Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Ecología austral
versión On-line ISSN 1667-782X
Ecol. austral vol.22 no.2 Córdoba mayo/ago. 2012
COMUNICACIÓN BREVE
Evaluation of soil-microbial communities by their CLPP. Standardization of a laboratory technique to replace commercial available microplates
Evaluación de comunidades microbianas edáficas mediante CLPP. Estandarización de una técnica de laboratorio para reemplazar microplacas comerciales
Luciana P. Di Salvo & Inés E. García De Salamone
Cátedra de Microbiología Agrícola, Facultad de Agronomía, Universidad de Buenos Aires, Avenida San Martín 4453, Ciudad Autónoma de Buenos Aires (C1417DSE), Argentina. Tel. +54-11-4524-8061. mail: igarcia@agro.uba.ar
Recibido: 2 de septiembre de 2011; Fin de arbitraje: 22 de diciembre;
Revisión recibida: 15 de febrero de 2012;
Aceptado: 20 de marzo.
ABSTRACT
Variation of soil-microbial communities are good bioindicators of human impacts in soils, such as different soils management or contamination. Considering that traditional methods of isolation and taxonomic analysis do not consider the functionality of the microbial community, Community-Level Physiological Profiles (CLPP) emerged as a complementary methodology to study microbial communities. Several studies have shown that Biolog® EcoPlates® are very useful for determining physiological differences between communities from different samples. However, commercial microplates have some disadvantages which led us to the idea of replacing them by microplates prepared in the laboratory (Laboratory's). Here, we compared both types of microplates using soil samples from a bioremediation assay. We compared a) the average well color development for each sample, b) the averages of absorbance values for each type of microplate, c) Principal Components, and d) Shannon-Weaver's diversity index (H). Although Laboratory's showed signifi cantly lower Average absorbance values than EcoPlates®, the principal component analysis and diversity index did not differ between types of microplates. In conclusion, both types of microplates showed a relatively similar ability to detect differences in the CLPP of the treatments studied. Consequently, microplates prepared in laboratory are a reliable and economical tool to study the physiology of soil microbial communities.
Keywords: Carbon-source utilization; Community-level physiological profiles; Functional diversity; Metabolic profiles
RESUMEN
La variación de la composición de algunas comunidades microbianas edáficas son buenos bioindicadores del impacto de la actividad antrópica sobre los suelos, tales como diferentes formas de manejo o su contaminación. Los métodos tradicionales de aislamiento y análisis taxonómico no consideran la funcionalidad de las comunidades microbianas, por lo que los perfiles fisiológicos de uso de fuentes carbonadas (CLPP) constituyen una metodología complementaria para su estudio. Numerosos trabajos demostraron que las microplacas de Biolog® EcoPlates® son útiles para determinar diferencias fi siológicas entre comunidades de diferentes suelos. Sin embargo, estas microplacas comerciales poseen algunas desventajas, por lo cual surgió la idea de reemplazarlas por microplacas preparadas en el laboratorio. Comparamos ambos tipos de microplacas con muestras de suelo provenientes de un ensayo de biorremediación. Analizamos a) el desarrollo promedio de color para cada tratamiento, b) los valores promedio de absorbancia para cada tipo de microplaca, c) los análisis de componentes principales, y d) el índice de diversidad de Shannon-Weaver (H) para cada muestra. Si bien los valores promedio de absorbancia difi rieron signifi cativamente entre ambos tipos de microplacas, los resultados del análisis de componentes principales y de diversidad fueron relativamente similares. En conclusión, ambos tipos de microplacas resultaron similares para detectar diferencias en los CLPP de los distintos tratamientos. Es por ello que las microplacas preparadas en el laboratorio constituyen una herramienta confi able y económica para el estudio de la fisiología de comunidades microbianas de suelo.
Palabras clave: Perfiles fisiológicos de uso de fuentes carbonadas; Diversidad funcional; Perfiles metabólicos
INTRODUCTION
It is well known the importance of soil microbial communities in ecosystem functioning. Besides their role in nutrient cycling and pollutant degradation, many authors showed that different soil-microbial communities are good bioindicators of human impact in soils (Schnürer et al. 1985; Beare et al. 1992; Abril 2003). Other authors evaluated the effects of different soil management regimes in agroecosystems by the use of microbiological parameters (Bending et al. 2004; Bucher & Lanyon 2005).
