Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Ecología austral
On-line version ISSN 1667-782X
Ecol. austral vol.26 no.2 Córdoba Aug. 2016
ARTÍCULO ORIGINAL
Mixotrophic ciliate dynamics in two zones of a temperate and highly turbid estuary in South America, Argentina
Rosa E. Pettigrosso1,*; Maximiliano D. García2; Román Uibrig2; M. Sofía Dutto2; María López Morales1& Mónica S. Hoffmeyer2,3
1 Departamento de Biología, Bioquímica y Farmacia, Universidad Nacional del Sur, Bahía Blanca, Argentina.
2 Instituto Argentino de Oceanografía (Centro Científco Tecnológico Bahía Blanca-CCTBB, CONICET), Bahía Blanca, Argentina.
3 Facultad Regional de Bahía Blanca, Universidad Tecnológica Nacional, Bahía Blanca, Argentina.
*Editor asociado: Pedro Daleo fpetti@criba.edu.ar
Recibido: 24 de julio de 2015 Aceptado: 14 de marzo de 2016
Abstract
Mixotrophy is a feeding strategy by which some organisms combine autrotrophic and heterotrophic activities. The seasonal dynamics of the fve most common mixotrophic ciliates were studied monthly in surface layers of the inner and middle zone of the Bahía Blanca estuary, a nutrient-rich, shallow and highly turbid environment in Argentina, from January to December 2009. Temperature, salinity, turbidity and chlorophyll a were recorded, as well as the abundance and biomass of Strombidium capitatum, Strombidium acutum, Cyrtostrombidium sp., Lohmanniella oviformis and Tontonia appendiculariformis. The highest mixotrophic ciliate abundance was recorded during the austral winter (June-July) in the inner zone, meanwhile in the middle zone of the estuary the presence of these ciliates was almost nuil. The highest chlorophyll contribution derived from mixotrophic ciliates to total chlorophyll a ranged from 6% in the inner zone to 23% in the middle zone, both registered in autumn. The low abundance of mixotrophs in the middle zone of the estuary during the winter, as is usually observed in other coastal ecosystems, could be explained through a higher grazing pressure in this zone (top-down control) by mesozooplankton (e.g., copepods) in comparison to the inner zone. The secondary bloom of phytoplankton consistently observed during the last summers dominated by small sized diatoms and nanoplankton phytofagelates, could have sustained the high abundance of mixotrophic ciliates registered in the middle zone in autumn and summer.
Keywords: Mixotrophy; Chlorophyll concentration; Turbidity; Southern Atlantic
Resumen.
Dinámica de los ciliados mixótrofos en dos zonas de un estuario templado y turbio en Sud América, Argentina. La mixotrofía es una estrategia de alimentación por medio de la cual un organismo combina la autotrofa y la heterotrofa. La dinámica estacional de cinco ciliados mixótrofos comúnmente presentes en las aguas superfciales de la zona interna y media del estuario de Bahía Blanca, Argentina, rico en nutrientes, somero y con alta turbidez, se estudió mensualmente desde enero a diciembre de 2009. Se registraron los valores de temperatura, salinidad, turbidez y clorofla a, así como también la abundancia y biomasa de Strombidium capitatum, Strombidium acutum, Cyrtostrombidium sp., Lohmanniella oviformis y Tontonia appendiculariformis. La abundancia más alta de los mixótrofos se registró en el invierno en la zona interna, mientras que en la zona media del estuario la presencia de estos ciliados fue casi nula. La contribución más alta de clorofla proveniente de los mixótrofos a la clorofla a total fue 6% en la zona interna y 23% en la zona media (ambas estimaciones registradas en otoño). La baja abundancia de mixótrofos en la zona media del estuario durante el invierno, como usualmente es observado en otros sistemas costeros, podría ser explicada por una presión de pastoreo elevada en esta zona (control de tipo "top-down") por parte de mesozooplancton (e.g., copépodos), en comparación con la zona interna. La foración secundaria del fitoplancton observada durante los últimos veranos, dominada por diatomeas de pequeño tamaño y ftofagelados del nanoplancton, podría haber sustentado la alta abundancia de ciliados mixótrofos que se registró en la zona media en otoño y verano.
