Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Ecología austral
versión On-line ISSN 1667-782X
Ecol. austral vol.23 no.1 Córdoba ene./abr. 2013
COMUNICACIÓN BREVE
Does habitat specificity by frugivorous birds result in uneven seed rain within Bolivian mixed plantations?
Flavia A. Montaño-Centellas
Instituto de Ecología, Universidad Mayor de San Andrés, La Paz, Bolivia mail: flamontano@gmail.com
Editor asociado: Fernando Milesi
Recibido: 1 de junio de 2012,
Fin de arbitraje: 30 de julio,
Versión revisada: 24 de agosto,
Aceptado: 1 de octubre
ABSTRACT
Small-scale habitat specificity by frugivorous birds might affect their patterns of use of space and, consequently, may influence the range of habitats over which seeds are disseminated. These differences may translate into non-redundancy among bird assemblages using different habitats. I tested this hypothesis by capturing birds in two habitats within shaded mixed plantations in a Bolivian Andean forest (plantation interior and plantation edges with the forest matrix) and comparing the seeds found in their droppings. I found that, despite differences in bird assemblage composition and activity, the overall seed rain was similar among habitats: the seed rain in edges was only slightly richer in species than in the plantation interior, but species composition did not differ significantly between habitats. Although some seed species showed a biased distribution among habitats that might be partially explained by habitat specificity of frugivorours birds, the overall relative importance of seed species in the seed rain was similar for both habitats. These findings suggest that even when bird assemblages differ among habitats, from the plant community perspective these assemblages might be ecologically redundant.
Keywords: Agriculture; Dropping; Miconia; Ornitochory; Redundancy; Richness; Seed dispersa
RESUMEN
La especificidad de hábitat por aves frugívoras ¿resulta en diferentes lluvias de semillas dentro de cultivos tradicionales bolivianos?: En paisajes fragmentados, las aves frugívoras pueden mostrar especificidad de hábitat a pequeñas escalas pudiendo, por ejemplo, utilizar o evadir los bordes entre parches de cultivo y la matriz boscosa que los rodea. Estos patrones de uso podrían afectar el rango de hábitats en que estas aves depositan las semillas que dispersan, generando diferentes lluvias de semillas y resultando en ensambles de aves ecológicamente no redundantes. En este trabajo examiné esta hipótesis comparando la lluvia de semillas generada por aves en el interior y en el borde de plantaciones mixtas, en un paisaje en mosaico de los Andes bolivianos donde las plantaciones constituyen pequeños parches de hábitat rodeados por una matriz de bosque húmedo montano. Para esto, identifiqué las semillas en las fecas de aves capturadas con redes de niebla en ambos hábitats a lo largo de un año. Pese a las diferencias de actividad de aves entre hábitats, la diversidad de semillas fue similar entre el interior y el borde. Aunque la lluvia de semillas en borde fue ligeramente más rica en especies que en interior, la composición de especies no varió significativamente entre estos hábitats. Algunas especies de semillas mostraron una distribución sesgada (i.e., fueron depositadas más frecuentemente en uno de los dos hábitats) que puede ser parcialmente explicada por las diferencias en actividad de frugívoros. Sin embargo, la importancia relativa de las especies de plantas dentro de la lluvia de semillas en ambos hábitats fue similar. Estos resultados sugieren que los ensambles de aves pueden resultar ecológicamente redundantes aún si difieren entre hábitats cercanos.
Palabras clave: Agricultura; Fecas; Miconia; Ornitocoria; Redundancia; Riqueza; Dispersión de semilla
INTRODUCTION
Small scale fragmentation in the tropics often creates mosaic landscapes, where small shaded-agricultural patches are embedded in a forest matrix (Lambin et al. 2003; Perfecto & Vandermeer 2008). Specific habitats within agricultural patches might be differentially perceived by the local avifauna, and taxa may experience differential permeability across habitats (Ries et al. 2004; Tscharntke et al. 2008; Reino et al. 2009) depending on habitat configuration and structural complexity (Tscharntke et al. 2008; Martin et al. 2012).
