Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Ecología austral
versión On-line ISSN 1667-782X
Ecol. austral vol.26 no.1 Córdoba abr. 2016
ARTÍCULO ORIGINAL
Indirect assessment of seed dispersal effectiveness for Solatium riparium (Solanaceae) based on habitat use and rate of fruitdisappearance
Silvia Beatriz Lomascolok*
Instituto de Ecología Regional, Universidad Nacional de Tucumán. CC 34. Residencia Universitaria Horco Molle, (4107)Yerba Buena, Tucumán, Argentina.
*slomascolo@gmail.com
Editor asociado: Martín Núñez
Recibido: 23 de junio de 2015 Aceptado: 16 de diciembre de 2015
Abstract.
The ability of a plant's propagule to reach microhabitats with the adequate conditions for seed germination and sapling establishment will have a direct effect on the plant's fitness. In the case of fleshy-fruited plants, the seeds are dispersed by frugivorous animals. One part of the seed dispersal process is the removal of the fruits, which contain the seeds, from the parental plant. Another important part of the dispersal process is where the seeds land, especially for species with specific light, temperature or humidity needs for germination, such as disturbance colonizers. Solanum riparium (Solanaceae) is a shade intolerant species found in forest gaps within a subtropical montane forest and along river and road edges in North-western Argentina. In this study I assess the relative importance of bats and birds as seed dispersers of S. riparium (Solanaceae) in forest gaps and river edges, based on data on fruit disappearance during the night and during the day. I also classify seed dispersers according to the habitat types in which they were caught with mist-nets. Diurnal and nocturnal fruit disappearance rate did not differ and neither did disappearance rate between habitats. The results of this study suggest that, based on habitat use, the best seed dispersers for S. riparium are the frugivorous birds Atlapetes citrinellus and Turdus rufventris, commonly caught at river edges and forest gaps. Based on habitat use of the frugivorous bats studied here, Sturnira lilium and S. eryihromos, they potentially disperse half of the seeds to forest gaps and river edges, which are appropriate sites for germination of S. riparium seeds, and half to the river bank, a place with high risk of seeds being washed away and destroyed by occasionally strong water currents.
Keywords: Yungas forest; Tabaquillo; Frugivory; Birds; Bats
Resumen
Evaluación indirecta de la efectividad de la dispersión de semillas de Solanum riparium (Solanaceae) en base al uso del hábitat y tasa de desaparición de frutos. La habilidad del propágulo de una planta para alcanzar un sitio con las condiciones adecuadas para la germinación de la semilla y el establecimiento del renoval tendrá un efecto directo en la aptitud ("fitness") de la planta. En el caso de plantas con frutos carnosos, las semillas son dispersadas por animales frugívoros. Una parte del proceso de dispersión de semillas es la remoción del fruto, que contiene las semillas, de la planta madre. Otra parte importante de este proceso es dónde aterriza la semilla, especialmente para especies con necesidades específicas de luz, temperatura, y humedad para su germinación, tales como las plantas colonizadoras de ambientes perturbados. Solanum riparium (Solanaceae) es una especie no tolerante a la sombra que se distribuye en los bosques de montaña subtropical del noroeste de Argentina, las Yungas, típicamente en sitios de abertura del dosel, y a lo largo de bordes de ríos y rutas. En el presente estudio evalúo la importancia relativa de murciélagos y aves como dispersores de semillas de S. riparium (Solanaceae) en sitios de abertura de dosel y bordes de ríos, en base a datos de desaparición de frutos durante el día y durante la noche. También clasifico a los dispersores de acuerdo al uso del hábitat en base a datos de captura en redes de niebla. La desaparición nocturna de frutos no difirió de la diurna, así como tampoco difirió la tasa de desaparición de frutos en los distintos hábitats. Los resultados de este estudio sugieren que, en base al uso de hábitat, los mejores dispersores de S. riparium serían las aves frugívoras Atlapetes citrinellus y Turdus rufventris, comúnmente atrapados en bordes de río y sitios de abertura del dosel. En base al uso del hábitat, los murciélagos frugívoros estudiados aquí, Sturnira lilium y S. erythromos, potencialmente dispersarían la mitad de las semillas consumidas a aberturas del dosel y bordes de ríos, sitios apropiados para la germinación de S. riparium, y la otra mitad al lecho del río, un lugar con alto riesgo de que las semillas sean acarreadas y destruidas por las fuertes corrientes de agua ocasionales.