For several years, ecological studies of microbial communities were based on methods of isolation and taxonomic analysis. These methodologies do not consider the functionality of the microbial community (Garland & Mills 1991). Community-Level Physiological Profiles (CLPP) emerged as a complementary methodology to study microbial communities. Inoculation of them in microplates with sole carbon sources produces a pattern of color development as a result of the reduction of a dye indicator when carbon sources were utilized. This can be used for sample differentiation. Color development is consequence of carbon source consumption by microorganism development. Because of this, CLPP technique is selective. In spite of these, the discrimination by selectivity may be useful for determining differences between microbial communities, because cultivable microorganisms could be most relevant in terms of both biomass and activity (Bakken 1997; Ellis et al. 2003).
The utilization of this methodology was extended through the time. Initially, Biolog® GN® microplates were used to identify and classify pure cultures of heterotrophic bacteria (Bochner & Savagneau 1977). Later, Garland & Mills (1991) extended it to characterize different soil microbial communities. Some years later, Campbell et al. (1997) reduced the number of carbon sources and used rhizospere substrates as sources. These changes could provide them greater discrimination between microbial communities. Finally, Insam (1997) proposed the carbon sources included in the Biolog® EcoPlates®. Complementarily, Derry et al. (1998) could obtain greater differentiation between diverse soil types when they perform analyses with only 23 sources of GN® microplates which were also present in EcoPlates® microplates in place of analyses performed with the total of 95 sources of GN® microplates.
Several studies have shown that Biolog® microplates are very useful for determining physiological differences between diverse microbial communities. Calbrix et al. (2005) reported 27 papers published between 1994 and 2001, which used any Biolog® microplates for microbial community analysis. Preston-Mafham et al. (2002) reported 122 papers published prior to 2001, which used this methodology to study highly different microbial communities. In some of these studies, authors analyzed microbial communities from different habitats (Garland & Mills 1991; Zak et al. 1994; Haack et al. 1995). Other authors measured impacts of flooding (Bossio & Scow 1995), agricultural management (Lawlor et al. 2000) or pollutants such as heavy metals (Kelly & Tate 1998) and pesticides (Engelen et al. 1998). Also, this methodology was used to follow bioremediation in hydrocarbon polluted soils (Dobler et al. 2000).
The CLPP methodology has become popular because it is a simple tool which provides a lot of information about an important functional attribute of microbial communities. Besides, its utility for determining physiological differences between microbial communities from different origins or under different treatments has been widely recognized (Garland & Mills 1991). Some authors showed that the metabolism of bacterial community differed both quantitatively and qualitatively with the developmental stage of the crop and the distance from roots (Baudoin et al. 2002, 2003; Naiman et al. 2009; García de Salamone et al. 2010).
Although the CLPP technique is vastly accepted and generalized to study functional diversity of soil-microbial communities, its use has some disadvantages. Composition of commercial microplates is a trade secret which involves the knowledge of the precise elements used. They can change during a particular assay (Nielsen & Winding 2002), affecting its repetitiveness. Furthermore, the carbon source in each well cannot be modified by the user in response to a particular aim. Finally, the access to commercial microplates in countries other than USA and Canada can be restricted due to their high cost and because their importation reduces the available time for use until the expiration date. For these reasons, the idea of replacing commercial microplates with others prepared in laboratory gives the possibility to apply the CLPP technique in a less restrictive way.
Microplates prepared in laboratory might show similar results than those obtained with the use of Biolog® EcoPlates®. However, there is no evidence comparing both methods. The aim of this study was to assess the reliability of microplates prepared in laboratory to replace commercial Biolog® microplates to differentiate microbial communities under different treatments using CLPP.