Habitat specificity might affect the patterns of habitat use by birds (Rodewald & Yahner 2001; Cruz-Angón et al. 2008) and, consequently, modify the ecological services that they provide in these landscapes. In the Andean humid forest of Bolivia, agricultural patches are often scattered small shaded plantations composed of a mix of land covers and native vegetation. Even though plantation-forest edges are not sharp in this landscape, some bird species seem to skew their activity to either plantation interior or edge (Montaño- Centellas unpublished data). Among them, several frugivores such as Chiroxiphia boliviana, Euphonia xanthogaster, Chlorosphingus ophthalmicus and Mionectes striaticollis are more active in edges, whereas other species such as Anisognathus somptuosus, Eubucco versicolor, Zimmerius bolivianus and Tangara xanthocephala are more active in plantation interior. The activity and behavioral patterns of these frugivores may influence the range of habitats over which seeds are disseminated, hence potentially creating different seed rain in these habitats (Alcántara et al. 2000; Martínez et al. 2008). These differences may translate into non-redundancy of bird assemblages as seed dispersers (Loiselle et al. 2007). In this study, I analyzed the seeds in the droppings of birds captured in these mixed plantations to test this hypothesis. Specifically I examined if there are differences in the species richness and composition of the seed rain produced by birds, in the edges with the surrounding forest matrix and in the plantation interior, in a fragmented landscape of the Andean humid montane forests of Bolivia. I predicted the different bird assemblages in plantation interior and edges not to be redundant in their seed dispersing role (Loiselle et al. 2007): different seed species were expected to compose seed rain in each habitat. As described in other mosaic landscapes in the tropics (Cubiña & Aide 2001; de Melo et al. 2006; Martínez-Garza et al. 2009; Cole et al. 2010), I expected a biased seed rain towards edges: seed rain in edges should be richer and include more forest species, whereas seed rain in the interior of plantations should be dominated by pioneer species.
MATERIALS & METHODS
Study area
The study was conducted in three traditional mixed-plantations nearby the former Tunquini Biological Station (TBS; 67°52' W and 16°11' S; 1450 m.a.s.l.), a research station located in the Eastern-Andean humid montane forest of Nor Yungas Province, La Paz, Bolivia. Landscape at TBS is dominated by evergreen old-growth forest with small agricultural and young-growth patches resulting both from abandoned plantations and from natural slides and gaps (Paniagua-Zambrana et al. 2003; Arteaga 2007). Plantations were 0.5-1 ha, completely surrounded by forest and separated by at least 1 km from one another. They were 12-15 year-old and consisted of a dense mosaic of small coffee plantations (Coffea arabica) and orchards growing plantain (Musa acuminata) and fruits (Citrus spp.). Beside the planted Inga spp. trees, scattered shrubs of Baccharis spp. and Miconia spp., as well as Solanum spp., Pouroma spp., Vismia spp. Piper spp. and Alchornea triplinerva trees are found in the plantations, mostly in edges. Most forest species in the area have ripe fruits in the wet season (from September to March), whereas several early-growth species (Solanum spp., Piper spp., Miconia spp.) may produce fruits along the year though still less abundantly in the driest months (June and July) (Roldán & Larrea 2003; Loayza et al. 2006; Arteaga 2007).
Data collection
From April to October 2002 and February to April 2003, birds were captured monthly with 4-6 mistnets placed in two habitats within each plantation: the plantation interior (hereafter 'interior') and the plantation-forest edges (hereafter referred as 'edge'; ~1 m from the forest matrix). As cutting plants within plantations was not allowed, nets in interior were set haphazardly in any available area (i.e., open enough for a straight 12 m mistnet), avoiding edges and walking paths. When possible, nets were located in different places in consecutive months. Nets were operated for four consecutive days per month per plantation from sunrise until 17:00 h (non-stopping at noon) except with heavy rain. Netting effort was proportional to the extent of each habitat (~2.5:1 relation between interior and edge) comprising a total of 909.3 NH for edge and 2155.5 NH for interior (1 NH=one 12 m net opened for 1 h).
