Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Ecología austral
versión On-line ISSN 1667-782X
Ecol. austral vol.25 no.1 Córdoba abr. 2015
ARTÍCULO ORIGINAL
Saline waters and macroinvertebrates in subtropical Andean streams
Ana Lucía González Achem1,2, *; María Laura Rolandi1 & Hugo Rafael Fernández1,2
1. Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán.
2. Instituto de Biodiversidad Neotropical, CONICET, Argentina.
* anagonzalezachem@gmail.com
Editora asociada: María Diéguez
Recibido: 13 de junio de 2014; Fin de arbitraje: 1 de agosto; Última versión: 16 de octubre; Aceptado: 25 de octubre.
Abstract.
Salinity of rivers is an expression of the concentration of salts dissolved in the water body. Secondary salinization is the increase in salinity of a water body as a result of human activity. Salinity may restrict the species composition of aquatic biota to tolerant species. In this study we analyzed benthic communities and their relationship with physicochemical variables in natural pristine systems and impacted environments. We collected benthic community samples using a D-net and water samples for laboratory major ions analysis. We noted a critical limit of electrical conductivity above 800µS/cm breaking up taxa pools of benthic macroinvertebrates. We observed that taxonomic richness is a sensitive indicator to detect effects of secondary salinization. We hypothesized that the monsoonal precipitation regime of the studied region is an important driver of environmental conditions for the benthic fauna since it regulates the concentration of major ions. The evidence collected shows that seasonal rainfall may affect differentially the benthic communities of Andean streams, improving the equitability below 800µS/cm in saline sites while changing the community structure from equitable to one dominated by a certain taxa in less saline locations.
Keywords: South America; Secondary salinization; Benthic community; Seasonal rainfall; Salt mining
Resumen.
Aguas Salinas y macroinvertebrados en arroyos subtropicales andinos: La salinidad de los ríos es una expresión de la concentración de sales solubles disueltas en la masa de agua. Salinización secundaria es el aumento de la salinidad de un cuerpo de agua como resultado de las actividades humanas. La salinidad puede afectar la composición de la biota acuática, restringiendo las comunidades a la presencia de especies tolerantes. En este estudio se analizó la relación entre las comunidades bentónicas y variables fisicoquímicas en sistemas naturales prístinos y ambientes con impacto antrópico. Se colectaron muestras de la comunidad bentónica con Red D y muestras de aguas para el análisis de los iones mayoritarios en el laboratorio. Los resultados señalan que existe un límite crítico de conductividad eléctrica, por encima de 800µS/cm, a partir del cual se produce un cambio de los taxones de macroinvertebrados bentónicos en los arroyos. La riqueza taxonómica resultó un indicador sensible para detectar los efectos de la salinización secundaria. El régimen monzónico de precipitaciones en la región ejercería una influencia importante en las condiciones del hábitat para la fauna bentónica debido a que controla la concentración de los iones may ores. En este sentido, los resultados obtenidos indican que las lluvias afectaron diferencialmente a las comunidades de macroinvertebrados de los arroyos andinos, mejorando su equitativita por debajo de 800µS/cm en sitios de elevada salinidad pero cambiando la estructura de la comunidad de equitativa a dominada por un taxa, en sitios con menor salinidad.
Palabras clave: Sudamérica; Salinización secundaria; Comunidad bentónica; Lluvias estacionales; Minería de sales
Introduction
Surface waters can be classified according to their salt content. The salinity of a wetland is an expression of the concentration of soluble salts dissolved in the water body. Salinity can be stated as parts per million (ppm), milligrams of salt per liter (mg/L) or measured by an indirect metric, such as the electrical conductivity (EC= S/cm). There are several classifications that sort out waters as function of their electrical conductivity values, such as the one proposed by Rodier (1989), the Median Range Guidelines for Surface Water Conductivity (Department of Environmental and Heritage and Department of Natural Resources 1999), and others (Cañedo-Argüelles et al. 2013).
A saline water body is considered to be the result of both natural factors (e.g; lithology of the substrate through which it flows) and human factors. Natural salinization of water bodies involves the accumulation of salts from rainwater and from terrestrial sources at rates unaffected by human activities; and it is restricted to endorheic basins in semi-arid and arid regions of the world (Williams 1999). In addition, superficial waters that are fed by groundwater or rivers with limited drainage, and/or experiencing water loss due to evapotranspiration, generally have saline waters (Williams 2001). A particular case of salinization involves extended periods of drought in regions affected by marked rainfall seasonality.
Secondary salinization is the increase in the salinity of a water body as a result of human activities (Williams 1999). In this process, catchment changes and other anthropogenic disturbances to hydrological cycles increase salt loads to water bodies: fresh waters become saline and saline waters become even more saline (Williams 2001). Recently, Cañedo-Argüelles et al. (2013) have drawn attention to the growing threat of secondary salinization in rivers and streams worldwide.
Several authors have pointed out that changes in species richness along salinity gradients are negligible even when the water bodies have a different community composition (Williams et al. 1990; Metzeling 1993; Kefford 1998). Therefore, taxonomic richness may not be an accurate proxy of secondary salinization, due to the fact that sensitive species are simply replaced by more tolerant ones, ultimately reaching a similar species number (Horrigan et al. 2005).
In South America, the three most important basins draining to the Atlantic Ocean are the Amazon, Orinoco and Paraná rivers. These basins have high discharges and low electrical conductivity values throughout the year (Neiff 1996). There are also numerous endorheic basins in regions of the pre-cordillera and cordillera of Bolivia, Peru, Chile and Argentina, many of them geographically related to salt flats and salt pond systems (Sylvestre et al. 2001; Alonso et al. 2006).