MATERIALS & METHODS
A bioremediation assay was performed under controlled conditions as described by Di Salvo et al. (2007). Four strategies of bioremediation on a non-contaminated soil and the same soil contaminated with phenanthrene or anthracene were included (Table 1). Each treatment had three replicates. The soil used was a Typic Hapludol. After two months of plant emergence, soil samples were taken from the 12 treatments and soil suspensions were performed in NaCl solution (9 g/L in water). Ten-fold dilutions were prepared for each sample. Standardized mixing was applied. As manufacturer recommends, aliquots of 150 μl from the 10-4 dilution were inoculated in commercial available microplates named Biolog® EcoPlates® to study soil microbial communities, including three samples per microplate. The 31 carbon sources included in these microplates were: β-methyl-glucoside, galactonic acid γ-lactone, arginine, pyruvic acid methyl ester, xylose, galacturonic acid, asparagine, tween 40, ierythritol, 2-hydroxy benzoic acid, phenylalanine, tween 80, mannitol, 4-hydroxy benzoic acid, serine, α-cyclodextrin, N-acetyl-glucosamine, γ-hydroxybutyric acid, threonine, glycogen, glucosaminic acid, itaconic acid, glycyl-glutamic acid, cellobiose, glucose-1-phosphate, α-ketobutyric acid, phenylethylamine, α-lactose, α-glycerol phosphate, malic acid, putrescine.
Table 1. Description of treatments: contaminants added and bioremediation strategies used.
Tabla 1. Descripción de tratamientos: contaminantes agregados y estrategias de biorremediación utilizadas

Microplates prepared in laboratory (named Laboratory's microplates) were inoculated with 50 μl from the 10-4 dilution per each well. Four samples were included per microplate. Each well also contained 50 μl of the respective carbon sources, 100 μl of buffer medium (NH4Cl 0.03%, NaNO3 0.05%, K2HPO4 0.1%, MgSO4.7H20 0.03%, CaCl2 0.01% y FeCl3 0.005%) and 50 μl of tetrazolium violet (0.0025%) as redox dye indicator, which inhibits fungal growth (Preston-Mafham et al. 2002). The concentration of each source was 0.2%. The sources used in these microplates were 23: arginine, glutamine, glycine, phenylalanine, proline, histidine, cellobiose, dextrose, maltose, rhamnose, xylose, fructose, glycerol, mannitol, lactic acid, malic acid, citric acid, oxalic acid, salicylic acid, benzoic acid, Tween 20, putrescine and itaconic acid (García de Salamone et al. 2010; Semmartin et al. 2010).
Both types of microplates were incubated at 30 °C for 96 h. Absorbance values were taken every 24 h with a microplate reader Multiskan® EX® (Labsystems, Finland) at 590 nm as indicator of color development. These data was used to obtain Average Well Color Development (AWCD) values for each sample, as described by Garland and Mills (1991). In this work, when significant differences between treatments at 24 h record were not observed, it was assumed that the samples contained similar number of microbial cells and AWCD standardization of absorbance values was not applied.
Absorbance values at 72 and 96 h were used to perform Principal Component Analyses with carbon source as dependent variable, to calculate both averages of absorbance values for each type of microplate and Shannon-Weaver's diversity indexes (H), following the methodology performed by Gómez et al. (2004).
This study was performed to determine whether commercial microplates and microplates prepared in laboratory can show the same CLPP for bacterial communities under different treatments of contamination. Accordingly of this, absorbance, principal components values and H index values of the four treatments of the phenanthrene group and the four treatments of the anthracene group (P and A groups, respectively) were contrasted with those values of the four treatments of the control non-contaminated group (C group) (Table 1). Data were subjected to analysis of variance followed by Tukey's tests (P<0.05). The statistical package InfoStat® (1.1 Version-Universidad Nacional de Córdoba) was used.
RESULTS
Absorbance values used for analyses were not standardized with the AWCD values because differences between treatments after 24 h of incubation were not detected. This situation can be adjudicated to the absence of differences among inoculum's densities (data not shown). Average absorbance values obtained at 72 and 96 h differed between the two types of microplates (P<0.05, Table 2). A source of variability in AAV which was taken into account was the difference in the level of carbon source use due to microplates dissimilar composition. Therefore, three sources (mannitol, arginine and putrescine) were selected depending on their Pearson's correlation value in the PCA, and their presence in both types of microplates. At 72 and 96 h of incubation, the utilization of these carbon sources corresponding to EcoPlates® was higher than those corresponding to Laboratory's microplates (Figure 1).
Table 2. Comparisons of the average absorbance values (AAV)(1) between two microplate types.
Tabla 2. Comparaciones de los valores de absorbancia promedio entre los dos tipos de microplacas.


Figure 1. Color development of three different carbon sources, present both in Laboratory's microplates (solid symbols) and EcoPlates® (open symbols). Points with letters denote significant differences between types of microplates at each incubation time and for each carbon source, with Tukey's test (P<0.05). Non-italic lowercase letters are for arginine's mean comparison and uppercase are for mannitol's mean comparison. Italic lowercase letters are for putrescine's mean comparison.