Palabras clave: Yungas; Tabaquillo; Frugivoría; Aves; Murciélagos
Introducción
Seed dispersal effectiveness (SDE) assesses the overall "service" received by a plant from its assemblage of seed dispersers (Schupp et al. 2010). The SDE concept includes quantity and quality components. The quantity component includes data on the number of seeds dispersed by a seed dispersal agent, while the quality component measures the probability that a dispersed seed will germinate and establish to produce a reproductive adult plant. Dispersal quality is in part determined by the conditions of the site where seeds land (Harms et al. 2000; Rey & Alcántara 2000; Schupp et al. 2010). The capability of a plant's propagule for reaching microhabitats with the adequate conditions for seed germination and sapling establishment will have a direct effect on the fitness of both the maternal plant and the seedling (Howe & Miriti 2004), and on plant community composition (Harms et al. 2000). Where seeds land is especially important for species with specific light, temperature or humidity needs for germinating, such as disturbance colonizers (i.e., a gap in the forest canopy, land slide, habitat openings caused by flood or fire, etc.).
For fleshy-fruited plants, seed deposition will be highly dependent on the activity patterns of the frugivores that consume the fruits and defecate the seeds (Calviño-Cancela 2002; Calviño-Cancela & Martín-Herrero 2009; Martínez & García 2015). For example, many frugivorous birds from the understory concentrate their activity in forest gaps and edges, where food resources are more abundant than in the forest interior (Thompson & Willson 1978; Blake & Hoppes 1986; Hoppes 1988; Levey 1988), while frugivorous bats may often use riparian corridors to move between sites (de la Peña-Cuéllar et al. 2015). Therefore, the seeds from a plant located in a forest gap that are consumed by birds that frequently visit that habitat type will have high probabilities of landing in another forest gap. Frugivorous bats, instead, have not been reported, to my knowledge, to be particularly attracted to canopy gaps, and their activity in such sites may be lower than bird activity. On the other hand, bats use rivers and large creeks that run through the forest as corridors for daily displacement to and from feeding sites (Giannini, CONICET - PIDBA UNT personal communication). Therefore, seeds consumed by bats on the river edge have high probabilities of being transported to another river edge site through river corridors.
Solanum riparium Pers. (Solanaceae), whose common name is Tabaquillo, is a shade intolerant species and is only found in gaps within a subtropical montane forest in Northwestern Argentina, Peru and Bolivia. It is also a frequent colonizer of river and road edges, or any site where solar radiation is abundant (Grau 2002; Sirombra & Mesa 2010). The seeds of S. riparium are unlikely to germinate in the forest interior and, therefore, arrival to disturbed sites is crucial for seedling establishment and survival. The fruits of S. riparium are consumed by a great variety of bird families, such as Emberizidae, Turdidae, Thraupidae, Corvidae and Vireonidae, among others, and bats of the family Phyllostomidae (Giannini 1999; Sánchez et al. 2012; Ruggera 2013). In this study I tried to get a first approximation to the seed dispersal effectiveness of frugivorous birds and bats for S. riparium based on rate of fruit disappearance during the day and night (indirect measure of dispersal quantity) and on habitat use by dispersers (indirect measure of dispersal quality). I expect that birds will remove more fruits and, hence, disperse more seeds from trees located in forest gaps, while bats will disperse more seeds from trees along the river edge. Therefore, I expect that diurnal fruit removal in gaps will be higher than nocturnal removal, and the opposite will occur at river habitats.
Materials and Methods
Study area
This study was done between January and March 2000 in Sierra de San Javier Biological Park (hereafter San Javier; 24°47' S, 65°22' W) in Tucumán province, Argentina. The park is in north-western Argentina and is part of the Austral Yungas ecosystem represented by a subtropical montane forest (Brown 1995; Brown et al. 2001). Common canopy species at San Javier are Parapiptadenia excelsa (Fabaceae), Ocotea porphyria (Lauracae), Juglans australis (Juglandaceae), Blepharocalyx salicifolius (Myrtaceae) and Myrsine laetevirens (Myrsinaceae). The subcanopy is dominated by Piper tucumanum (Piperaceae), Allophylus edulis (Sapindaceae) and Psychotria carthagenensis (Rubiaceae) (Grau et al. 2010). The native pioneers Heliocarpus popayanensis (Tiliaceae), Tecoma stans (Bignoniaceae), Solanum riparium (Solanaceae) and exotic colonizers including Morus spp. (Moraceae), Ligustrum lucidum (Oleaceae) and Citrus spp. (Rutaceae) (Grau & Aragón 2000), are also common at San Javier.
