probabilidades de sobrevida (ϕ), tasa de crecimiento poblacional (λ) y recaptura (p); los símbolos (*) y (+) significan efectos combinados y aditivos respectivamente. Sólo se muestran modelos con ΔAICc o ΔQAICc≤2. AICc y QAICc: medida de ajuste de cada modelo; K: número de parámetros estimables.

In both periods, population densities showed a peak at the end of summer and beginning of autumn, and lower numbers afterwards (Figure 3). In reference period abundance pattern was similar among the three enclosures, except that enclosure IV peaked one month earlier than the other two. In treatment period, population densities were estimated for control and experimental enclosures separately. The pattern of abundance was similar between control and experimental enclosures except that experimental enclosures peaked one month earlier than control enclosures. The estimation of population growth rates (Figure 2) reflected the abundance pattern observed in the enclosures in both periods (Figure 3). Due to the fact that recapture probabilities were always best modelled with constant values; we used the Null model to estimate population densities in CAPTURE program.

Figure 3. Population abundances of C. musculinus in reference and treatment (removal of adult females) periods.
Figura 3. Abundancias poblacionales de C. musculinus en los períodos de referencia y tratamiento (remoción de hembras adultas).
DISCUSSION
Calomys musculinus females are territorial, they have exclusive home ranges and aggressive behaviour towards other females in association with the defense of their own home range as an adaptation of pup defense (Steinmann et al. 2006; Steinmann et al. 2009; Coda et al. 2011). Considering this behaviour, it would be hope that the presence of adult females would cause poor survival and/or lack of reproduction of the females reaching reproductive age. In this experimental study, the most parsimonious model in survival analysis showed time effects but not sex effect. Recruitment probabilities were higher in presence of adult females (control enclosures), possibly due to the fact that these females would contribute with a higher number of new recruited individuals until juvenile females started to reproduce. In spite of the differences in survival and recruitment probabilities, there were not any differences in population growth rates between control and experimental enclosures. The positive effect that the higher recruitment probability would have on growth rate of C. musculinus in control enclosures would be damped by lower survival in these populations. Thus, the population growth rates at the end of the study were similar between control and experimental enclosures.
The population demography of both reference and treatment (i.e., removal of adult females) periods showed similar time pattern, but they seemed to be also influenced by other different factors. In reference period sex and time were the most important factors that influenced both survival and recruitment probabilities. In treatment period there were two models with similar support to explain recruitment, one including the same factors than in the first period and the other with treatment and time effects. On the other hand, in this period survival was only explained by time. Although in this period the survival models including treatment had similar support to the most parsimonious model, they were discarded due to their higher number of parameters for estimating.
Considering the demographic parameters analyzed in this study, it would be important to emphasize some results. Pradel's model does not differentiate between reproduction and immigration as different sources of recruitment. However, recruitment in our study, developed with enclosed populations, would be almost entirely from reproduction (Priotto et al. 2010). Temporal variations in recruitment would be in relation to the annual reproductive pattern observed in this species, where a peak in reproduction is registered in summer (Polop & Suárez 2010). This reproductive pattern would cause an increase of the summer-autumn population numbers as we observed in the enclosed populations. Besides greater numbers of captured males, as we observed, are common in sigmodontine rodents due to their high movement rates (Polop & Suárez 2010; Sommaro et al. 2010a). In relation to survival probability, there is not any information about capture-markedrecapture data in natural populations but a winter declination in numbers associated with breeding interruption and mortality increase was observed (Polop & Suárez 2010; Simone et al. 2010; Sommaro et al. 2010a). This would be in agreement with the decreasing survival probabilities observed in this study. Finally, temporal variation in growth rates was a result of temporal variation in both survival and seniority. In both periods population growth rates peaked at the end of summer (February- March), this observation is in agreement with the highest population numbers registered in late summer and early autumn (March and April) in the enclosures. This pattern of population abundance matches that observed in C. musculinus natural populations (Mills& Childs 1998; Sommaro et al. 2010a).