In Argentina, inland saline aquatic ecosystems are well represented by arheic systems located in a treeless windswept high tableland region (Puna plateau), and by streams included in more than 20 endorheic basins distributed in the northwest and central west of the country (Iriondo 1989).
Salinity affects aquatic biota, thereby limiting the composition of the biota driving it towards the prevalence of tolerant species. Most invertebrates have internal ionic concentrations 1000-1500 mg/L (Hart 1991). They can passively maintain constant internal ionic concentrations in freshwater and thus, while salinity increases so does the capture of ions from the medium (Beadle 1969). It is accepted that freshwater ecosystems experience very little ecological stress below 1500 µS/cm of electrical conductivity (EC) (Hart et al. 1991). However, Horrigan et al. (2005) and González Achem (2012) have noted that there is a range of EC between the 800-1000 µS/cm in which the most significant switch occurs between groups of salinity tolerant and non-tolerant macroinvertebrates. An increase in the EC gradient in natural environments may decrease the probability of occurrence of some macroinvertebrate groups such as the Tipulidae (Diptera) and the Leptophlebiidae (Ephemeroptera), whereas other groups such as Copepoda (Crustacea) and Hydraenidae (Coleoptera) may show an increasing trend (Horrigan et al. 2005). In search of a metric to synthesize the response of organisms to variations in salts within the system, these authors suggested the use of a Salinity Index (SI) based on a scale SSS (salinity sensitive score) that indicates the tolerance to salt concentration of a given taxon, thereby measuring the change in the macroinvertebrate community produced by salinity. The tolerance to salinity of some species has been evaluated in several studies in relation with ionic composition (Bayly 1972; Williams 1998; Sylvestre et al. 2001). In this line, Kefford et al. (2004) emphasized the greater toxicity of anion sulfate, and Bayly (1969) suggested that monovalent cations (Na+ and K+) are more toxic than the divalent ones (Ca++).
Due to the various forms of human impact on ecosystems (irrigation channels from water courses, solid waste disposal and saline solutions), secondary salinization can affect aquatic systems in multiple ways. The impact may involve direct toxic effects, changes in chemical processes and the consequent habitat loss in water bodies, riparian areas and adjacent floodplains, as well as the decrease in biodiversity and productivity (Williams 1999).
Secondary salinization is the major cause of degradation in Australian freshwater (James et al. 2003). Because of this, most of the available literature comes from this region. There are also records of streams subjected to this process in Central Asia, USA, Europe and South Africa (Cañedo-Argüelles et al. 2013). Although it has been stated that anthropogenic salinization is significant in some regions of South America due to the aridity and the growth of human populations (Williams 1987; 2001), macroinvertebrate responses to salinity in inland streams have received little attention in South America. At present, in Argentina, the main causes of secondary salinization are agriculture and mining. The aim of this work was to study the benthic macroinvertebrate community and its relation to physicochemical variables in saline streams at the headwaters of the Salí River, an endorheic basin in Tucumán province (Argentina). We hypothesized that: (i) sites with higher salinity will have relatively similar macroinvertebrate community composition than less saline sites; (ii) the precipitation is the main driver of environmental conditions for macroinvertebrates in saline waters because of its dilution effect.
Materials and Methods
Study system
The Sail River Basin is the main hydrological basin of Tucumán province, covering 10000 km2, almost 45% of the total area of the province. The Sail River is the main course of an endorheic system, which drains to a saline lake named Mar Chiquita located in Córdoba province (Martinez 1995). The Sail Rver basin integrates twelve sub-catchments. We selected two saline sub-catchments differing in the origin of the salt, due to the particular lithology in the case of the Lules River or to the contribution of anthropogenic activity in the Calera River sub-catchment (Figure 1). Lules Rver sub-catchment presents mostly mountain forest typical of the Yungas, characterized by large-sized tree species (average height ~30 m). The waterways traverse the geological formations La Yesera, Rio Loro and R'o Sail (Bossi et al. 1998). Agriculture and livestock have been identified as the most important activities (Grau & Brown 2000; Quiroga et al. 2011). Calera River sub-catchment is part of the Chaco forest, characterized by species of comparatively smaller size trees (average height ~15 m) with lower water requirements. The waterways traverse the geological formation Río Sail. The main economic activities are agricultural, logging and livestock.

Figure 1. Map of tributaries of Salí River (Lules River sub-catchment and Calera River sub-catchment). Arrows show the sampled sites. Pt: Potrerillo Stream; SJ: San Javier River; LP1: La Perdiz stream upstream of the salt factory; LP2: La Perdiz stream downstream of the salt factory.
Figure 1. Mapa de tributarios del Río Sali (subcuenca del Río Lules y subcuenca del Río Calera). Las flechas muestran los sitios de muestreo. Pt: Arroyo Potrerillo; SJ: Río San Javier; LP1: Arroyo La Perdiz aguas arriba de la industria salinera; LP2: Arroyo La Perdiz aguas debajo de la industria salinera.
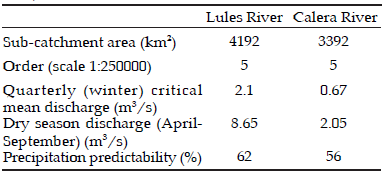
Tucumán province has a tropical climate with high temperatures prevailing during most the year. The rainfall concentrates in summer (90% from November to March), and the region endures a marked water deficit period (dry season) extending from winter to spring (June to November) (Brown & Malizia 2004). The total annual rainfall in the mountain areas of the province ranges between 1000 and 2000 mm (Table 1).