The study was done in two different sites within San Javier Biological Park, which were at least 3 km apart. Both sites were composed of well-conserved forest, and crossed by medium-sized river banks (approximately 10-25 m wide) that most of the time carried little water and, hence, allowed for setting mist-nets across the bank (see below). During days of heavy summer rains, the rivers carry abundant water, and this is how the bank is maintained fairly open for the rest of the year. Hence, each site was considered a replicate, and in each site nets were set up in the forest interior, in forest gaps, along the river edge and across the river bank (see below) to quantify frugivore activity. Fruit removal from trees located in gaps and the river edge was only quantified in one of the sites as the other did not have many trees with good visibility for a reliable fruit count (see below).
Fruit removal rate
Solanum riparium fruits are yellowish-orange round berries, 10-12 mm in diameter, supported by a persistent calyx. The removal of a fruit can be assumed by the empty calyx. This species has an extended phenology and therefore trees with fruits can be found year-round. A fructification peak occurs during the end of the spring and the summer, when this study was done. The trees chosen to record fruit disappearance had similar harvest size that was representative of the fruit offer seen in most trees in the area at that moment (see number of trees in the next paragraph). Importantly, they also had clear visibility of fruits. At each site, I established two treatments: forest gap trees and river trees. Within each treatment, I recorded nocturnal and diurnal removal, as follows.
With the help of a field assistant, I identified 3-12 distinct groups of fruits in each tree, and we counted the fruits at dawn with the first morning light (approximately between 06:50 h and 07:50 h) and 1 h before dusk (approximately between 19:00 h and 20:00 h) for 3 days to quantify diurnal and noctunal removal (Figure 1). Fruits that disappeared between the dusk and the dawn counts were assumed to have been removed by bats, as fruits had exclusively been exposed during the night (hereafter, nocturnal removal). Fruits that disappeared between dawn and dusk were assumed to have been removed by birds, since fruits had exclusively been exposed during the day (hereafter, diurnal removal). I did not carry out direct observations of fruit removal by dispersers, consequently fruit disappearance may also be attributable to fruits falling to the ground due to senescence or wind. However, there is no reason to assume that fruits fall to the ground differentially during the day and night. Thus, given that I focus on the difference between fruit disappearance during diurnal and nocturnal periods from trees located in gaps and river edges, and the incidence of senescence and wind are probably equal during these periods and sites, I consider that this methodology might be a good approximation to the real removal rate.
The four-day counts for each treatment were done twice: the first count was done from February 1 to February 4 on seven trees placed in different forest gaps (total of 268 fruits counted at the start of the four-day count) and six trees placed along the river bank (254 fruits counted at the start of the four-day count). The second count was from February 28 to March 2 and was done on seven trees in forest gaps (508 fruits counted at the start of the four-day count) and 10 trees along the river bank (229 fruits counted at the start of the four-day count). All trees were located at least 1 km apart. The same trees were counted in both instances, with the exception of four trees added along the river bank during the second count. Fruit removal was not recorded in the forest interior because no S. riparium trees were found there. In summary, I did two counts, each including three estimations for diurnal and three for nocturnal fruit removal (Figure 1), on a total of 13 trees for the first count (seven in forest gaps and six at the river edge), and 17 for the second count (seven in forest gaps and 10 at the river edge).
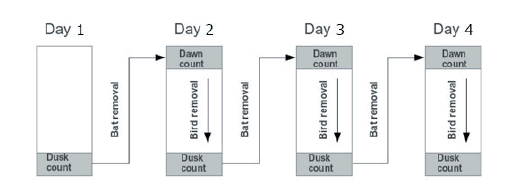
Figure 1. Graphical representation of fruit counts to quantify disappearance of Solanum riparium fruits during the night (between dusk and dawn counts, presumably done mainly by bats) and during the day (between dawn and dusk counts, presumably done by birds). This four-day count was replicated twice, once between February 1-4, and the other between February 28-March 2 of 2000.