Palabras clave: Mixotrofía; Concentración de clorofla; Turbidez; Atlántico Sur
Introduction
In aquatic food webs, mixotrophy represents a spectrum of nutritional strategies from absolute heterotrophy to total autotrophy (Flynn et al. 2012). Whereas acquired phototrophy with endosymbiotic associations generally prevails in nutrient-poor environments, organelle retention is typical of more productive environments (Stoecker et al. 2009; Johnson 2011a-b; Flynn et al. 2012; Caron & Hutchins 2013). Therefore, mixotrophic planktonic protists diversify the flow of energy and nutrients by placing them at different trophic levels. Planktonic ciliates, many of which are mixotrophs (Pérez et al. 1997), are one of the most important ecological groups within microzooplankton, both in abundance and in composition in several marine environments. Mixotrophic ciliates are frequent in the water column mixing zone, particularly during stratification periods (Stoecker et al. 2009). Under these conditions, they generally reach their highest abundances, the most common being the kleptoplastidic oligotrichs (that retain algal plastids derived from their prey). In marine surface layers, mixotrophs are generally dominant among oligotrich ciliates (Stoecker et al. 2009), being also common in coastal and estuarine mixed environments. However, knowledge on the ecology of mixotrophic ciliates is still fragmentary (Sanders 1995; Pérez et al. 1997, 2000; Dolan and Marrasé 1995; Modigh 2001; Urrutxurtu et al. 2003; Mironova et al. 2011, 2013, 2014) and data are rather scarce worldwide.
Estuaries are very complex coastal environments. Local physicochemical and biological factors, such as the water column depth, light availability, nutrient turnover, grazing pressure and species-specific interactions, in addition to the particular longitudinal hydrological gradient and seasonal dynamic (temperature, salinity and turbidity), induce high variability in the distribution and structure of planktonic populations (McLusky and Elliott 2004). Primary production usually decreases towards the estuary head where turbidity, nutrient concentration and particulate organic matter are high (Kocum et al. 2002). Phytoplankton development is mainly regulated by light and nutrient availability, nevertheless, in turbid and shallow estuaries of temperate regions light is the chief factor acting on phytoplankton dynamics (Cloern 1987; Kocum et al. 2002). Light availability depends on the water turbidity whose concentration usually varies over the year due to the influence of tides, winds velocity and intensity, and freshwater inputs (Smayda 1983; Cole et al. 1992; Kocum et al. 2002).
The Bahía Blanca estuary, located on the southwest temperate Atlantic coast in Argentina, is a shallow, highly turbid and eutrophic ecosystem (Freije and Marcovecchio 2004; Guinder et al. 2009). A study carried out by Popovich and Marcovecchio (2008) on the spatial and temporal phytoplankton variability and the associated environmental factors in the main navigation channel of this estuary, showed that both phytoplankton abundance and biomass decrease from the inner to the outer estuary. So, these authors determined three physiographic zones along this spatial gradient (inner, middle and outer zones), concluding that the inner zone displays the maximum production of phytoplankton biomass during winter-early spring. The environmental conditions which promote the phytoplankton bloom seems be the high nutrient concentration in autumn, which coincides with the highest rainfall period for the region (Piccolo and Perillo 1990), the low grazing pressure by mesozooplankton due to low water temperature (Popovich and Marcovecchio 2008; Popovich et al. 2008; Pettigrosso and Popovich 2009) and the marked reduction in water turbidity (Guinder et al. 2009). The middle zone is euhaline, being the phytoplankton biomasses and nutrient contents lower than those registered in the inner zone and showing a less pronounced temporal (seasonal) variability (Popovich and Marcovecchio 2008). The outer estuarine zone is characterized by a higher influence from the shelf region yet, with lower phytoplanktonic biomass and nutrient content. The most representative area of the inner zone of the Bahía Blanca estuary is Puerto Cuatreros (Freije and Marcovecchio 2004), characterized as a semi-closed area with a restricted circulation and low advection, a relatively high residence time and a well-mixed water column all year round due to the effect of tides and winds (Perillo et al. 2001).
Although ciliates are one of the most important links in planktonic food webs, and in the Bahía Blanca estuary several studies on species composition, abundance and biomass of aloricate ciliates have been carried out (Pettigrosso 2003; Barría et al. 2003; Pettigrosso and Popovich 2009), there is no quantitative information to date on the spatial and seasonal variability of mixotrophic ciliates, particularly during an annual cycle. Thus, the objective of this study was to analyse the seasonal dynamics of five mixotrophic ciliates commonly found in the superficial waters of the inner and middle zones of the Bahía Blanca estuary. We hypothesized that a higher availability of food (phytoplankton bloom) in winter at the inner zone of the estuary favours the development and high abundances of mixotrophic ciliates. This fact could have effects on the magnitude of the primary production and efficiency of the plankton food web.