Table 1. Morphometric variables, discharge and precipitation of the studied sub-catchments (Modified from Fernández 2003).
Tabla 1. Variables morfométricas, caudal y precipitaciones de las sub-cuencas esrudiadas (Modificado de Fernández 2003)
Field and laboratory work
Samples were collected in June, August and October of 2010 using a paired difference design (Kefford 1998). We selected four sites, two for each sub-catchment, and located 200 m apart from each other. They were coded as follows: Pt for Potrerillo stream; SJ for San Javier River; LPlfor La Perdíz stream (upstream of a salt factory); and LP2 for La Perdíz stream (downstream of the salt factory). In both sub-catchments, the location with the lower EC was considered as the reference site: SJ for Lules River sub- catchment and LP1 for Calera sub-catchment, whereas Pt and LP2 were considered contrasting sites (Table 2).
Table 2. Main characteristics of the sampled sites.
Tabla 2. Principales características de los sitios muestreados.

At each site, we determined in situ, water temperature, EC, total alkalinity, flow velocity, width and depth of the river segment. We collected and preserved water samples for chemical analysis, using validated and quality controlled methodology according to IRAM (Instituto Argentino de Normalización y Certificación 2012). Benthos duplicated samples were collected on each site with D-net of 200 µm mesh, on transects perpendicular to the river and along it, from shore to shore. The width measured at the 4 sampling points resulted similar (width range = 1- 2.5 m). Samples were preserved with ethanol 76%.
In the laboratory, we performed several chemical analyses to the water samples including the determination of the major ions concentrations following the standard methodology (APHA 1998; Rodier 1989). We calculated discharge values from flow velocity data (Global Flow Probe; accuracy 0.03 meters per second), and the width and depth of the river. We separated organisms from the benthic samples and identified them to the lowest possible taxonomic category. The identification was based on general and regional keys (Lopretto & Tell 1995; Domínguez & Fernández 2009).
Data analysis
We organized the collected data in two matrices: a matrix of taxa abundance per sample (2 samples per site, 3 sampling dates, 4 sites), and a matrix of physicochemical variables.
The software Rockworks 15 was used for the analysis of chemical data. Piper diagrams were used to summarize the ionic composition of the different sampling points and to rank them depending on their predominant water soluble salt. To classify the sites according to their salinity levels, we used the EC values and applied the classification by Rodier (1989): < 250 µS/cm: non saline waters; 250 µS/cm - < 750 µS/cm: medium salinity waters; 750 µS/cm - < 2250 µS/cm: strong salinity waters; 2250 µS/cm - < 5000 µS/cm: very strong salinity waters; 5000 µS/cm - 20000 µS/cm: waters with excessive salinity. This classification, which is used for irrigation standards, was applied here because its range of values fit suitably to the water characteristics, and allowed the separation of the samples based on their electrical conductivity values. To evaluate the dilution effect of rainfall on the salt loading in streams, we analyzed the relationship between EC and discharge on each site at a sub-catchment scale.
In order to interpret the biological records we compared macroinvertebrate abundances between reference and comparison sites using paired-sample t- test and Richness (S) was compared using the Wilcoxon matched pairs test (Zar 1999). The tests were performed using the R platform (R Core Team 2012). Biological structure of communities was compared using S and Simpson index (D) and taxa importance curves per site and sampling date. To facilitate the interpretation of graphs, we calculated the corrected Simpson index (D '=1-D), according to which higher values correspond to higher community diversity (Magurran 1988). The effect of increasing rainfall on community abundance and composition(from June to October)was calculated applying the proportional similarity index (Ludwig & Reynolds 1988) between the pairs of sampling sites SJ-Pt, LP1-LP2 and Pt-LP2.Rainfall data was obtained from the experimental station "Estación Experimental Agroindustrial Obispo Colombres".
A Canonical Correspondence Analysis (CCA) was performed using the statistical program CANOCO version 4.0 (Ter Braak & Smilauer 1998) in order to evaluate the ordination of macroinvertebrate communities of each site based on physicochemical parameters. A total of 14physicochemical variables measured were considered in the analysis: bicarbonate (HCO3-), chloride (Cl-), sulfate (SO4=), calcium (Ca++), magnesium (Mg++), sodium (Na+), potassium (K+), nitrite (NO2-), nitrate (NO3-), phosphate (PO43-), Electrical conductivity (EC), Water temperature (T), pH and Discharge rate (Q). The Monte Carlo permutation test was applied, with all canonical axes resulting insignificant and a total of 499 permutations.
Results
Physicochemical water characterization
The Piper diagram (Figure 2) shows that the predominant soluble salt in SJ was calcium bicarbonate. Pt stream water was found to be calcic sulfated in June, varying to calcic bicarbonated in August and October. LP1 presented calcic sulfated waters and after passing through the salt factory (LP2), sodium chloride was the predominant soluble salt. San Javier River and Potrerillo stream flow through the geological formations La Yesera, Río Salí and Río Loro. These rocks provide calcite (calcium carbonate), gypsum (calcium sulfate dihydrate) and several oxidized metals (Fernández & Hidalgo 2011).

Figure 2. Piper Diagram of the chemical composition of major ions at each sampling point and date. SeeTable 2 for sites codes.