Figura 1. Desarrollo de color de tres fuentes carbonadas diferentes, presentes en Laboratory's microplates (símbolos negros) y en las EcoPlates® (símbolos blancos). Puntos con letras indican diferencias significativas entre tipos de microplacas para cada tiempo de incubación y para cada fuente de carbono, según prueba de Tukey (P<0.05). Letras minúsculas corresponden a la comparación de medias para arginina y mayúsculas corresponden a la comparación de medias para manitol. Letras minúsculas cursivas corresponden a la comparación de medias para putrescina.
The principal components and diversity index analyses were made at 72 h for Laboratory's microplates because AAV at this time did not differ from AAV at 96 h (Figure 2). In the case of EcoPlates®, significant differences were observed between AAV at 72 h and 96 h (Figure 2). Because of this, the last record was considered for the further analyses of this type of microplate. Results showed significant differences (P<0.05) between C and P groups (Table 3) and between C and A groups (Table 4), and there were no significant differences (P<0.05) accounted for the types of microplates (Tables 3 and 4).

Figure 2. Color development of both types of microplates. Different letters indicate significant differences in the average absorbance values among incubation times, with Tukey's test (P<0.05).
Figura 2. Desarrollo de color para ambos tipos de microplacas. Letras distintas indican diferencias significativas en los valores de absorbancia promedio entre tiempos de incubación, según prueba de Tukey (P<0.05).
Table 3. Analysis of Principal Component (PC) values between C and P groups of treatments. Comparisons between treatments and mean values of Laboratory's microplates (at 72 h) and EcoPlates® (at 96 h).
Tabla 3. Análisis de los valores de Componentes Principales (PC) entre los grupos C y P. Comparaciones entre tratamientos y valores medios de Laboratory's microplates (a las 72 h) y EcoPlates® (a las 96 h).
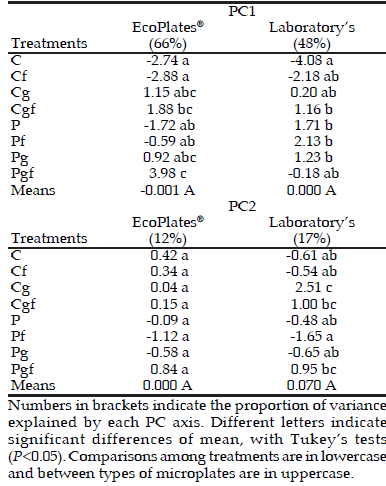
Table 4. Analysis of Principal Component (PC) values between C and A groups of treatments. Comparisons among treatments and mean values of Laboratory's microplates (at 72 h) and EcoPlates® (at 96 h).
Tabla 4. Análisis de los valores de Componentes Principales (PC) entre los grupos C y A. Las comparaciones entre tratamientos y valores medios de Laboratory's microplates (a las 72 h) y EcoPlates® (a las 96 h).

Diversity indexes also showed significant differences among treatments (P<0.05) whereas there were no differences (P<0.05) between types of microplates (Table 5). Despite significant differences were not found for both types of microplates, the discrimination between treatments of Laboratory's microplates was dissimilar to EcoPlates® discrimination. On the one hand, Laboratory's microplates showed significantly different diversity's index between microbial communities for C and Cf treatments. Also, both treatments showed significantly less diversity than the other treatments. On the other hand, EcoPlates® did not show these differences, neither C-P groups' nor C-A groups' comparisons (Table 5). In this case, EcoPlates® showed that microbial communities' diversity indexes from C and Cf treatments were lower than Pg, Pgf, Ag and Agf treatments.
Table 5. H index of each treatment and mean comparison between Laboratory's microplates (at 72 h) and EcoPlates® (at 96 h).
Tabla 5. Índice H de cada tratamiento y comparación de medias entre Laboratory's microplates (a las 72 h) y EcoPlates® (a las 96 h).