Fifty seven species composed the frugivore bird assemblage in the study area (Table 1), of which ten species were found only in edge and seven were only captured in interior. Captured frugivorous birds were kept in a cloth bag with a plastic dish at the bottom to collect droppings, and released after a maximum time of one hour. Seeds in each sample were carefully separated and identified to species level by comparing them with a reference collection at the National Herbarium of Bolivia. Droppings with fruit pulp only or unidentifiable seeds were discarded. I noted only the presence (or absence) of any given seed species in each fecal sample, and corrected the number of droppings containing each plant species by sampling effort to allow comparisons between habitats. A relative frequency for each seed species is used as relative abundance for calculations (Gorchov et al. 1995; Montaño-Centellas 2012). I assumed that capture sites of birds are likely to represent the habitats were seeds were potentially deposited (Loiselle & Blake 1993; Loiselle et al. 2007) therefore, here I considered the expected seed rain in each habitat as the sum of the seeds in all fecal samples collected on it.
Table 1. Bird species with a (at least partially) frugivorous diet captured in interior and edges of mixed plantations in a Bolivian Andean forest, and their contribution to the seed rain. The number of individuals captured in each habitat and the number of droppings containing identifiable seeds is presented.
Tabla 1. Especies de aves con dieta (al menos parcialmente) frugivora, capturadas en el interior y en los bordes de plantaciones mixtas de un bosque Andino boliviano, y su contribución a la lluvia de semillas. Se presenta el número de individuos capturados en cada hábitat y el número de heces fecales conteniendo semillas identificables.

Analyses
I followed Moreno & Halffter (2000) to assess the completeness of the seed rain in each habitat: I created smoothed sample-based species accumulation curves (1000 randomizations) performed with EcoSim v. 7 (Gotelli & Entsminger 2001), and fitted observed accumulation curves to the linear dependence model S=a/b(1-e-bx), where S is the seed species richness and x is the added sample number, to estimate (a/b), which is the expected species richness when the asymptote is reached. The seed rain in each habitat was described with (1) species richness and (2) species composition. Species richness was compared between habitats with sample-based rarefaction curves performed with EcoSim v. 7 (Gotelli& Entsminger 2001). I followed Pitman et al. (2001) to compare the relative importance of seed species between the seed rain of the two habitats. Initially, I used a Spearman's non-parametric correlation to test for the relationship between the occurrences of seeds in the seed rain of both habitats. Then, I used a Model II regression (major axis, MA) to calculate the slope of the line between edge and interior, to test for the null hypothesis that the two habitats are equivalent in terms of species composition. If the relative abundances of each seed species were the same in the two habitats, the slopes of the line should be equal to one. Finally, I used a nonparametric analysis of similarity (ANOSIM) ordination procedure with 999 permutations to explore the differences in species composition between edge and interior. For this, I separated the seeds from droppings collected in each plantation and used the seed rain in each habitat/plantation as response unit (N=3). The model II regression was performed with the lmodel2 package v. 1.7 (Legendre 2011) and the ANOSIM with the Vegan package v. 2.0-0 (Oksanen et al. 2011) within R (R Core Team 2009).
RESULTS
I collected 417 fecal samples, of which only 127 contained identifiable seeds: 51 were collected in edge and 76 in interior. A total of 23 plant species were identified in the seed rain potentially produced by birds (18 in edges and 17 in interior, Table 2). Observed accumulation curves fitted the linear dependence model (edge: R2=0.93, P<0.00001; interior: R2=0.97, P<0.001; uA). According to the asymptotes estimated by these models, I sampled 81% of the species richness in the seed rain of edge and 70% of interior. Rarefaction curves showed a greater expected species richness at the edge (18 species in edge and 14 species in plantation interior; cutoff at N=51; Figure 1B).
Table 2. Plant species, and their relative abundance, in the seed rain generated by birds in interior and edges of mixed plantations in a Bolivian Andean forest. The number of collected droppings containing seeds (sample size) is presented in parenthesis beside each habitat name.