Figure 2. Diagrama de Pipar de la composición química de iones mayoritarios para cada sitio de muestreo y fecha. Ver tabla 2 para los códigos de los sitios.
Table 3 shows the EC values for each sample, which allowed us to sort the sites based on Rodier (1989) classification system. SJ river samples were always at the medium salinity level, while Pt, LP1 and LP2 varied between strong, very strong and excessive salinity conditions. The main discharges for all sites corresponded to August and October.
Table 3. Measured values of Discharge and Electrical Conductivity (EC) during the study. Salinity classification of the samples according to Rodier (1989).
Tabla 3. Valores de caudal y conductividad eléctrica (EC) medidos durante el estudio. La clasificación de salinidad de las aguas realizada de acuerdo a Rodier (1989).

EC rate of comparison to reference sites was > 2 in June declining to < 2 in two other sampling dates. A regression line fitted to EC versus discharge on each sub-catchment showed an inverse relationship for SJ-Pt (b= -3869, n= 6, r2= 0.76, F 1,4 = 12.7, P= 0.024). In the case of LP1-LP2EC and discharge were not related (P> 0.05).
Composition and structure of benthic communities
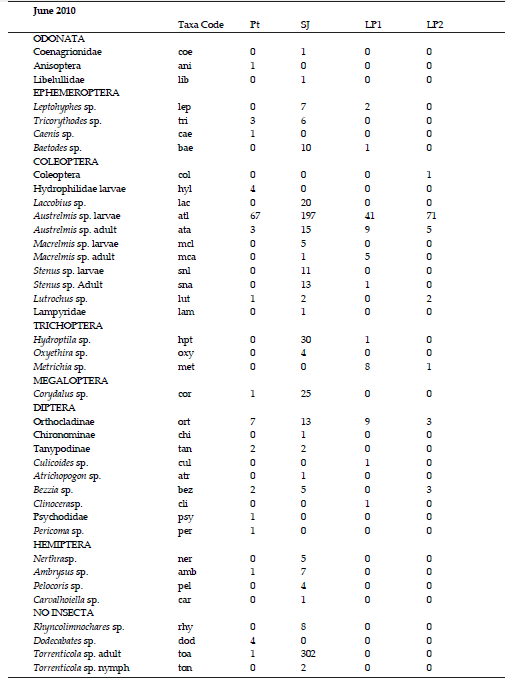
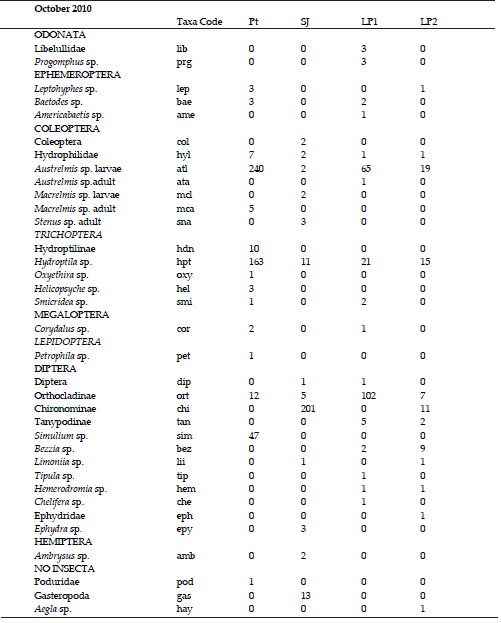
A total of 5265 individuals belonging to 76 different taxa were counted and classified (Appendix, Supplementary Information).The highest richness corresponded to Pt stream in August (S = 36 taxa), and the lowest richness was recorded in LP2 (S= 8 taxa) in June (Figure 3). In the drier sampling dates (June and August), LP2 was the site with the highest values of the corrected Simpson index (D´). When it started raining in October, SJ River had the maximum D´. The curve to the right in Fig. 3 shows a typical pluviometric profile of the region, with rainfall concentrated in the summer. Nonetheless, the amount of precipitation during that year was lower than the corresponding to the regional average. Similarity percentage between pairs of selected sites changed markedly with the increasing precipitation in October, decreasing in all the three cases (Table 4).


Figure 3. Panel A: Temporal variation of taxa richness (S) and corrected Simpson diversity (D') values per site and month. Panel B: temporal variation of electrical conductivity (EC) per site along the study superimposed with a rainfall curve for the year 2010.SeeTable 2 for sites codes.
Figure 3. Panel A: Variación temporal de la Riqueza de taxa (S) y el Índice de Simpson corregido (D´) por sitio y por mes. Panel B: variación temporal de la conductividad eléctrica (EC) por sitio a lo largo del estudio, superpuesta con una curva de precipitaciones para el año 2010. Ver tabla 2 para los códigos de los sitios.
Table 4. Percentage of similarity for pair of selected sites and month. Sites coded as in Table 2.
Tabla 4. Porcentaje de similitud para los pares de sitios seleccionados por mes. Los sitios se encuentran codificados como en la tabla 2.

Richness was similar in SJ and Pt sites (z= 1.13, P= 0.25), while differed significantly between LP1 and LP2 (z= 2.43, P= 0.01).The abundance of macroinvertebrates was similar between SJ and Pt sites (t-test= 0.02, P= 0.98) and between LP1 and LP2 (t-test= 1.97, P= 0.05). The regression analysis applied to study the relationship between richness and EC showed a significant negative trend (b= -0.0037, n= 12, r2= 0.39, F1, 10= 6.4, P= 0.030).