DISCUSSION
Although CLPP technique is very useful, it is necessary to keep in mind that the use of commercial microplates has some disadvantages. At this respect, Laboratory's microplates make it possible to know its precise composition and would allow changing the types or number of carbon sources used in each study, according to different criteria. Furthermore, they reduce costs of the technique because researchers can prepare them when they require and with the carbon sources they need; their components are less costly than commercial microplates; Laboratory's microplates allow the inoculation of four samples instead of three in EcoPlates®. Thus, if the number of samples is high, researchers will need significantly less number of microplates to complete each assay. However, in order to replace confidently the use of EcoPlates® by Laboratory's microplates, comparisons between results obtained with both types of microplates were necessary.
Some authors recommend standardizing absorbance values of each sample with its AWCD values before the analyses (Garland & Mills 1991; Garland 1996) to reduce any probable interference in results due to different densities of inocula. However, the AWCD standardization method reduces differentiation between treatments (Palmroth et al. 2005). Due to the fact that in this work it was not necessary, absorbance values were not standardized with the AWCD values.
The average absorbance values obtained in this assay with EcoPlates® were similar to other published results (Campbell et al. 1997; Dobler et al. 2001; Moynahan et al. 2002; Calbrix et al. 2005). Laboratory's microplates showed lower AAV than EcoPlates® (Figure 2) because both types of microplates have very different composition. Despite of this, Laboratory's microplates were able to show differences between treatments. Differences between AAV of both types of microplates are not explained by differences in incubation times, because comparison at each incubation time showed differences between both types of microplates (Table 2). Taking into account that this technique is based on development of cultivated-microorganisms, it is important to define both time and incubation conditions (Preston-Mafham et al. 2002; Calbrix et al. 2005). A literature review shows that it is chosen a particular incubation time to perform the statistical analyses without any justified explanation. In contrast, in this work, the applied methodology is an alternative in the way to bring a better use of CLPP technique.
In this work, different average absorbance values were due to differences in the level of use of each carbon source. This was demonstrated with the color development of mannitol, arginine and putrescine for each type of microplate (Figure 1). This analysis demonstrated that the behaviour of color development depended on both the type of microplate and the carbon source considered. Furthermore, other variability sources were a) the different number of carbon sources used per microplate type (23 in Laboratory's microplates vs. 31 in EcoPlates®), b) carbon source concentration (0.2% for Laboratory's microplates and unknown for EcoPlates®), c) redox dye indicator concentration (0.0025% for Laboratory's microplates and unknown for EcoPlates®), d) composition and concentration of the buffer medium (unknown for EcoPlates® ), e) different inocula (50 μl for Laboratory's microplates and 150 μl for EcoPlates®), f) other unknown ingredients contained in EcoPlates®, such as additives added for the drying process, which were probably included in order to adequate them for the commercialization process. All of these differences between both types of microplates may also affect the absorbance values recorded.
According to comparisons of PCA values, it was possible to demonstrate that both types of microplates were not significantly different. Despite Laboratory's microplates could explain 20% less of total variance of PC1 than commercial microplates, both types of microplates could show differences between treatments (Tables 3 and 4). However, differences in PCA values of each treatment were not the same in both types of microplates. We found two reasons to explain why differences between treatments were not the same in both types of microplates. First, total variance of PC1 explained by commercial microplates was 18% and 27% more than Laboratory's microplates, when C and P groups and C and A groups were compared, respectively. Despite this, both types of microplates showed differences between treatments (Table 3 and 4). However, Laboratory's microplates could separate in both comparisons (C-P groups and C-A groups) better than EcoPlates® when H indexes were analyzed (Table 5). Second, despite the differences in total variances of PC2 explained with both types of microplates were lower than PC1, differences between treatments were also observed (Table 3 and 4). However, in the C and A group's comparison, Laboratory's microplates could distinguish differences between treatments, while EcoPlates® could not find differences among them (Table 3).
All these results demonstrate that preparing microplates in the laboratory enabled us to detect differences among treatments as when using commercial microplates. In previous studies using Laboratory's microplates, authors could find differences between treatments with separations in PCA and/or differences in Shannon-Weaver's diversity index (Naiman et al. 2009; Semmartin et al. 2010; García de Salamone et al. 2010). Besides in order to distinguish differences between microbial communities, this work showed that Laboratory's microplates could exhibit higher level of sensitivity to detect differences between treatments than EcoPlates® when the Principal Components and Shannon-Weaver's diversity indexes were analyzed. Based on these results, we conclude that Laboratory's microplates can be used as a reliable and economical tool for studying the physiology of soil microbial communities of different environments or subjected to different treatments.