Materials and Methods
Study area
The Bahía Blanca estuary is located at 38°45'-39°40' S and 61°45'-62°30' W, in a temperate, semiarid zone on the Southern Atlantic coast in Argentina (Figure 1). The estuary is a mesotidal coastal plain environment formed by a series of NW-SE channels separated by interconnected tidal channels, islands, extensive tidal flats, and low marshes. The main navigation channel is funnel-shaped, with a total length of ~68 km and a width that varies from 200 m at the head (3 m depth) to 3-4 km at the mouth (22 m depth) (Perillo et al. 2001). Based on its physiographic characteristics, the main navigation channel is divided into three sections, namely: inner, middle and outer zone (Popovich and Marcovecchio 2008). The annual average transparency (depth of light penetration measured by Secchi disk) is 0.55 m in the inner zone, 2.04 m in the outer zone and 0.65 m at the middle zone (Popovich and Marcovecchio 2008). The water column is vertically homogeneous throughout the year although it could be partially mixed in the inner zone depending on the intensity of freshwater runoff. Surface water temperature is slightly higher at the head of the estuary. Water temperature seasonally varies between 4 and 26 °C, with an average of 21 and 8.5 °C in summer and in winter, respectively (Piccolo and Perillo 1990).

Figure 1. Map of the Bahía Blanca estuary showing the location of the two sampling sites: the inner zone (Puerto Cuatreros) and the middle zone (Boya 26).
Figura 1. Mapa del estuario de Bahía Blanca mostrando la ubicación de los dos sitios de muestreo: zona interna (Puerto Cuatreros) y zona media (Boya 26).
Mean surface salinity normally increases from the head to the mouth. Historical values of salinity vary throughout the year from 17.3 to 41.9 ups, with an average annual value of 31.61 ups in the inner zone, 32.91 ups in the middle, and 32.80 ups in the outer zone (Freije and Marcovecchio 2004; Popovich and Marcovecchio 2008). Oxidation and remineralization of organic matter is stimulated by the usually high levels of dissolved oxygen which generally are close to saturation level values due to the dynamics of the environment (Popovich et al. 2008). Freshwater input is, in general low, the main tributaries being the Sauce Chico and the Napostá Grande Rivers with a mean annual run-off of 1.9 and 0.8 m3/s, respectively (Perillo and Piccolo 1991).
Sampling was carried out monthly during daylight hours on the main navigation channel of the estuary from January 2009 to December 2009. Only one sample of both biotic and abiotic variables was taken at each two fixed stations. These were Puerto Cuatreros (PC) in the inner zone of estuary and Boya 26 (B26) located in its middle zone (Figure 1). Surface water samples (1 L) were collected using a 2.5 L Van Dorn bottle to determine chlorophyll a concentration (Chl-a), and to analyse mixotrophic ciliates. Temperature (°C), salinity and turbidity (NTU) were measured with a multisensor HORIBA U-10. Chlorophyll a was determined spectrophotometrically following Lorenzen and Jeffrey (1980). Mixotrophic ciliates were identified following Maeda and Carey (1985), Maeda (1986), Agatha and Riedel-Lorje (1997), Montagnes and Lynn (1991), Montagnes and Taylor (1994), Pettigrosso (2003). The genera and species considered in this study have been documented as mixotrophs from the literature (Pierce and Turner 1992; Bernard and Razouzadegan 1995; Modigh 2001; Stelfox-Widdicombe et al. 2004; Stoecker et al. 2009) and they have already been recognized as mixotrophs in this estuary (López Abbate et al. 2015). Samples for the estimation of abundance and biomass of mixotrophic ciliates were preserved in 2% acidified Lugol's solution (Stoecker et al. 1994). After gently mixing, a subsample of 50 ml was removed and sedimented in a cylinder combined chamber for 24-48 h following the Utermöhl's method after Hasle (1978). The mixotrophs in the chamber were counted and the linear dimensions (length and diameter) of each cell were measured at 400X magnification using a Nikon® Eclipse TE 300 inverted microscope and a Nikon® Sight DS-U2 digital camera. Biovolume of mixotrophs was estimated by associating the shape of each ciliate with standard geometric configurations (Montagnes and Lynn 1991). Carbon biomass (Biom) was estimated by converting cell volume into carbon weight using a factor of 0.19 pg C/µm3 (Putt and Stoecker 1989), and expressed as µg C/L.