Tabla 2. Abundancia relativa de las especies de plantas en la lluvia de semillas producida por aves, en el interior y el borde de plantaciones mixtas de un bosque Andino boliviano. El número de heces fecales colectadas que contenían semillas (tamaño muestral) se presenta en paréntesis junto a cada hábitat.
t

Figure 1. Seed species in the seed rain generated by birds in interior (open circles) and edges (black circles) of mixed plantations in a Bolivian Andean forest. (A) Observed accumulation curves showing seed species accumulation as a function of sample effort, and (B) sample-based rarefaction curves showing the expected seed species richness in the seed rain for different sample sizes. The cutoff line at N=51 (dashed line) shows higher species richness in edge. Linear dependence models in A correspond to the model S=a/b(1-e-bx).
Figura 1. Especies en la lluvia de semillas generada por aves en el interior (círculos abiertos) y en el borde (círculos negros) de plantaciones mixtas de un bosque montano boliviano. (A) Curvas de acumulación de especies observada en función del esfuerzo de muestreo, (B) curvas de rarefacción de riqueza de especies esperada en la lluvia de semillas para distintos tamaños muestrales. Más detalles en leyenda en inglés
There was no higher dissimilarity in the species composition between edge and interior seed rain than expected by chance (ANOSIM, R=0.11, P=0.31). There was a positive correlation between the occurrence of seeds in the seed rain of plantation interior and edge (rs=0.63; P<0.001). The habitats were equivalent in terms of plant species composition (i.e. the relative importance of the seed species within the seed rain of each habitat was similar): the slope of the regression (R2=0.64, P<0.01) between edge and interior did not differed from one (slope= 0.94; 95% CI=0.66-1.32; Figure 2). However, the deposition of some seed species was slightly biased towards one of the habitats. For example, M. calvescens and M. amabilis were more frequent in edge (33% of the samples) and interior (42%) respectively. A. triplinerva was frequently dispersed in edges but not in interior, whereas Leandra carassana was present only in edges (Figure 2).
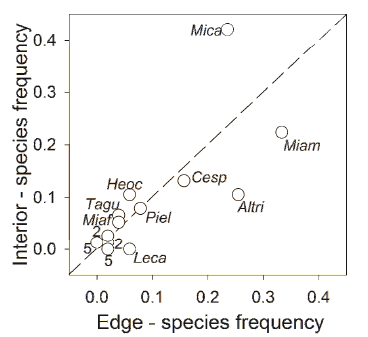
Figure 2. Proportion of fecal samples containing seeds of each plant species deposited on forest edges (x-axis) and plantation interior (y-axis), in mixed plantations in an Andean montane humid forest in Bolivia. The straight discontinuous line represents a 1:1 relationship between the two habitats. The slope of the actual MA regression is 0.94 (95% CI=0.66-1.32). Numbers adjacent to a point indicate the number of species represented by that point. Species codes are Mica: Miconia calvescens, Miam: Miconia amabilis, Miaf: Miconia affinis, Leca: Leandra carassana, Altri: Alchornea triplinervia, Cesp: Cecropia sp., Piel: Piper elongatum, Tagu: Tapirira guianensis.
Figura 2. Proporción de muestras fecales conteniendo semillas de cada especie vegetal, depositadas en bordes de bosque (eje x) y en interior (eje y) de las plantaciones mixtas de un bosque montano andino de Bolivia. Más detalles en leyenda en inglés
DISCUSSION
The dispersal of seeds by birds seems to result in a relatively uniform seed rain between the interior and edges of the traditional mixedspecies plantations at TBS. Although different bird species used plantation edges and interior, these assemblages were ecologically redundant: overall the same plant species were deposited into plantation interior and edges.