In this study to consider some methodological aspects would be relevant. Firstly, there were not any clear differences among the best approximating models; there were various models with similar support in almost all analyses of demographic parameters. Considering that habitat conditions and trapping schedule were similar among enclosures we did not expect enclosure effects in recapture and demographic parameters. The low number of replicates could reduce our statistical power to find clear differences in these parameters. In a study developed in the same enclosures with Calomys venustus, Priotto et al. (2010) found that the statistical power to find differences in survival estimations would increase 40%, considering the quadruple number of replicates. One needs to be cautious since some biologically significant effects in reducing juvenile performance (survival and reproduction) might have been missed due to the small sample sizes. Secondly, we set up a monthly time scale to experimentally test population changes in a short time interval (7 months) although most demographic studies in mammals involve long term analysis with annual and/or seasonal time scales (Lima et al. 2001; Crespin et al. 2002; Pocock et al. 2004; Ozgul et al. 2007). This was possible due to the fact that C. musculinus has a short life expectancy, a multivoltine life cycle and a clear breeding period (Mills & Childs 1998; Polop & Suárez 2010). Finally, our study attempts to analyze the demography of populations under experimental conditions using methods that account for variation in capture probabilities and that provide unbiased estimates of demographic parameters (CMR methods). Despite the lack of clear treatment effects, the use of this methodology allows us to estimate demographic parameters of C. musculinus populations. The development of statistical models for demographic analyses based on CMR data has great potential in rodent management studies (Lima et al. 2003). The demographic parameters would be useful to be incorporated in population dynamics models of this species. The link between demography and population dynamics could be very useful for gaining an understanding of rodent population fluctuations (Lima et al. 1999, 2003).
In summary, the results of this study could indicate that the low number of adult females and the absence of them in experimental enclosures were not enough to cause a clear effect in juvenile female performance. Nevertheless, female density values higher than those manipulated in this study, with a greater number of females with regard to the number of available reproductive spaces, might show the effect of female spacing behaviour on population performance in C. musculinus populations.
ACKNOWLEDGEMENTS: We thank the help of S. Vilor for the English version. This research was made possible by grants from Conicet and Secyt, Universidad Nacional de Río Cuarto.
REFERENCES
1. ANDERSON, DR; K BURNHAM & G WHITE. 1994. AIC model selection in overdispersed capture-recapture data. Ecology, 75:1780-1793. [ Links ]
2. BOND, ML & JO WOLFF. 1999. Does access to females or competition among males limit male home-ranges in a promiscuous rodent? J. Mammal., 80:1243-1250. [ Links ]
3. BURNHAM, K & D ANDERSON. 1998. Model selection and inference: A practical information-theoretic approach. Springer-Verlag, New York. [ Links ]
4. BUSCH, M; MH MIÑO; JR DADON & K HODARA. 2000. Habitat selection by Calomys musculinus (Muridae, Sigmodontinae) in crop areas of the Pampean region, Argentina. Ecología Austral, 10:15-26. [ Links ]
5. CHOQUET, R; AM REBOULET; R PRADEL; O GIMENEZ & J-D LEBRETON. 2003. User´s Manual for U-CARE, Version 2.0. [ Links ]
6. CODA, J; J PRIOTTO & A STEINMANN. 2011. Behavioral counterstrategies to infanticide in corn mouse females, Calomys musculinus. Mastozoología Neotropical, 18(2):207-215. [ Links ]
7. CRESPIN, L; R VERHAGEN; N STENSETH; N YOCCOZ; AC PRE´VOTJULLIARD; ET AL. 2002. Survival in fluctuating bank vole populations: seasonal and yearly variations. Oikos, 98: 467-479. [ Links ]
8. CRESPIN, L & M LIMA. 2006. Supervivencia adulta y dinámica poblacional del lauchón orejudo Phyllotis darwini en Chile central. Rev. Chil. Hist. Nat., 79:295- 308. [ Links ]
9. DE VILLAFAÑE, G & SM BONAVENTURA. 1987. Ecological studies in crop fields of the endemic area of Argentine hemorrhagic fever. Calomys musculinus movements in relation to habitat and abundance. Mammalia, 51: 233-248. [ Links ]
10. DOBSON, F & M OLI. 2001. The demographic basis of population regulation in Columbian ground squirrels. Am. Nat., 158:236-247. [ Links ]
11. ECCARD, J; I KLEMME; T HORNE & H YLONEN. 2002. Effects of competition and season on survival and maturation of young bank vole females. Evol. Ecol., 16:85-99. [ Links ]