Statistical analysis
To determine spatio-temporal variations in the studied biotic (abundance and biomass of mixotrophic ciliates) and environmental variables (temperature [T°], turbidity [Tu], salinity [S], chlorophyll a [Chl a] and depth [D]) between sites and seasons, two-way ANOVAs were performed by grouping the data corresponding to the three months of each season (n=24; df=3; dferror=20) and to the sites (n=24; df=1; dferror=22), respectively. No interaction between the factors (site and period) was found so variables were compared separately between each factor level (PC vs. B26, and among seasons). In all cases, mean comparisons were performed using ANOVA followed by Fisher's least significant difference (LSD) after checking the assumptions of normality and homoscedasticity. If assumptions were not met for ANOVA, data were log-transformed (Sokal and Rohlf 1999). Pearson correlations considering abundance, biomass, chlorophyll a, temperature, salinity and turbidity were performed for each sampling site using INFOSTAT software.
In addition, log transformed compositional mixotrophic ciliate data was converted into a similarity matrix using the Bray-Curtis index. Similarity percentage routines (SIMPER) were performed to detect the species, which contributed most to each sampling site or season. Analysis of similarity (ANOSIM) was applied to detect statistical differences in the species compositions between sampling sites and seasons (Clarke and Warwick 1994). These analyses were made using PRIMER-E6.
For comparison with other studies, the chlorophyll a content of mixotrophic ciliates was estimated using the relationship between cell volume and volume-specific chlorophyll a content in mixotrophic oligotrichs based on literature reports of direct measurements (Pérez et al. 2000). This relationship was applied to estimate chlorophyll content per cell, based on the cell size of the mixotrophic ciliates found in this study.
Results
Environmental variables
Water surface temperature ranged from 7.4 °C in July to 23.4 °C in December in the inner zone, while at the middle zone temperature varied from 7.4 °C in July to 22.9 °C in March (Figure 2a). There were no significant differences in temperature between the inner and middle zone (ANOVA: F=2.6x10-3; P=0.96).

Figure 2. Temporal variability of a) surface water temperature (°C), b) salinity and c) turbidity (NTU) at Puerto Cuatreros and Boya 26 from January 2009 to December 2009.
Figura 2. Variación temporal de a) temperatura superficial del agua (°C), b) salinidad y c) turbidez (NTU) en Puerto Cuatreros y Boya 26, desde enero a diciembre de 2009.
Water surface salinity fluctuated from 27.24 ups in July and August to 42.92 ups in January at the inner zone, and from 26.7 ups in July to 40.9 ups in January, at the middle zone (Figure 2b). No significant differences were shown in salinity between the sites all along the year (ANOVA: F=0.19; P=0.66), but it was different among the seasons (ANOVA: F=7.33; P<0.05) being significantly lower during winter in comparison with the other seasons, at the two sites studied (LSD Test: P<0.05) (Table 1).
Table 1. Seasonal mean and standard deviation (in brackets) of temperature, salinity, turbidity, chlorophyll a, abundance and biomass of mixotrophic ciliates in both sampling sites of the Bahía Blanca estuary: Puerto Cuatreros (inner zone) and Boya 26 (middle zone). Letters on the side of each variable indicate ANOVA and LSD test.
Tabla 1. Media estacional y desviación estándar (entre paréntesis) de temperatura, salinidad, turbidez, clorofila a, abundancia y biomasa de los ciliados mixotrófos en ambos sitios de muestreo en el Estuario de Bahía Blanca. Puerto Cuatreros (zona interna) y Boya 26 (zona media). Las letras al lado de cada variable indican el resultado de ANOVA y LSD test.
Surface turbidity varied from 23.4 and 35 NTU in July to 136 and 186 NTU in January, at the inner and middle zone respectively (Figure 2c). No differences in turbidity were detected between the sites (ANOVA: F=3.58; P=0.07), but a significant decrease was shown in winter differing with spring and summer (ANOVA: F=7.26; P<0.05; LSD Test: P<0.05) (Table 1).
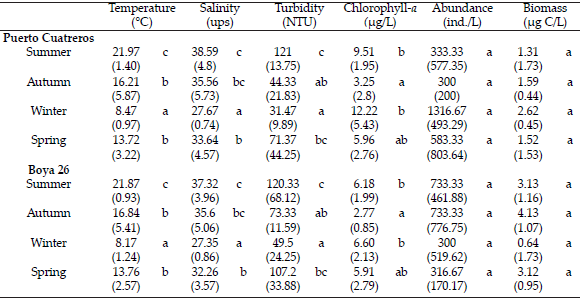
Chlorophyll a concentration showed the highestvalueattheinnerzone (17.33 µg/L) in July and the lowest valué (1.52 i-ig/L) in May. At the middle zone, it varied from 2.16 l-ig/L in April to 8.42 ug/Lin December (Figure 3). Chlorophyll a was no significantly different between the sites (ANOVA: F=4.11; P=0.06). It showed significant differences amongthe seasons (ANOVA: F=5.55;P<0.05), showing lower valúes in autumn and higher ones in surnmer and winter (LSD Test: P<0.05) (Table 1).