Seed rain was strongly dominated by few melastome species, a common component in the diet of understory tropical birds (Stiles & Rosselli 1993; Loiselle et al. 1996; Silva et al. 2002). Although one genus, Miconia, was the most frequent in the seed rain, different species were dominant in each habitat: M. calvescens strongly dominated the seed rain in plantation, whereas M. amabilis was dominant in that of edge. The dominance of few zoochorous species in seed rain is a common pattern in tropical areas (Loiselle et al. 2007; Cole et al. 2010). However, in agroecosystems these few species might define vegetation recovery and forest dynamics (Cubiña & Aide 2001), as agriculture often eliminates or at least strongly reduces seed banks. Thus, seed dispersal becomes the main source of forest species in land released to ecological succession (Wijdeven & Kuzee 2000; Cramer et al. 2008).
Contrary to expectations, the overall input of bird-disseminated seeds did not differ significantly between plantation interior and edges, though seed rain in edges was slightly richer, more equitative and included more forests species than seed rain in interior. McDonnell & Stiles (1983) suggested that the input of bird-disseminated seeds is positively correlated with structural complexity in old field vegetation. Larger numbers of pioneers and late successional species in (and nearby) edges will attract fruit-eating birds and offer perching sites, and consequently increase the chances of more seed species to be deposited in them (Harvey 2000; Cubiña & Aide 2001; Pejchar et al. 2008; Martínez-Garza et al. 2009; Cole et al. 2010). Structural complexity at TBS plantations is increased by frequent native trees of the genera Inga, Alchornea, Pourouma, Piper and Vismia and native shrubs of the genera Solanum and Miconia. Although most of these elements are concentrated in edges, their presence in plantation interior might create a 'soft edge' between forest and traditional plantations and reduce edge effects on birds (Reino et al. 2009). For instance, Pizo & dos Santos (2011) found that fruiting trees in different habitats within an agricultural landscape are visited by frugivorous birds (regardless of frugivore identity) in similar rates. A similar pattern may occur at TBS, and in consequence, the structurally complex plantation interior would support a different yet redundant frugivore assemblage.
The similarity in bird-disseminated seeds between habitats (redundancy) might also highlight the importance of traditional agriculture practices to sustain ecosystem functional assemblages. Furthermore, as suggested by Pejchar et al. (2008) in such human-dominated landscapes where fruiteating birds feed broadly, we would expect frugivore abundances (activity patterns) rather than frugivore diversity to drive seed dispersal. Although not statistically significant, differences in seed rain between plantation edge and interior might partially be explained by differences in the frugivore assemblage using these habitats. For example, Alchornea triplinervia (Euphorbiaceae), the second most important component of the seed rain in edges, was found in droppings of Mionectes striaticollis, Buarremon torquatus and Chiroxiphia boliviana, frugivores that seem to prefer plantation edges. Similarly, the higher (yet low) frequency of Hebanthe occidentalis in plantation interior was due to the occasional dispersal of this seed species by frugivores such as Tangara xanthocelaphala and Eubucco versicolor, which were more frequent in plantation interior. Nevertheless, from the plant community perspective, these differences in habitat specificity by frugivorous birds seem not to be sufficient to create differences in the input of seeds.
ACKNOWLEDGEMENTS: I would like to acknowledge the staff of the former Tunquini Biological Station, M. Villegas, J. Urrelo and R. Gutierrez for their help on the field and the owners of plantations for allowing me to work on their properties; J. Tordoya from the National Herbarium (LBP) helped with seed identification. Financial support was provided by Instituto de Ecología (UMSA) and Sigma Xi.