Taxa importance curves (Figure 4) showed the presence of dominant taxa in the communities of Potrerillo and La Perdíz streams, a condition also revealed by the Simpson index values. Austrelmis sp. larvae (Coleoptera) was the dominant taxa in most saline sites (Pt, LP1, LP2) in June and August samplings. San Javier River showed more equitable communities in June and August; the dominant taxon (81%) in October was Dicrotendipes sp. (Chironominae, Diptera).

Figure 4. Taxa importance curves of the studied sites. Only some of the genera codes are shown. See Table 2 for sites codes and appendix for taxa codes and the complete list of genera for site.
Figure 4. Curvas de importancia de taxa de los sitios estudiados. Solo algunos de los códigos de géneros pueden ser observados en la gráfica. Ver tabla 2 para los códigos de los sitios y Anexos para los códigos de los taxa y el listado completo de géneros por sitio.
In the Canonical Correspondence Analysis (Figure 5), the axis I (? = 0.66) explained 33.5% of the total variation, and the axis II (? = 0.43) explained 22%. The axis I represents a gradient along which the communities of each sample ordered from right to left according to discharge values (Table 3). Alkalinity, sodium, chloride, sulfate, calcium, nitrite and nitrate further contributed to the ordination along this axis. Axis II allows the communities to be ordered according to the electrical conductivity from top to bottom (Table 3).
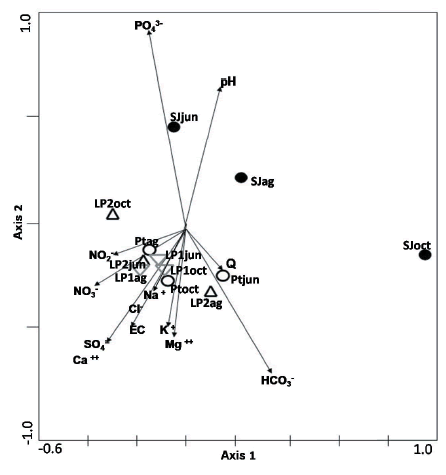
Figure 5. Canonical Correspondence Analysis plot showing association between each physicochemical variable and sampled communities, in relation to the first two ordination axes. Sites code same than in Table 2.
Figure 5. Gráfico del Análisis de Correspondencia Canónica mostrando la asociación entre las variables fisicoquímicas y las comunidades muestreadas en relación a los dos primeros ejes del ordenamiento. Ver tabla 2 para los códigos de los sitios.
Discussion
This study showed that the composition of soluble salts in this river and streams was primarily due to the acquisition of solutes from different sediments. In October, when rainfall increased, the variation of Pt from calcic sulfated to calcic bicarbonated water was due to changes in the solubility of sulfated and bicarbonated mineral salts from the substrate, as suggested by Rolandi et al. (2011). In La Perdíz site, upstream of the salt factory, calcium sulfate was predominant, which also characterizes the Río Salí formation. In contrast, the predominance of sodium chloride at the site downstream of the salt factory was almost entirely due to the industrial effluents, because minerals providing these species in appreciable amounts to the aqueous medium are lacking lithology minerals (Rolandi et al. 2011). Baldwin et al. (2006) have observed that the increase in NaCl concentrations led to the immediate release of NH+ and Fe2+ from sediments of the River Murray floodplain (Australia) due to cation competition. We observed that in the salinized site LP2, anion competition led to the replacement of SO4= by Cl-.
Several authors have distinguished degraded areas related with the streams considered in our study (García et al. 2007; Fernández et al. 2009; Mesa 2010). However, these studies have not recorded a significant impact on the benthic community of the water courses. Mesa (2010) concluded that the variables contributing to explain the community structure (> 10% of the variation) were EC, channel width and temperature; while discharge, nitrate, dissolved oxygen, pH and the area affected by agriculture and cattle grazing contributed at a lesser extent (< 10% of the variation). Thus, it can be inferred from our results that salinity levels have greater effects on the benthic community structure than human activities. In Lules River sub-catchment (Pt and SJ) the relationship between EC and discharge coincides with that expected for the region (Fernández et al. 2009; Mesa 2010): electrical conductivity decreases as the discharge increases. The relationship between EC and discharge in Calera River sub-catchment is attributed to two causal factors: in the upstream site the interaction water-substrate (lithology) prevails as a driver, while in the downstream site the driver is likely the salt factory influence. Although precipitation was negligible during August, horizontal precipitation typically occurring in the Yungas region may have contributed water to the systems. This phenomenon involves the release of the water droplets intercepted by the foliage from the clouds (Hunzinger 1977), and may contribute significant amounts of water during the dry season. In addition, we consider that horizontal precipitation may contribute to maintain the volume of the discharges during August. Our results are in agreement with the findings by Ziemann & Schulz (2011) in terms of the strength of the interaction between salinity and other abiotic parameters influencing the responses of organisms.
Lules River sub-catchment is naturally affected by precipitation, showing effects of dilution concomitant to the increase in the discharge. This situation influences the structure of benthic communities, either improving the environmental conditions as reflected by the diversity of Pt in August, or disturbing them as reflected by richness and the community structure found in SJ during October. Calera River sub-catchment was affected by a secondary salinization caused by the discharge of effluents from the salt factory.