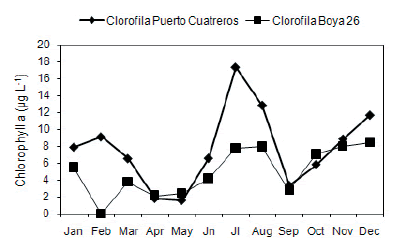
Figure 3. Temporal variability of chlorophyll a (µg/L) at Puerto Cuatreros and Boya 26 from January 2009 to December 2009.
Figura 3. Variación temporal de la concentración de clorofila a (µg/L) en Puerto Cuatreros y Boya 26, desde enero a diciembre de 2009.
Temporal and spatial variability and species composition of mixotrophic ciliates
The abundance and biomass of mixotrophic ciliates showed no clear pattern of distribution at any sampling site during the annual cycle (Figure 4a-b). Positive correlations between abundance and biomass were observed both at the inner and middle zone (r=0.89 and 0.72; P<0.05, respectively) (Figure 5a-c). The highest abundance valué of mixotrophic ciliates was 1.65xl03 cells/L, recorded at the inner zone during winter (July), along with the lowest valúes of temperature and turbidity. Significant negative correlation was registered at inner zone between abundance of mixotrophs and turbidity (r=-0.72; P<0.05) (Figure 5b). In winter, the máximum total aloricate ciliate abundance was also recorded, of which, mixotrophic ciliates accounted for 39.8%. At the middle zone, mixotrophic ciliates showed the highest abundances in autumn (March) and in surnmer (December and January), reaching 1.6x103 cells/L and 1x103 cells/L, respectively. A significantly positive correlation between abundance with turbidity and temperature was registered (r=0.69 and 0.61; P<0.05 respectively) (Figure 5d-e).
Figure 4. a) Temporal variability of mixotrophic ciliates abundance (ind./L) and b) biomass (ug C/L)at Puerto Cuatreros and Boya 26 from January 2009 to December 2009.
Figura 4. a) Variación temporal de la abundancia (ind./L) y b) de la biomasa (µg C/L) de los ciliados mixótrofos en Puerto Cuatreros y Boya 26 desde enero a diciembre de 2009.


Figure 5. Main statistically significant (p 0.05) Pearson correlations between variables, (N = 24, f.deg = 22). PC: a) abundance vs. biomass. b) abundance vs. turbidity. Boya 26: c) abundance vs. biomass. d) abundance vs. turbidity. e) abundance vs. temperature. f) biomass vs. temperature.
Figura 5. Principales correlaciones de Pearson estadísticamente significativas (p 0,05) entre las variables, (N =24, g. l. = 22). PC: a) abundancia vs. biomasa. b) abundancia vs. turbidez. Boya 26: c) abundancia vs. biomasa. d) abundancia vs. turbidez. e) abundancia vs. temperatura. f) biomasa vs. temperatura.
Regarding mixotrophic biomass, at the in-ner zone, the highest values were recorded, in spring (November) representing 28% in relation to total aloricate biomass. At the middle zone, the maximum pick of biomass was registered in autumn (March), representing 90% of the total aloricate biomass. Posi-tive correlation between mixotrophic ciliates biomass and temperature (r=0.58; P<0.05) was registered (Figure 5f). There were not statistical dif f erences between sites and among seasons neither in abundance ñor in biomass (ANOVA: P>0.05).
The mixotrophic genera and species studied were Cyrtostrombidium sp. Lynn and Gilron 1993; Strombidium acutum Leegaard 1915; Tontonia appendiculariformis Fauré-Fremiet 1914; Strombidium capitatum (Leegaard 1915) Kahl 1932, and Lohmanniella oviformis Leegaard 1915. In terms of species composition, ANOSIM showed no difference between the sampling sites (Global R=-0.031; P=0.054), whereas differences were found among the seasons (ANOSIM, Global R=0.179; P<0.05). According to SIMPER, Cyrtostrombidium sp. was the only species which contributed to summer (100%), while S. capitatum was the main contributor to autumn (71.97%), and T. appendiculariformis contributed (100%) to winter and spring, of total aloricate ciliates.