Figura 1. Representación gráfica de los conteos de frutos para cuantificar la desaparición de los frutos de Solarium riparium durante la noche (entre los conteos del atardecer y del amanecer, supuestamente realizado por murciélagos) y durante el día (entre los conteos del amanecer y el atardecer, supuestamente realizado por aves). Este conteo de cuatro días fue repetido dos veces, una vez del 1 al 4 de febrero y otra vez del 28 de febrero al 2 de marzo de 2000
All the data for nocturnal fruit disappearance were added for all trees across the four days of each count and across both counts. The same was done for diurnal fruit disappearance. Data were analysed with a Chi-squared test, adequate for count/frequency data.
Use of habitat
Use of habitat by dispersers was evaluated using mist-nets, which were 12 m long x 2 m high. I set up 16 mist-nets located as follows: two nets at each of two gap sites, two nets at each of two forest interior sites, two nets at each of two river edge sites, and two nets at each of two river sites. This design was replicated in two similar locations, separated by 3 km. River edge sites differed from river sites in that river edge nets were oriented along the river to catch animals that intended to cross the river or that simply visited that type of habitat, while river nets were oriented across the river to catch the animals that travelled along the river, using it as a corridor. Moreover, river edge nets were located right at the border between forest interior vegetation and the completely open canopy found on the river. River nets were located in completely open habitat, on rocks or sandy soil. Nets were opened during two consecutive days from 06:30 h to 11:30 h for bird capture, and from 20:00 h to 02:00 h for bat capture. This procedure was repeated in January, March and April, except for river edge nets, which were only setup in March and April. February sampling was skipped due to constant rain. Birds caught in mist-nets were kept in plastic boxes with holes to allow normal breathing until they defecated, for a maximum of 1 h. Bats were kept in fabric bags. Faecal samples were collected and later observed under a dissecting scope to look for seeds. Birds and bats that defecated whole seeds of any plant species at least once were considered potential seed dispersers.
Net hours for bats added up to 184.5 for forest gap, interior and river nets, and 116 for river edge nets. For birds, net hours added up to 208 for forest gap, interior and river nets, and 132 for river edge nets. I ran a correspondence analysis to see how frugivorous bird and bat species were ordinated on the basis of their use of habitat types (forest gap, forest interior, river edge and river). Correspondence analysis is the multivariate version of the chi-squared test, appropriate for data on frequency of capture in the different types of habitat (McCune & Grace 2002). In order to correct for the uneven sampling hours for each site, the frequency of capture of each species was scaled up to the greatest sampling time. That is, I estimated how many more times would each species be captured in 208 sampling hours given the observed capture rate. Numbers were rounded to the nearest integer.
Results
Use of habitat by frugivore species
Species considered for this analysis were those that defecated whole seeds when caught in the mist-nets during this study and for which there is evidence in the literature that they consume fruits of S. riparium (Iudica & Bonaccorso 1997; Rougès & Blake 2001; Giannini 1999; Sánchez et al. 2012; Ruggera 2013; Blendinger et al. 2015; Blendinger et al. in press). The frugivorous species caught were the bat species Sturnira lilium and S. erythromos (Phyllostomidae), and the bird species Turdus rufiventris, T. nigriceps, Catharus ustulatus (Turdidae), Thraupis sayaca (Thraupidae), Chlorospingus ophthalmicus, Atlapetes citrinellus and Arremon flavirostris (Emberizidae) (Table 1).
Table 1. Frequency of capture of bird and bat species in mist-nets placed at the edge of rivers (River edge), in forest gaps (Gap), within closed forests (Forest interior) and across rivers to capture individuals that use them as corridors (River) in a subtropical montane forest of the Sierra de San Javier Biological Park, Tucumán, Argentina. Numbers in parenthesis represent capture frequency scaled up to the greatest sampling time, considering the observed capture rate.