Taxa richness showed higher values for Lules sub-catchment than for Calera sub-catchment. This is coincident with the higher degree of human impact in the latter and the effect of drought which may have affected particularly Calera as compared to Lules sub-catchment. Calera River traverses a region with high abundance of xerophytic species of small size that makes the riverside more vulnerable to the impact of human activities (Fernández 2003).
Within Lules sub-catchment, the average richness was similar in SJ and Pt in spite of the higher richness observed in San Javier River, also noted by Romero et al. (2011). These authors calculated a total historical richness of 75 species for the sub-catchment (SmaxPt = 36, Smax SJ = 30 in this work). On the other hand, significant differences were observed between LP1 and LP2 (Calera sub-catchment), which may be attributed to the dramatic effect of the secondary salinization. In their review about salinization as a global environmental issue, Cañedo-Argüelles et al. (2013) propose that secondary salinization produces structural changes in freshwater communities affecting density, species richness and functional aspects. They argue that at the community level more information is needed regarding the relationship between salinity and species richness, because many studies have considered species density instead of species richness. According to our study, taxonomic richness may be a sensitive indicator to detect effects of secondary salinization; however, it is not as sensitive when natural salinity is involved. The values obtained in the regression between taxa richness and CE (r2= 0.39) would indicate that richness declines in approximately 4 taxa by 1000 µS/cm. However, when we consider each sub-catchment separately, this model does not fit well to the observed data, probably due to the different types of salt.
Precipitation (vertical) plays an important and different role in each of the studied basins. When seasonal precipitation increased, the communities in more saline sites were structured more equitably, while SJ community (the less saline site) was dominated by Dicrotendipes sp. (Chironominae). This chironomid and the presence of Physaacuta (Gasteropoda) shift the typical structure of SJ community (Fernandez et al. 2009). The taxonomic composition was different between paired sites with increasing precipitation in October. In agreement with these observations, Reynaga & Dos Santos (2012) found in neighboring streams an important component in the flow regimes capable to define a temporal habitat or templet. Although our study was performed during the dry season we can infer that the macroinvertebrate richness would be lower during the wet season due to the fact that increased flow causes instability in the substrate. Reynaga & Dos Santos (2013) performed a spatio-temporal analysis at Lules sub-catchment and showed that richness changed between successive periods of the flow regime, thereby emphasizing the rainfall effect on the landscape. Williams (2001) has suggested that in regions with high and low rainfall seasons and presumably, low and high salinity, the taxa remaining in saline waters are those tolerant to such fluctuations.
Reynaga & Dos Santos (2012) defined a templet for the same basin analyzed in the present study, using EC as a synthetic and powerful variable associated with biological traits. In the same line, the CCA allowed us to affirm that there is a critical limit of electrical conductivity that separates taxa of benthic macroinvertebrate communities, as has been previously pointed out by Horrigan et al. (2005). This critical limit was observed in our study in waters with EC above 800µS/cm (samples from Pt, LP1 and LP2). In the seasonal frame, this could be observed when EC in Pt took values below the limit of 800 µS/cm in August when its Richness resembled to SJ´s.
Aboal (1989) and Moreno et al. (1997; 2001) observed that saline streams in southeastern Spain show marked differences in taxonomic composition compared with their freshwater counterparts in the same region. Additionally, Williams (1991) observed that the taxonomic composition of aquatic insects is markedly similar in salinized rivers in Australia. In our study, taxa which characterize samples from sites with EC above 800 µS/cm are Austrelmis sp. larvae (Coleoptera) and Metrichia sp. (Trichoptera). As revealed by the CCA, these samples were more similar to each other than San Javier River samples. It is worth noting that taxa Carvalhoiella sp. (Hemiptera), Hydrochus sp. (Coleoptera), Macrelmis sp. (Coleoptera), Leuronectes sp. (Coleoptera) are restricted to San Javier River (below 800 µS/ cm). The importance of EC as a factor beyond a salinity measure was stressed by Reynaga & Dos Santos (2012). They used EC in Lules River as a variable summarizing the spatial-temporal configuration of factors influencing both the faunal and functional structure of associated macroinvertebrate community and their biological traits.
In conclusion, taxonomic richness may be a sensitive indicator to detect effects of secondary salinization due to human impact; however, this index is not such a good proxy in cases of natural salinization. Seasonal rainfall appears to structure benthic communities differently depending on site-specific salinity. In saline sites the community becomes more equitable while in less saline sites the community appears to be disturbed. There is a critical limit of EC above 800µS/cm that separates taxa of benthic macroinvertebrate communities based on their tolerance to salinity. In addition, when salinity falls within the categories strong, very strong or excessive in these subtropical Andean streams, one elmid and one caddisfly species are the dominant taxa.
SUPPLEMENTARY INFORMATION
Appendix
List of taxa, taxa codes and total abundances per taxa for each site. Pt: Potrerillo stream, SJ: San Javier River; LP1: La Perdíz stream upstream the salt factory, LP2: La Perdíz stream downstream the salt factory. The sampling month is at the beginning of each list.



Aknowledgements: We are grateful to F Romero for her assistance with macroinvertebrate identification. For the identification of taxa, we thank to V Manzo (Elmidae), E Tejerina helping (Chironomidae) and X Ovando (Gasteropoda). We thank to the anonymous referees who made numerous suggestions that improved the manuscript. We thank Laboratorio de Química de la Facultad de Ciencias Naturales, at the University National of Tucumán for helping with chemical analysis. Research was partially supported by the Consejo de Investigaciones de la UNT (CIUNT) projects 26-G416 and 26/G422 (University National of Tucumán, Argentina).