Estimation of chlorophyll content in mixotrophic ciliates
The chlorophyll content of L. oviformis, S. capitatum, S. acutum, Cyrtostrombidium sp. and T. appendiculariformis in this ecosystem were 3.62xl-3, 1.4xl0-3, 3.5xl0-3, 3.8xl0-3 and 1.5xl0-3 pg chlorophyll a/µm3, respectively. The valúes were then transformed to µg Chl/L to estímate the contribution of chlorophyll content in mixotrophic ciliates to total chlorophyll.
In the inner zone, the highest valué of chlorophyll derived f rom mixotrophic ciliates was observed in winter and in the middle zone, in autumn. In relation to the contribution of the chlorophyll derived from mixotrophic ciliates to the total phytoplankton chlorophyll, the highest valúes were observed in autumn at both sites. In the inner zone, the highest valué (6.5%) of chlorophyll was reached mainly by the presence of S. capitatum and T. appendiculariformis sp., whereas in the middle zone (23%), by the contribution of S. capitatum and Cyrtostrombidium sp.
Discussion
Mixotrophic ciliates and environmental variables
The variables analyzed in the present study (temperature, salinity, turbidity and chlorophyll a) were not significantly different at the two sampling sites, but along the year, chlorophyll a concentration showed its highest value in winter along with the lowest turbidity, especially at the inner zone. The latter is consistent with findings from previous reports in the estuary (Popovich et al. 2008; Popovich and Marcovecchio 2008; Guinder et al. 2012). Following the trend of the cholorophyll a, mix-otrophic ciliates showed their highest abundance in the inner zone in winter, even though correlations between these variables were not significant. Even though in estuaries there are no reports, to our knowledge, in some of early studies carried out in non-estuarine environments (Catalan Sea), the lack of correlation between mixotrophic ciliates and chlorophyll a was considered a reasonable result because mixotrophs appear to be mainly restricted to the surface and show distributional patterns affected by vertical migrations which may vary at different hours of the day and from ecosystem to ecosystem (Dolan and Marrasé 1995). On the other hand, in the Liguria Sea (NW Mediterranean), Pérez et al. (2000) studied the vertical distribution of planktonic ciliates in relation to vertical distribution of chlorophyll and found positive correlation between mixotrophs and chlorophyll only in discrete samples, but reported a lack of correlation when samples were integrated through the water column, although a positive relationship was found for some species when considered independently.
In our study, turbidity showed a great variability throughout the year, however, at both sampling sites, inner and middle zone turbidity showed similar values during winter but slightly higher in the middle estuary zone. In shallow estuaries, turbidity is considered one of the main factors influencing the seasonal variation of the phytoplankton community. Studies carried out by Popovich and Marcovecchio (2008) and Guinder et al. (2012), showed that under similar conditions of turbidity in the inner and middle zones of the Bahía Blanca estuary, phytoplankton bloom develops only in the inner zone. In the inner zone, phytoplankton blooms occur even in a thin euphotic zones in a completely mixed water column due to the shallowness of the area and the decreasing turbidity, the phytoplankton cells remain below the euphotic zone during short periods of time as they are constantly resuspended into the surface layers by vertical mixing processes (Popovich and Marcoveccio 2008; Guinder et al. 2012). Given that mixotrophic ciliates are in general restricted to the euphotic zone of the water column (Stoecker et al. 2009; Dolan and Marrasé 1995), the food availability in the water surface layer, represented by the chlorophyll concentration, could be one of the main conditions that sustained the high abundance of mixotrophic ciliates during winter in the inner zone. On the other hand, although in this study mesozooplankton abundance was neither addressed nor estimated, a decrease in the number of planktonic metazoans (e.g., copepods) is usually observed in this estuary in winter (Hoffmeyer 2004; Pettigrosso and Popovich 2009). The consequent low predation pressure (e.g., from copepods and invertebrate larvae) may have supported the increase in the ciliates abundance.
In contrast, in the middle zone during winter, a low abundance of mixotrophic ciliates was observed along with a low chlorophyll a concentration. In this zone, a strong decrease of phytoplankton biomass was recorded as a consequence of both, deep-mixed depths and a low euphotic/mixing depth ratio (Popovich and Marcovechio 2008; Guinder et al. 2012). Considering that mixotrophic ciliates are selective consumers, retain functional plastids from ingested algal, that there is no evidence of plastid reproduction inside ciliate cytoplasm and are dependent on algal food to replace their plastids, the scarce number of mixotrophs in winter may be related to the lower availability of suitable food. On the other hand, in the last years, a secondary bloom of phytoplankton was consistently observed during summer dominated by small sized diatoms and nanoplankton phytoflagellates (Guinder et al. 2012; Popovich and Marcovecchio 2008) which could have sustained the high abundance of mixotrophic ciliates observed in the middle zone in autumn and summer.