Tabla 1. Frecuencia de captura de especies de aves y murciélagos en redes de niebla ubicadas a lo largo de bordes de ríos (River edge), sitios de abertura de dosel (Gap), dentro de bosque cerrado (Forest interior) y a través de ríos para capturar individuos que los usan como corredores, en un bosque subtropical de montaña del Parque Biológico Sierra de San Javier, Tucumán, Argentina. Los números entre paréntesis representan la frecuencia de captura a una escala correspondiente al mayor tiempo muestreado, dada la tasa de captura observada

The correspondence analysis resulted in strong differentiation of river sites from all the other sites, especially on Dimension 1, where river sites showed a high positive score (Figure 2). This was due to the fact that there was little overlap of species caught in river sites and those caught in forest interior, gap and river edge sites. The two bat species were more often caught in river sites while the bird species most commonly caught, A. flavirostris, T. rufiventris, A. citrinellus and T. nigriceps were found mainly in the other three habitat types (Tables 1 and 2).
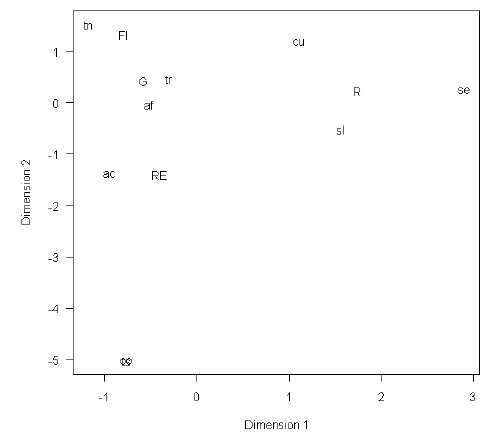
Figure 2. Plot of Dimension 1 vs. Dimension 2 of the correspondence analysis based on capture rate of frugivorous bird and bat species caught in Sierra de San Javier Biological Park, Tucumán, Argentina. Sites are represented by upper case letters: RE=river edge, G=gap, FI=forest interior, R=river. Birds are represented by the following abbreviations: af=Arremon flavirostris, ac=Atlapetes citrinellus, cu=Catharus ustulatus, co=Chlorospingus ophthalmicus, ts=Thraupis sayaca, tn=Turdus nigriceps, tr=Turdus rufiventris. Bats are represented by the following abbreviations: se=Sturnira erythromos, sl=Sturnira lilium.
Figura 2. Gráfico de la Dimensión 1 vs Dimensión 2 del análisis de Correspondencia basado en las tasas de captura de especies de aves y murciélagos frugívoros atrapados en el Parque Biológico Sierra de San Javier, Tucumán, Argentina. Los sitios están representados por letras mayúsculas: RE=borde de río, G=abertura del dosel, FI=interior de bosque, R=río. Las aves están representadas por las siguientes abreviaturas: af=Arremon flavirostris, ac=Atlapetes citrinellus, cu=Catharus ustulatus, co=Chlorospingus ophthalmicus, ts=Thraupis sayaca, tn=Turdus nigriceps, tr=Turdus rufiventris. Los murciélagos están representados por las siguientes abreviaturas: se=Sturnira erythromos, sl=Sturnira lilium
Table 2. Correspondence analysis based on capture frequency of frugivorous birds and bats at river, river edge, gap, and forest interior sites in Sierra de San Javier Biological Park, Tucumán, Argentina. Species and site scores on dimensions 1 and 2 are shown.
Tabla 2. Análisis de correspondencia basado en frecuencia de captura de aves y murciélagos frugívoros en sitios de río, borde de río, interior de bosque y aperturas de dosel en el interior del bosque en el Parque
Biológico Sierra de San Javier, Tucumán, Argentina. Se presentan los valores de especies y sitios en las dimensiones 1 y 2

The river edge habitat is also contrasted against river, forest gap and interior habitats in Dimension 2. This was mainly produced by the presence of the frugivorous tanagers T. sayaca and C. ophthalmicus, which were caught exclusively in river edge nets. These results should be taken with caution as sample size for most species is quite small and may produce some artificial correlations (Table 1). The two most commonly caught, potentially seed dispersing, bird species were T. rufiventris (81 captures) and A. flavirostris (47captures) (a granivore that defecated viable seeds [i.e., that germinated successfully, personal observation]), which scored very close to zero on both dimensions (Table 2, Figure 2).