References
1. Aboal, M. 1989. Epilithic algal communities from River Segura Basin, southeastern Spain.Arch. Hydrobiol, 116: 113-124. [ Links ]
2. Alonso, RN; B Bookhagen; B Carrapa; I Coutland; M Haschke; GE Hilley; et al. 2006. Tectonics climate and landscape evolution of the Sothern Central Andes: The Argentina Puna Plateau and adjacent regions between 22 and 30° S latitudes. Springer Verlag, Heidelberg. Pp. 265-283. [ Links ]
3. American Public Health Association. 1989 Standard Methods for the Examination of Water, Sewage and Wastewater.17th edition. [ Links ]
4. Baldwin, D; G Rees; A Mitchell; G Watson & J Williams. 2006. The short-term effects of salinization on anaerobic nutrient cycling and microbial community structure in sediment from a freshwater wetland. Wetlands, 26:455-464. [ Links ]
5. Bayly, IAE. 1969. The occurrence of calanoids copepods in athalasic saline waters in relation to salinity and anionic proportions. Verh. Internat. Verein. Limnol, 17:449-455. [ Links ]
6. Bayly, IAE. 1972. Salinity tolerance and osmotic behavior of animals in Athalassic saline and Marine Hypersaline waters. Annu. Rev. Ecol. Evol. Syst, 3:233-268. [ Links ]
7. Beadle, LC. 1969. Osmotic regulation and the adaptation of freshwater animals to saline inland waters. Verh. Internat. Verein. Limnol, 17:421-429. [ Links ]
8. Bossi, GE; IJC Gavriloff & G Esteban. 1998. Terciario. Estratigrafía, Bioestratigrafía y Paleogeografía. Pp. 87-108. In: M Gianfrancisco; M Puchulu; J Durango de Cabrera; G Aceñaloza (Eds.). Geología de Tucumán segunda edición. Publicación Especial del Colegio de Graduados en Ciencias Geológicas de Tucumán. [ Links ]
9. Brown, AD & LR Malizia. 2004. Las selvas pedemontanas de las yungas. Ciencia Hoy, 14:53-63. [ Links ]
10. Cañedo-Argüelles, M; BJ Kefford; C Piscart; N Prat; RB Schäfer & S Claus-Jürgen. 2013. Salinisation of rivers: An urgent ecological issue. Environ. Pollut, 173:157-167. [ Links ]
11. Department of Environmental and Heritage and Department of Natural Resources. 1999. Testing waters. A report of the quality of Queensland waters.Report 21. Queensland Government, Brisbane. [ Links ]
12. Domínguez, E & HR Fernández (Eds.). 2009. Macroinvertebrados bentónicos sudamericanos. Sistemática y biología. Fundación Miguel Lillo. Tucumán. Argentina. [ Links ]
13. Estación Experimental Agroindustrial Obispo Colombres. Tucumán, Argentina. http://www.eeaoc.org.ar/. [ Links ]
14. Fernández, DS &M del V Hidalgo. 2008. Mapeo geoquímico ambiental de la Cuenca del Río Lules mediante el uso de muestras de sedimentos de corriente. Pp. 33-46. In: Fernández, HR & HM Barber (Eds.). La cuenca del Río Lules: una aproximación multidisciplinaria a su complejidad. [ Links ]
15. Fernández, HR. 2003. Structure of water mite taxocenoses in two northwestern Argentinean subtropical sub-catchments. Syst. Appl. Acarol, 8:55-66. [ Links ]
16. Fernández, HR; F Romero &E Domínguez. 2009. Intermountain Basins use in Subtropical Regions and their infuences on benthic fauna. River. Res.Appl, 25: 181-193.
17. García, MG; M Hidalgo & M Blesa. 2007. Impacto del hombre sobre la calidad del agua en los Humedales de la Cuenca del Río Salí, Provincia de Tucumán, Argentina. Pp. 127-144. In: D Cicerone & M Hidalgo (Eds.). Los Humedales de la Cuenca del Río Salí [ Links ].