12. GUREVITCH, J; L MORROW; A WALLACE & J WALSH. 1992. A meta-analysis of fi eld experiments on competition. Am. Nat., 140:539-572. [ Links ]
13. LEBRETON, J-D; K BURNHAM; J CLOBERT & D ANDERSON. 1992. Modeling survival and testing biological hypotheses using marked animals: a unifi ed approach with case studies. Ecol. Monogr., 62:67-118. [ Links ]
14. LIMA, M; JE KEYMER & F JAKSIC. 1999. ENSO-driven rainfall variability and delayed density dependence cause rodent outbreaks in western South America: linking demography and population dynamics. Am. Nat., 153:476-491. [ Links ]
15. LIMA, M; R JULLIARD; N STENSETH & F JAKSIC. 2001. Demographic dynamics of a neotropical small rodent (Phyllotis darwini): feedback structure, predation and climatic factors. J. Anim. Ecol., 70:761-775. [ Links ]
16. LIMA M, N STENSETH, H LEIRS & F JAKSIC. 2003. Population dynamics of small mammals in semi-arid regions: a comparative study of demographic variability in two rodent species. Proce. R. Soc. Lond. B, 270:1997-2007. [ Links ]
17. MILLS, JN & JE CHILDS. 1998. Ecologic studies of rodent reservoirs: their relevance for human health. Emerg. Infect. Dis., 4:529-537. [ Links ]
18. NICHOLS, JD; JA HINES; J-D LEBRETON & R PRADEL. 2000. The relative contributions of demographic components to population growth: a direct estimation approach based on reverse-time capture-recapture. Ecology, 81: 3362-3376. [ Links ]
19. OZGUL, A; M OLI; L OLSON; L BLUMSTEIN; D BLUMSTEIN & K ARMITAGE. 2007. Spatiotemporal variation in reproductive parameters of yellow-bellied marmots. Oecologia, 154:95-116. [ Links ]
20. POCOCK, M; SEARLE J & P WHITE P. 2004. Adaptations of animals to commensal habitats: population dynamics of house mice Mus musculus domesticus on farms. J. Anim. Ecol., 73: 878-888. [ Links ]
21. POLOP, JJ & O SUÁREZ. 2010. Ecología de poblaciones de roedores Sigmodontinos de la zona central de Argentina. Pp. 289-324 in: Polop, JJ & M Busch (eds.). Biología y Ecología de pequeños roedores en la región Pampeana de Argentina. Editorial Universidad Nacional de Córdoba. Córdoba, Argentina. [ Links ]
22. PRADEL, R. 1996. Utilization of capture-mark-recapture for the study of recruitment and population growth rate. Biometrics, 52:703-709. [ Links ]
23. PRIOTTO, J; D GÓMEZ & J POLOP. 2010. A demographic analysis of population responses to the manipulation of adult males in Calomys venustus (Rodentia, Sigmodontinae). Ecol. Res., 25:521-529. [ Links ]
24. PUSENIUS, J & J VIITALA. 1993. Varying spacing behaviour of breeding fi eld voles, Microtus agrestis. Ann. Zool. Fenn., 30:143-152. [ Links ]
25. SIMONE, I; F CAGNACCI; C PROVENSAL & J POLOP. 2010. Environmental determinants of the small mammal assemblage in an agroecosystem of central Argentina: The role of Calomys musculinus. Mamm. Biol., 75:496-509. [ Links ]
26. SOMMARO, L; D GÓMEZ; F BONATTO; A STEINMANN; M CHIAPPERO; ET AL. 2010a. Corn mice (Calomys musculinus) movement in linear habitats of agricultural ecosystems. J. Mammal., 91(3):668-673. [ Links ]
27. SOMMARO, L; A STEINMANN; M CHIAPPERO & J PRIOTTO. 2010b. Effect of high density on the short term Calomys musculinus spacing behaviour: A fencing experiment. Acta Oecol., 36:343-348. [ Links ]
28. STEINMANN, A; J PRIOTTO; E CASTILLO & J POLOP. 2005. Size and overlap of home range in Calomys musculinus (Muridae: Sigmodontinae). Acta Theriol., 50:197-206. [ Links ]
29. STEINMANN, AR; J PRIOTTO; L SOMMARO & J POLOP. 2006. The influence of adult female absence on the spacing behaviour of juvenile corn mice, Calomys musculinus: a removal experiment. Ann. Zool. Fennici, 43:366-372. [ Links ]
30. STEINMANN, A; J PRIOTTO & J POLOP. 2009. Territorial behaviour in corn mice, Calomys musculinus (Muridae: Sigmodontinae), with regard to mating system. J. Ethol., 27:51-58. [ Links ]
31. TELFER, S; M BENNETT; K BOWN; R CAVANAGH; L CRESPIN; ET AL. 2002. Effects of cowpox virus on survival in natural rodent populations: increases and decreases. J. Anim. Ecol., 71:558-568. [ Links ]
32. WHITE, GC & KP BURNHAM. 1999. Program MARK: survival estimation from populations of marked animals. Bird Study, 46:S120-A139. [ Links ]
33. WHITE, GC; DR ANDERSON; KP BURNHAM & DL OTIS. 1982. Capture-recapture and removal methods for sampling closed populations. Report Los Alamos. Los Alamos National Laboratory. [ Links ]
34. WOLFF, JO. 1992. Parents suppress reproduction and stimulate dispersal in opposite sex juvenile white-footed mice. Nature, 359:409-410. [ Links ]
35. WOLFF, JO; DW EDGE & G WANG. 2002. Effect of adult sex ratios on recruitment of juvenile gray-tailed voles, Microtus canicaudus. J. Mammal., 83:947-956. [ Links ]
36. WOLFF, JO & DW MACDONALD. 2004. Promiscuous females protect their offspring. Trends Ecol. Evol., 19:127-134. [ Links ]