Fruit removal rate
During the night, 31 fruits disappeared from trees located in gaps (mean of 0.74 fruits.tree-1.day-1, SD=1.16), and 20 from trees along the river edge (mean of 0.42 fruits.tree-1.day-1, SD=0.59). During the day, 40 fruits disappeared from trees located in gaps (mean of 0.95, SD=1.05) and 11 from trees along the river edge (mean of 0.23, SD=0.24). Overall, nocturnal and diurnal disappearance from gaps and river edges did not differ statistically (X2=3.754, d.f.=1, P=0.053) (Table 3).
Table 3. Contingency table for X2 test of frequency of fruit disappearance during the day and night from trees located along the river edge and in canopy gaps. Expected values are in brackets next to observed frequencies.
Tabla 3. Tabla de contingencia para la prueba de X2 de desaparición de frutos durante el día y la noche, de árboles localizados sobre el borde del río y en aberturas del dosel. Los valores esperados se encuentran entre corchetes, al lado de las frecuencias observadas

Discussion
It is interesting to note that the fruits of Solanum riparium are not brightly pigmented and, therefore, would not be expected to be conspicuous enough to be found by birds. Based on the Dispersal Syndrome hypothesis, which states that fruit traits are a result of selective pressure by seed dispersers (McKey 1975; Janson 1983), the fruits of this species would be expected to be mainly bat-dispersed. However, fruits of S. riparium disappeared similarly often during the day and night, which suggests that they are equally attractive to diurnal and nocturnal dispersers, presumably birds and bats, respectively. When comparing between diurnal and nocturnal disappearance, although there was a trend that matched my prediction that fruits from the river edge disappeared more often during the night than during diurnal hours, and fruits located in gap trees more often during the day than during nocturnal hours, the difference was not statistically different. Hence, based on the quantitative component I measured, birds and bats seem to be equally effective as seed dispersers of S. riparium for trees in the two habitat types studied. While bats seemed to commonly travel along the river (Giannini CONICET - PIDBA UNT personal communication, and this study) and therefore may often find fruiting trees located on river edges, birds used river edge habitat much more often than bats did and may, therefore, easily find fruiting trees in that habitat type as well. Because fruit disappearance at night from forest gaps and from river edges did not differ, these results suggest that although bats did use rivers more often than they used forest gaps, they did not seem to remove fruits differently from these habitats. Bats might use rivers to travel to different sites and may not be so focused on searching for fruits as they may be during their less frequent visits to forest gaps. In fact, river sites were differentiated from all others, based on the high capture rate of the two frugivorous bats, Sturnira lilium and S. erythromos in river nets.
Although nocturnal and diurnal frugivores had a comparable quantity component of seed dispersal effectiveness, some slight differences in the proportional use of habitat types may lead to differences in the quality component of dispersal. Despite the fact that sample size if very limited in this study, I think that there are some trends worth discussing. Although the overall number of individuals of birds and bats was very similar along the river bank, bats used this habitat type proportionally more often than birds did. Hence, seeds ingested by bats seem to have a high probability of being deposited on the river bank, where they may be washed away by the water, a lower probability of being deposited either in river edges or gaps, and null probability of being deposited in the forest interior. The fact that bats do not seem to visit the forest interior means that the S. riparium seeds that they eat will not be deposited in this unfavourable habitat. Bats seem to use river sites quite often, as half of the captures were in that habitat type. If the seeds resist being submerged in water and are later deposited on a place with good soil for germination, this may not be an issue. But, most likely, most of those seeds deposited there may be lost, either with the water or on the rocky and sandy soil. The other half of the captures were from river edge and gap sites, both favourable sites for S. riparium seeds to germinate. If seed deposition rate is proportional to habitat use rate, then these results suggest that frugivorous bats may potentially deposit half of the consumed seeds in sites that are favourable for S. riparium and half in sites with high risk of being lost.
Frugivorous birds, in general, seem to use all habitat types more or less evenly, so seeds consumed by birds will end up being evenly distributed in all habitat types. One of the species most commonly caught, T. rufiventris, is also one of the bird species responsible for the highest fruit consumption in San Javier (Blendinger et al. 2012). This species seemed to use all habitat types often and similarly; therefore, although it could potentially often take seeds to forest gaps and river edges where they will likely germinate and establish, these birds might take just as many seeds to sites that are not appropriate for recruitment of S. riparium. The same applies to A. flavirostris. The effect of Thraupis sayaca on dispersal of S. riparium seeds would be worth studying further because, even though the present study caught only four individuals of this species, it is a common frugivore, responsible for most of the fruit consumption events recorded in the Yungas forest (Blendinger et al. 2012; Blendinger et al. 2015; Ruggera 2013). Atlapetes citrinellus was only caught in gaps and river edges, which indicates that this bird species tends to use these types of habitat proportionally more often than others that are less appropriate for establishment of S. riparuim. In order to assess seed dispersal effectiveness of frugivores, and complement these results, the effect of these disperser species on S. riparium reproduction should be evaluated by looking at removal rate, gut passage effect, and probability of seed deposition according to habitat use.