18. González Achem, AL. 2012. Composición de la comunidad de macroinvertebrados de dos arroyos salinos de la Cuenca del Río Salí (Tucumán-Argentina). Thesis of Licenciature degree in Ciencias Biológicas de la Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, Argentina. [ Links ]
19. Grau, A & AD Brown. 2000. Development threats to biodiversity and opportunities for conservation in the mountain ranges of the upper Bermejo river basin, NW Argentina and SW Bolivia. Ambio, 29:445-450. [ Links ]
20. Hart, BT; P Bailey; R Edwards; K Hortle; K James & A McMahon. 1991. A review of the salt sensitivity of the Australian freshwater biota. Hydrobiologia, 210:105-144. [ Links ]
21. Horrigan, N; S Choy; J Marshall & F Recknagel. 2005. Response of streams macroinvertebrates to changes in salinity and the development of a salinity index. Mar. Freshwater. Res, 56:825-833. [ Links ]
22. Huzinger, H. 1997. Hydrology of montane forests in the sierra de San Javier, Tucumán, Argentina. Mt. Res. Dev, 17:299-308. [ Links ]
23. Instituto Argentino de Normalización y Certificación, 2012. IRAM 29012-2: Técnicas de muestreo. IRAM 29012-3: Calidad ambiental. Calidad del agua. Muestreo. Parte 3: Guía para la preservación y manipulación de las muestras. IRAM 29012-6: Calidad ambiental. Calidad del agua. Muestreo: Parte 6: Directivas para el muestreo en ríos y cursos de agua. IRAM 29012-14: Calidad ambiental. Calidad del agua. Muestreo: Parte 14: Directivas sobre aseguramiento de la calidad del muestreo y manipulación de agua. http://www.iram.org.ar/. [ Links ]
24. Iriondo, M. 1989.Quaternary lakes of Argentina. Paleogeo gr.nPaleoclimatol. Paleoecol, 70:81-88. [ Links ]
25. James, K; B Cant & T Ryan. 2003. Responses of freshwater biota to rising salinity levels and implications for saline water management: a review. Aust. J. Bot, 51:703-713. [ Links ]
26. Kefford, BJ. 1998. The relationship between electrical conductivity and selected macroinvertebrate communities in four river systems of south-west Victoria, Australia. Int. J. Salt Lake Res, 7:151-170. [ Links ]
27. Kefford, BJ; CG Palmer; L Pakhomova & D Nugegoda. 2004. Comparing test systems to measure the salinity tolerance of freshwater invertebrates. Water SA, 30: 499-506. [ Links ]
28. Lake, PS. 2000. Disturbance, patchiness and diversity in streams. J. N. Am. Benthol. Soc, 19:573-592. [ Links ]
29. Ludwig JA & JF Reynolds. 1988. Statistical Ecology. J. Wiley & sons. New York. Pp: 337. [ Links ]
30. Magurran, AE & AE Magurran. 1988. Ecological diversity and its measurement. Vol. 179. Princeton: Princeton University Press. [ Links ]
31. Martinez, DE. 1995. Changes in the ionic composition of a saline lake, Mar Chiquita, Province of Córdoba, Argentina. Int. J. Salt Lake Res, 4:25-44. [ Links ]
32. Mesa, LM. 2010. Effect of spates and land use on macroinvertebrate community in Neotropical Andean streams. Hydrobiologia, 641:85-95. [ Links ]
33. Moreno, JL; M Aboal; MR Vidal-Abarca & ML Suárez. 2001. Macroalgae and submerged macrophytes from fresh and saline waterbodies of ephemeral streams ('ramblas') in semiarid south-eastern Spain. Mar. Freshwat. Res, 52:891-905. [ Links ]
34. Moreno, JL; A Millán; ML Suárez; MR Vidal-Abarca & J Velasco. 1997. Aquatic Coleoptera and Heteroptera assemblages from ephemeral coastal streams ('ramblas') of south-eastern Spain. Arch. Hydrobiol, 141:93-107. [ Links ]
35. Neiff, JJ. 1996. Large rivers of South America: toward the new approach. Verh. Internat. Verein. Theor. Angew. Limnol, 26:167-180. [ Links ]
36. Organización Mundial de la Salud. 2008. Guía para calidad de agua potable. Tercera edición. http://www.who.int/es/. [ Links ]
37. R CoreTeam. 2012. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/. [ Links ]
38. Reynaga, MC & DA Dos Santos. 2012. Rasgos biológicos de macroinvertebrados de ríos subtropicales: patrones de variación a lo largo de gradientes ambientales espacio-temporales. Ecol. Austral, 22:112-120. [ Links ]
39. Reynaga, MC & DA Dos Santos. 2013. Contrasting taxonomical and functional responsesof stream invertebrates across space and time in a Neotropical basin. Fundam. Appl. Limnol,183:121-133. [ Links ]
40. Rockworks. 2006. Rockware Integrated Geological Software-Revision 6.9.7. Windsor, Ontario, Canada. [ Links ]
41. Rodier, J. 1989. Análisis de las Aguas. Aguas naturales, aguas residuales, aguas de mar. Editorial Omega. [ Links ]
42. Rolandi, ML; MC Galindo; HR Fernández & M del V Hidalgo.2011. Equilibrios de solubilidad en la cuenca media del río Lules. Pp: 47-59. In: HR Fernández & HM Barber (Eds.). La cuenca del Río Lules: una aproximación multidisciplinaria a su complejidad. [ Links ]
43. Sylvestre, F; S Servant-Vildary & M Roux.2001. Diatom-based ionic concentration and salinity models from the south Bolivian Altiplano (15-23°S). J. Paleolimnol, 25:279-295. [ Links ]
44. TerBraak, CJF. & P Smilauer. 1998. CANOCO for Windows Version 4.02. Centre for Biometry, Wageningen, the Netherlands. [ Links ]
45. Williams, WD. 1987. Salinization of rivers and streams: an important environmental hazard. AMBIO, 16:180-185. [ Links ]
46. Williams, WD. 1991. Longitudinal distribution of macroinvertebrates in two rivers subject tosalinizatio n.Hydrobiologia, 210:151-160. [ Links ]
47. Williams, WD. 1999. Salinization: A major threat to water resources in the arid and semi-arid regions of the world. Lakes and reservoirs: Research and Management, 4:85-91. [ Links ]
48. Williams, WD. 1998. Salinity as a determinant of the structure of biological communities in salt lakes. Hydrobiologia, 381:191-201. [ Links ]
49. Williams, WD. 2001. Anthropogenic salinisation of inland waters. Hydrobiologia, 466:329-337. [ Links ]
50. Ziemann, H & CJ Schulz. 2011. Methods for biological assessment of salt-loaded running waters-fundamentals, current positions and perspectives. Limnologica, 41:90-95. [ Links ]