REFERENCES
1. ALCÁNTARA, JM; PJ REY; F VALERA & AM SANCHEZ-LAFUENTE. 2000. Factors shaping the seedfall pattern of a bird dispersed plant. Ecology, 81:1937-1950. [ Links ]
2. ARTEAGA, L. 2007. Fenología y producción de semillas de especies arbóreas maderables en un bosque húmedo montano de Bolivia (PN ANMI Cotapata). Rev. Boliv. Ecol. Conserv. Amb., 21:57-68. [ Links ]
3. COLE, RJ; KD HOLL & RA ZAHAWI. 2010. Seed rain under tree islands planted to restore degraded lands in a tropical agricultural landscape. Ecol. Appl., 20:1255-1269. [ Links ]
4. CRAMER, VA; RJ HOBBS & RJ STANDISH. 2008. What's new about old fields? Land abandonment and ecosystem assembly. Trends Ecol. Evol., 23:104-112. [ Links ]
5. CRUZ-ANGÓN, A; TS SILLETT & R GREENBERG. 2008. An experimental study of habitat selection by birds in a coffee plantation. Ecology, 89:921-927. [ Links ]
6. CUBIÑA, A & MT AIDE. 2001. The effect of distance from forest edge on seed rain and soil seed bank in a tropical pasture. Biotropica, 33:260-267. [ Links ]
7. GORCHOV, DL; F CORNEJO; C ASCORRA & M JARAMILLO. 1995. Dietary overlap between frugivorous birds and bats in the Peruvian Amazon. Oikos, 74:235-250. [ Links ]
8. GOTELLI, NJ & GL ENTSMINGER. 2001. EcoSim: Null models software for ecology. Version 7.0. Acquired Intelligence Inc. & Kesey-Bear. http://homepages.together.net/ ~gentsmin/ecosim.htm. [ Links ]
9. HARVEY, CA. 2000. Windbreaks enhance seed dispersal into agricultural landscapes in Monteverde, Costa Rica. Ecol. Appl., 10:155-173. [ Links ]
10. LAMBIN, EF; HJ GEIST & E LEPERS. 2003. Dynamics of landuse and land-cover change in tropical regions. Annu. Rev. Environ. Resour., 28:205-241. [ Links ]
11. LEGENDRE, P. 2011. Package lmodel2: Model II regression. Version 1.7-0. http://www.R-project.org. [ Links ]
12. LOAYZA, AP; RS RIOS & DM LARREA. 2006. Disponibilidad de recurso y dieta de murciélagos frugívoros en la Estación Biológica Tunquini, Bolivia. Ecol. Boliv., 41:7-23. [ Links ]
13. LOISELLE, BA & JG BLAKE. 1993. Spatial dynamics of understory avian frugivores and fruiting plants in lowland wet tropical forest. Vegetatio, 107/108:177-189. [ Links ]
14. LOISELLE, BA; PG BLENDINGER; JG BLAKE & TB RYDER. 2007. Ecological redundancy in seed dispersal system: a comparison between Manakins (Aves: Pipridae) in two tropical forests. Pp. 178-195 in: Dennis, JA; RJ Green & DA Westcott (eds.). Seed dispersal: theory and its application in a changing world. CAB International, Oxford, UK. [ Links ]
15. LOISELLE, BA; E RIBBENS & O VARGAS. 1996. Spatial and temporal variation in seed rain in a tropical lowland wet forest. Biotropica, 28:82-95. [ Links ]
16. MARTIN, EA; M VIANO; L RATSIMISETRA; F LALOË & SM CARRIÈRE. 2012. Maintenance of bird functional diversity in a traditional agroecosystem of Madagascar. Agr. Ecosyst. Environ., 149:1-9. [ Links ]
17. MARTÍNEZ, I; D GARCÍA & JR OBESO. 2008. Differential seed dispersal patterns generated by a common assemblage of vertebrate frugivores in three fleshy-fruited trees. Ecoscience, 15:189-199. [ Links ]
18. MARTÍNEZ-GARZA, C; A FLORES-PALACIOS; D DE LA PEÑA & H HOWE. 2009. Seed rain in a tropical agricultural landscape. J. Trop. Ecol., 25:541-550. [ Links ]
19. MCDONNELL, MJ & EW STILES. 1983. The structural complexity of old field vegetation and the recruitment of bird-dispersed plant species. Oecologia, 56:109-116. [ Links ]