Although phytoplankton and mixotrophic ciliates have different nutrition modes, and being that food source is one of the main factors that modulates the community structure of ciliates, we consider that peculiar features between the inner and middle zone of the Bahía Blanca estuary, which affect the pelagic distribution of phytoplankton communities could also have influenced indirectly the distribution of mixotrophic ciliates. Thus, the greatest biomass of ciliates in temperate estuaries and coastal environments follows the pattern of the phytoplankton bloom (Lynn et al. 1991; Johansson et al. 2004). In the inner estuary, previous studies have shown that the maximum biomass of total aloricate ciliates occurred during winter, immediately after the phytoplankton peak (Pettigrosso & Popovich 2009) which agrees with our findings. However, it is noteworthy the almost null abundance of mixotrophs found in the middle zone of the estuary during the winter, as is usually observed in other estuaries and coastal ecosystems where mixotrophs commonly decrease in abundance and biomass during the coldest season (see Table 2). Even though we did not find significant differences in abundance and biomass between both sampling sites, the almost null abundance of mixotrophic ciliates in the middle zone during winter could be explained through a higher grazing pressure (top-down control) by mesozooplankton (e.g., copepods) in this zone, in comparison to the inner zone.
Table 2. Mixotrophic ciliates in different marine ecosystems. Abundance in ind./L and biomass in µg C/L.
Tabla 2. Ciliados mixótrofos en diferentes ecosistemas marinos. Abundancia en ind./L y biomasa en µg C/L.

Mixotrophic ciliates contribution to the abundance and biomass of aloricate ciliates and species composition
Mixotrophic ciliates contribution to total aloricate ciliates during the winter was higher than the relative values reported in other studies (Table 2). In Atlantic coastal waters, mixotrophs represent only 10% of the total ciliates abundance in the cold seasons (Stoecker et al. 2009). Except for winter, similar relative values of mixotrophic ciliates recorded in this study were observed in other environments, (Stoecker et al. 1996; Ota and Taniguchi 2003; Stoecker et al. 2009; Mironova et al. 2013).
Although previous research has reported low percentages of mixotrophic ciliates in nutrient-rich environments and higher abundances of mixotrophic ciliates in oligo-to-meso-oligotrophic waters (Bernard and Rassoul-zadegan 1995), our findings confirm recent studies on the presence of this type of ciliates in nutrient-rich environments (Stoecker et al. 2009; Mironova et al. 2013; López Abbate et al. 2015), such as the Bahía Blanca estuary (Freije and Marcovecchio 2004).
The mixotrophic oligotrichs and choreotrichs studied belong to the genera Cyrtostrombidium, Tontonia, Strombidium and Lohmanniella. These genera include the most common and abundant mixotrophic ciliates in the mixed layer of marine and estuarine waters (Bernard and Rassoulzadegan 1995; Stoecker et al. 2009). According to Agatha (2011), Cyrtostrombidium, Tontonia and Lohmanniella genera occur exclusively in marine and brackish sea waters, whereas Strombidium includes both marine and freshwater species. On the other hand, Tontonia, Lohmanniella and Strombidium have been reported showing a cosmopolitan distribution (Agatha 2011), while Cyrtostrombidium has been reported to be restricted to the northern hemisphere. Nevertheless, previous research has reported Cyrtostrombidium genus in the Bahía Blanca estuary (Pettigrosso 2003; Barría et al. 2003; Pettigrosso and Popovich 2009).
The association of the five species of mixotrophic ciliates reported in this study showed some seasonal differences in composition and it could be a response to the particular environmental characteristics of the Bahía Blanca estuary. This was evident, for example in the inner zone in winter when the biomass peak was dictated by a predominance of T. appendiculariformis. In the Catalan Sea, Dolan & Marrase (1995) considered to T. appendiculariformis one of the commonest type of the larger mixotrophic species which was generally restricted to 0 to 20 m in depth. Several studies on T. appendiculariformis showed a rapid response to the increase of nutrients in mesocosm experiments (Gismervik et al. 2002). This species alone represented 74.1% of the ciliate biomass and 51.9% of the ciliate abundance in a coastal area of the eastern English Channel (Grattepanche et al. 2011). Furthermore, T. appendiculariformis proved to be an important contribution of carbon ingested by copepods in Oregon coastal Waters (Fessenden and Cowles 1994).