Results should be interpreted with caution because sample size for this study is reduced. Sample size of species that consume S. riparium could be increased not only by adding net hours, but also by placing nets a bit higher, as fruiting trees from this species are often 3-6 m high. The fact that the two most frequently caught species, T. rufiventris and A. flavirostris had a poor correlation with all the sites may either suggest that these two species use equally all the habitats sampled, or that a large enough sample size for the other frugivorous species captured might yield this same pattern for all of them. Future studies testing the predicted quality of the frugivores included in this study as seed dispersers, would be important to confirm the results obtained by the method used here for doing preliminary or rapid assessments of seed dispersal effectiveness in fruit-frugivore systems.
Acknowledgments. I thank D. Juri, I. Paz Posse and J. Carilla for assistance in the field. Thanks also go to A. Brown and S. Caziani as advisors for this project. N. Giannini shared his collection of seeds for reference. This project was done under a fellowship from the Secretaría de Ciencia y Técnica (CIUNT) of the National University of Tucumán.
References
1. Blake, JG & WG Hoppes. 1986. Infuence of resource abundance on use of tree-fall gaps by birds in an isolated wood lot. The Auk, 103:328-340. [ Links ]
2. Blendinger, PG; NP Giannini; IC Zampini; R Ordonez; S Torres; et al. 2015. Nutrients in fruits as determinants of resource tracking by birds. Ibis, 157(3):480-495. [ Links ]
3. Blendinger, 1 G; J Jiménez; L Macchi; E Martín; MB Sánchez; et al. In press. Scale-dependent spatial match between fruits and fruit-eating birds during the breeding season in Yungas Andean forests. Biotropica. [ Links ]
4. Blendinger, PG; RA Ruggera; MG NuNez-Montellano; L Macchi; et al. 2012. Fine-tuning the fruit-tracking hypothesis: spatiotemporal links between fruit availability and fruit consumption by birds in Andean mountain forests. J. Anim. Ecol., 81:1298-1310. [ Links ]
5. Brown, AD. 1995. Las selvas de montaña del noroeste de Argentina: problemas ambientales e importancia de su conservación. Pp. 9-18 in: Brown, AD & HR Grau (eds.). Investigación, conservación y desarrollo en selvas subtropicales de montaña. Produced by Laboratorio de Investigaciones Ecológicas de las Yungas, Fac. Cs. Nat e I.M.L., Universidad Nacional de Tucumán, Argentina. [ Links ]
6. Brown, AD; HR Grau; LR Malizia & A Grau. 2001. Argentina. Pp. 623-659 in: Kappelle, M & AD Brown (eds.). Bosques nublados del Neotrópico. INBio, San José, Costa Rica. [ Links ]
7. CalviNo-Cancela, M. 2002. Spatial patterns of seed dispersal and seedling recruitment in Corema album (Empetraceae): the importance of unspecialized dispersers for regeneration. J. Ecol, 90:775-784. [ Links ]
8. CalviNo-Cancela, M & J Martín-Herrero. 2009. Effectiveness of a varied assembly of seed dispersers of a fleshy-fruited plant. Ecology, 90(12):3503-3515. [ Links ]
9. Giannini, NP. 1999. La interacción de aves-murciélagos-plantas en el sistema de frugivoría y dispersión de semillas en San Javier, Tucumán, Argentina. PhD Thesis, Universidad Nacional de Tucumán, Tucumán, Argentina. [ Links ]
10. Grau, HR. 2002. Scale-dependent relationships between tree fall gaps and tree species diversity in a subtropical montane forest. Ecology, 83:2591-2601. [ Links ]