ACKNOWLEDGEMENTS: This work was partly supported by the projects PICT 08-15014/2004-2007 and FAUBARepsol YPF agreement, CO12-64/ 2005-2009 Authors would express special thanks to F. D'Auria for her revisions of this manuscript. We also grateful to the editor and anonymous reviewers for their comments and suggestions.
REFERENCES
1. ABRIL, A. 2003. ¿Son los microorganismos edáficos buenos indicadores de impacto productivo en los ecosistemas? Ecología Austral 13:195-204. [ Links ]
2. BAKKEN, LR. 1997. Culturable and nonculturable bacteria in soil. In: van Elsas, JD; JT Trevors & EMH Wellington (eds.). Modern Soil Microbiology. Marcel Dekker. [ Links ]
3. BAUDOIN, E; E BENIZRI & A GUCKERT. 2002. Impact of growth stage on the bacterial community structure along maize roots, as determined by metabolic and genetic fi ngerprinting. Appl. Soil Ecol. 19:135-145. [ Links ]
4. BAUDOIN, E; E BENIZRI & A GUCKERT. 2003. Impact of artificial root exudates on the bacterial community structure in bulk soil and maize rhizosphere. Soil Biol. and Biochem. 35:1183-1192. [ Links ]
5. BEARE, MH; RW PARMELEE; PF HENDRIX; W CHENG; DC COLEMAN; ET AL. 1992. Microbial and faunal interactions and effects on litter nitrogen and descomposition in agroecosystems. Ecological Monographs 62:569-591. [ Links ]
6. BENDING, GD; MK TURNER; F RAYNS; MC MARX & M WOOD. 2004. Microbial and biochemical soil quality indicators and their potential for differentiating areas under contrasting agricultural management regimes. Soil Biol. and Biochem. 36:1785-1792. [ Links ]
7. BOCHNER, BR & MA SAVAGEAU. 1977. Generalized indicator plate for genetic, metabolic, and taxonomic studies with microorganisms. Appl. Environ. Microbiol. 33:434-444. [ Links ]
8. BOSSIO, DA & KM SCOW. 1995. Impact of carbon and flooding on the metabolic diversity of microbial communities in soils. Appl. Environ. Microbiol. 61: 4043-4050. [ Links ]
9. BUCHER, AE & LE LANYON. 2005. Evaluating soil management with microbial community-level physiological profi les. Appl. Soil Ecol. 29:59-71. [ Links ]
10. CALBRIX, R; K LAVAL & S BARRAY. 2005. Analysis of the potential functional diversity of the bacterial community in soil: a reproducible procedure using sole-carbonsource utilization profi les. European J. of Soil Biol. 41: 11-20. [ Links ]
11. CAMPBELL, CD; SJ GRAYSTON & DJ HIRST. 1997. Use of rhizosphere carbon sources in sole carbon source tests to discriminate soil microbial communities. J. of Microbiol. Methods 30:33-41. [ Links ]
12. DI SALVO, LP; J ESCOBAR ORTEGA; S TORRI; MS ZUBILLAGA & IE GARCÍA DE SALAMONE. 2007. Influencia del raigrás y la fertilización sobre las comunidades microbianas de un suelo contaminado con fenantreno. Actas XI Congreso Argentino y Latinoamericano de Microbiología. Córdoba, Argentina. [ Links ]
13. DERRY, AM; WJ STADDON & JT TREVORS. 1998. Functional diversity and community structure of microorganisms in uncontaminated and creosote-contaminated soils as determined by sole-carbon-source-utilization. World J. of Microbiol. and Biotechnology 14:571-578. [ Links ]
14. DOBLER, R; P BURRI; K GRUIZ; H BRANDL & R BACHOFEN. 2001. Variability in microbial populations in soil higly polluted with heavy metals on the basis of substrate utilization pattern analysis. J. Soils and Sediments 1(3): 151-158. [ Links ]
15. DOBLER, R; M SANER & R BACHOFEN. 2000. Population changes of soil microbial communties indiced by hydrocarbon and heavy metal contamination. Bioremediaton J. 4:41-56. [ Links ]
16. ELLIS, RJ; P MORGAN; AJ WEIGHTMAN & JC FRY. 2003. Cultivation-dependent and independent approaches for determining bacterial diversity in heavy-metalcontaminated soil. Appl. and Environ. Microb. 69(6): 3223-3230. [ Links ]