20. DE MELO, FPL. 2006. Biased seed rain in forest edges: evidence from the Brazilian Atlantic forest. Biol. Conserv., 132:50-60. [ Links ]
21. MONTAÑO-CENTELLAS, FA. 2012. Are males and females of Yungas Manakin (Chiroxiphia boliviana) ecologically redundant as seed dispersers? Ornitol. Neotrop., 23: 185-192. [ Links ]
22. MORENO, CE & G HALFFTER. 2000. Assessing the completeness of bat biodiversity inventories using species accumulation curves. J. Appl. Ecol., 37:149-158. [ Links ]
23. OKSANEN, J; FG BLANCHET; R KINDT; P LEGENDRE; PR MINCHIN; ET AL. 2011. Vegan: Community Ecology Package. Version 2.0-0. http://www.R-project.org. [ Links ]
24. PANIAGUA-ZAMBRANA, N; C MALDONADO-GOYZUETA & C CHUMACERO-MOSCOSO. 2003. Mapa de vegetación de los alrededores de la Estación Biológica Tunquini, Bolivia. Ecol. Boliv., 38:15-26. [ Links ]
25. PEJCHAR, L; RM PRINGLE; J RANGANATHAN; JR ZOOK; G DURAN; ET AL. 2008. Birds as agents of seed dispersal in a human-dominated landscape in southern Costa Rica. Biol. Conserv., 141:536-544. [ Links ]
26. PERFECTO, I & J VANDERMEER. 2008. Biodiversity conservation in tropical agroecosystems: A new conservation paradigm. Ann. N.Y. Acad. Sci., 1134:173-200. [ Links ]
27. PITMAN, NCA; JW TERBORGH; MR SILMAN; VP NUÑEZ; DA NEILL ET AL. 2001. Dominance and distribution of tree species in upper Amazonian terra firme forests. Ecology, 82:2101-2117. [ Links ]
28. PIZO, MA & BTP DOS SANTOS. 2011. Frugivory, post-feeding flights of frugivorous birds and the movement of seeds in a Brazilian fragmented landscape. Biotropica, 43: 335-342. [ Links ]
29. R CORE TEAM. 2009. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.Rproject. org [ Links ]
30. REINO, L; P BEJA; PE OSBORNE; R MORGADO; A FABIÃO; ET AL. 2009. Distance to edges, edge contrast and landscape fragmentation: interactions affecting farmland birds around forest plantations. Biol. Conserv., 142:824-838. [ Links ]
31. RIES, L; RJJ FLETCHER; J BATTIN & TD SISK. 2004. Ecological responses to habitat edges: mechanisms, models, and variability explained. Annu. Rev. Ecol. Syst., 35:491- 522. [ Links ]
32. RODEWALD, AD & RH YAHNER. 2001. Influence of landscape composition on avian community structure and associated mechanisms. Ecology, 82:3493-3504. [ Links ]
33. ROLDÁN, AI & DM LARREA. 2003. Fenología de 14 especies arbóreas y zoócoras de un bosque yungueño en Bolivia. Ecol. Boliv., 38:125-140. [ Links ]
34. SILVA, WR; P DE MARCO; E HASUI & VSM GOMES. 2002. Patterns of fruit-frugivore interactions in two Atlantic forest bird communities of southeastern Brazil: implications for conservation. Pp. 423-435 in: Levey, DJ; WR Silva & M Galetti (eds.). Seed dispersal and frugivory: ecology, evolution and conservation. CAB International, Wallingford. [ Links ]
35. STILES, FG & L ROSSELLI. 1993. Consumption of fruits of the Melastomataceae by birds: how diffuse is coevolution? Vegetatio, 107/108:57-73. [ Links ]
36. TSCHARNTKE, T; CH SEKERCIOÐLU; TV DIETSCH; NS SODHI; P HOEHN; ET AL. 2008. Landscape constraints on functional diversity of birds and insects in tropical agroecosystems. Ecology, 89:944-951. [ Links ]
37. WIJDEVEN, SMJ & ME KUZEE. 2000. Seed availability as a limiting factor in forest recovery processes in Costa Rica. Rest. Ecol., 8:414-424. [ Links ]