11. Grau, HR & R Aragón. 2000. Árboles invasores de la Sierra de San Javier, Tucumán, Argentina. Pp. 5-20 in: Grau, HR & R Aragón (eds.). Ecología de árboles exóticos en las Yungas. Produced by Laboratorio de Investigaciones Ecológicas de las Yungas, Fac. Cs. Nat e I.M.L., Universidad Nacional de Tucumán, Argentina. [ Links ]
12. Grau, HR; L Paolini; A Malizia & J Carilla. 2010. Distribución, estructura y dinámica de los bosques de la Sierra de San Javier. Pp. 33-48 in: Grau, HR (ed.). Ecología de una transición natural-urbana: el gran San Miguel de Tucumán y la Sierra de San Javier. Editorial de la Universidad Nacional de Tucumán. San Miguel de Tucumán, Tucumán, Argentina. [ Links ]
13. Harms, KE; SJ Wright; O Calderón; A Hernández & EA Herre. 2000. Pervasive density dependent recruitment enhances seedling diversity in a tropical forest. Nature, 404:493-495. [ Links ]
14. Hoppes, WG. 1988. Seed fall pattern of several species of bird-dispersed plants in an Illinois woodland. Ecology, 69(2): 320-329. [ Links ]
15. Howe, HF & MN Miriti. 2004. When seed dispersal matters. BioScience, 54(7):651-660. [ Links ]
16. Iudica, C & F Bonaccorso. 1997. Feeding of the bat Sturnira lilium, on fruits of Solanum riparium infuences dispersal of this pioneer tree in forests of Northwestern Argentina. Stud. Neotrop. Fauna Environ., 32(1):4-6. [ Links ]
17. Janson, CH. 1983. Adaptation of fruit morphology to dispersal agents in a Neotropical forest. Science, 219:187-189. [ Links ]
18. Levey, DJ. 1988. Tropical wet forest tree fall gap sand distributions of understory birds and plants. Ecology, 69(4): 1076-1089. [ Links ]
19. McCune B & JB Grace. Ordination. Pp. 152-158 in: Analysis of ecological communities. Chapter 4. MjM Software Design, Oregon, USA. [ Links ]
20. McKey, DS. 1975. Co-evolution of animals and plants. Gilbert, LE & PH Raven (eds.). University of Texas Press, Austin, TX. Pp. 159-191. [ Links ]
21. Martínez, D & D García. 2015. Disentangling habitat use of frugivorous birds: constant interactive effects of forest cover and fruit availability. Basic App. Ecol, 16(5):460-468. [ Links ]
22. de la PeNa-Cuéllar, E; J Benítez-Malvido; LD Ávila-Cabadilla; M Martínez-Ramos & A Estrada. 2015. Structure and biodiversity of phyllostomid bat assemblages on riparian corridors in a human-dominated tropical landscape. Ecol. Evol., 5(4):903-913. [ Links ]
23. Rey, PJ & JM Alcántara. 2000. Recruitment dynamics of a fleshy-fruited plant (Olea europaea): connecting patterns of seed dispersal to seedling establishment. J. Ecol, 88:622-633. [ Links ]
24. Rougès, M & JG Blake. 2001. Tasas de captura y dieta de aves del sotobosque en el Parque Biológico Sierra de SanJavier, Tucumán. Hornero, 16(1):7-15. [ Links ]
25. Ruggera, RA. 2013. Equivalencia ecológica en mutualismos de dispersión-frugivoría y su relación con la estructura y función de las comunidades de las Yungas australes. PhD Thesis, Universidad Nacional de Tucumán, Tucumán, Argentina. [ Links ]
26. Schupp, EW; P Jordano & JM Gómez. 2010. Seed dispersal effectiveness revisited: a conceptual review. New Phyt., 188: 333-353. [ Links ]
27. Sirombra, MG & LM Mesa. 2010. Composición florística y distribución de los bosques ribereños subtropicales andinos del Río Lules, Tucumán, Argentina. Rev. Biol. Trop., 58(1):499-510. [ Links ]
28. Sanchez, MS; NP Giannini & RM Barquez. 2012. Bat frugivory in two subtropical rain forests of Northern Argetina: Testing hypotheses of fruit selection in the Neotropics. Mamm. Biol., 77:22-31. [ Links ]
29. Thompson, JN & MF Willson. 1978. Disturbance and the dispersal of fleshy fruits. Science, 200(4346):1161-1163. [ Links ]