17. ENGELEN, B; K MEINKEN; F VON WINTZINGERODE; H HEUER; HP MALKOMES; ET AL. 1998. Monitoring impact of a pesticide treatment on bacterial soil communities by metabolic and genetic fi ngerprinting in addition to conventional testing procedures. Appl. Environ. Microbiol. 64:2814- 2821. [ Links ]
18. GARCÍA DE SALAMONE, IE; LP DI SALVO; JS ESCOBAR ORTEGA; PMF BOA SORTE; S URQUIAGA; ET AL. 2010. Field response of rice paddy crop to Azospirillum inoculation: physiology of rhizosphere bacterial communities and the genetic diversity of endophytic bacteria in different parts of the plants. Plant and Soil 336:351-362. [ Links ]
19. GARLAND, JL. 1996. Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil. Biol. and Biochem. 28(2):213-221. [ Links ]
20. GARLAND, JL & AL MILLS. 1991. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbonsource utilization. Appl. and Environ. Microbiol. 57(8): 2351-2359. [ Links ]
21. GÓMEZ, E; J GARLAND & M CONTI. 2004. Reproducibility in the response of soil bacterial community-level physiological profi les from a land use intensifi cation gradient. Appl. Soil Ecol. 26:21-30. [ Links ]
22. HAACK, SK; H GARCHOW; MJ KLUG & L FORNEY. 1995. Analysis of factors affecting the accuracy, reproducibility, and interpretation of microbial community carbon source utilization patterns. Appl. Environ. Microbiol. 61(4): 1458-1468. [ Links ]
23. INSAM, H. 1997. A new set of substrates proposed for community characterization in environmental samples. In: Insam, H & A Rangger (eds.). Microbial communities: Functional versus structural approaches. Springer, Berlin. [ Links ]
24. KELLY, JJ & RLI TATE. 1998. Use of Biolog for the analysis of microbial communities form zinc-contaminated soils. J. Environ. Qual. 27:600-608. [ Links ]
25. LAWLOR, K; BP KNIGHT; VL BAROSA-JEFFERSON; PW LANE; AK LILLEY; ET AL. 2000. Comparison of methods to investigate microbial populations in soils under different agricultural management. FEMS Microbiol. Ecol. 33:129-137. [ Links ]
26. MOYNAHAN, OS; CA ZABINSKI & JE GANNON. 2002. Microbial community structure and carbon-utilization diversity in a mine tailings revegetation study. Restoration Ecology 10:77-87. [ Links ]
27. NAIMAN, AD; A LATRÓNICO & IE GARCÍA DE SALAMONE 2009. Inoculation of wheat with Azospirillum brasilense and Pseudomonas fluorescens: Impact on the production and culturable rhizosphere microfl ora. European J. of Soil Biol. 45:44-51. [ Links ]
28. NIELSEN, MN & AWINDING. 2002. Microorganisms as indicators of soil health. National Environmental Research Institute, Denmark. Technical Report No. 388. [ Links ]
29. PALMROTH, MRT; U MÜNSTER; J PICHTEL & JA PUHAKKA. 2005. Metabolic responses of microbiota to diesel fuel addition in vegetated soil. Biodegradation 16:91-101. [ Links ]
30. PRESTON-MAFHAM, J; L BODDY & PF RANDERSON. 2002. Analysis of microbial community functional diverstiy using sole-carbon-source utilisation profi les - a critique. FEMS Microbiol. Ecol. 42:1-14. [ Links ]
31. SCHNÜRER, J; M. CLARHOLM & ROSSWALL T. 1985. Microbial biomass and activity in an agricultural soil with different organic matter contents. Soil Biol. and Biochem. 17:611- 618. [ Links ]
32. SEMMARTIN, M; C DI BELLA & IE GARCÍA DE SALAMONE. 2010. Grazing-induced changes in plant species composition affect plant and soil properties of grassland mesocosms. Plant and Soil 328:471-481. [ Links ]
33. ZAK, HC; MR WILLIG; DL MOORHEAD & HG WILDMAN. 1994. Functional diversity of microbial communities: a quantitative approach. Soil Biol. and Biochem. 26: 1101-1108. [ Links ]