Contribution of mixotrophic ciliates to chlorophyll a
Given that literature data on the contribution of the chlorophyll derived from mixotrophs are sparse and variable, and the contribution of ciliates to chlorophyll is very sensitive to the factors used to estimate chlorophyll per biovolume unit and to the mix of occurring species, it is difficult to make comparisons (Stoecker et al. 2014). However our findings are in the range of those reported in the literature (Stoecker et al. 2009).
The estimated chlorophyll a contribution from the mixotrophic ciliates to the total phytoplankton chlorophyll a showed that in both, the inner and middle zones of the Bahía Blanca estuary, the highest values (6% and 23%, respectively) were observed in autumn, when the average of total chlorophyll a at both sites was the lowest: ≤3.2 µg Chl/L. Phytoplankton is composed by phytoflagellates, commonly documented in the inner and middle zone of the estuary during these seasons by Popovich and Marcovecchio (2008). Under these conditions photosynthesis from mixotrophs in the > 20 microns size class of the upper layer of the water column can be important (Stoecker et al. 1989-2009; Putt 1990).
On the other hand, the size fraction of the mixotrophic ciliates observed in this estuary are particularly important as prey for copepods (Dutz and Peters 2008) and young fish larvae (Figueiredo et al. 2007). Studies have demonstrated that photosynthesis in ciliates may promote the microzooplankton efficiency and could represent a significant flow of carbon to higher trophic levels. In addition, an increase in the gross growth efficiency in planktonic ciliates may increase nutrient regeneration (Dolan and Marrasé 1995; Dolan and Pérez 2000; Stoecker et al. 2009). According to Hammer and Pitchford (2005), mixotrophs due to their capacity of being both producers and consumers, would influence the structure and productivity of microbial food webs and the coupling to metazoan production.
If we consider that the estimations of chlorophyll of mixotrophs registered in this study were obtained only from five mixotrophic species of the superficial layer, the contribution of the chlorophyll from the mixotrophic ciliates in the Bahía Blanca estuary would be underestimated, and the phytoplankton biomass would be probably overestimated.
Despite the preliminary character of this study, our findings showed that the environmental factors in the time scale analyzed did not determine spatial differences in the abundance and biomass of mixotrophic ciliates. Thus, the planted hypothesis could not be verified. Further research is required to better understand the dynamic of mixtrophic ciliates and its environmental response in this turbid and mixed estuary.
Acknowledgements
The present research was supported by grant PICT 2011 - 2096 (ANPCYT) to MSH, Argentina. Authors are grateful to Ana Martínez for her help in statistical analyses and to the staff involved in the field work. Authors also acknowledge W. D. Melo for drawing the map.
References
1. Agatha, S., and J. C. Riedel-Lorjé. 1997. Taxonomy and ecology of some Oligotrichea from brackish water basins at the west coast of Schleswig-Holstein (Northern Germany). Journal of Eukaryotic Microbiology 44:27A. [ Links ]
2. Agatha, S. 2011. Global diversity of aloricate Oligotrichea (Protista, Ciliophora, Spirotricha) in marine and brackish sea water. PLoS ONE 6:e22466. [ Links ]
3. Barría De Cao, M. S., R. E. Pettigrosso, E. R. Parodi, and H. Freije. 2003. Abundance and species composition of the planktonic ciliates from the waste water discharge zone in the Bahía Blanca Estuary, Argentina. Iheringia. Serie Zoologia 93:229-236. [ Links ]
4. Bernard, C., and F. Rassoulzadegan. 1995. Seasonal variations of mixotrophic ciliates in the northwest Mediterranean Sea. Marine Ecology Progress Series 108:295-301. [ Links ]
5. Caron, D. A., and D. A. Hutchins. 2013. The effects of changing climate on microzooplankton grazing and community structure: drivers, predictions and knowledge gaps. Journal of Plankton Research 35:235-252. [ Links ]
6. Clarke, K. R., and R. M. Warwick (eds.). 1994. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation. Natural Environment Research. Council. Plymouth. United Kingdom. Pp. 144. [ Links ]
7. Cloern, J. E. 1987. Turbidity as a control on phytoplankton biomass and productivity in estuaries. Continental Shelf Research 7:1367-1381. [ Links ]
8. Cole, J. J., N. F. Caraco, and B. L. Peierls. 1992. Can phytoplankton maintain a positive carbon balance in a turbid, freshwater, tidal estuary? Limnology and Oceanography 37:1608-1617. [ Links ]
9. Dolan, J. R., and C. Marrase. 1995. Planktonic ciliate distribution relative to a deep chlorophyll maximum: Catalan sea, NW Mediterranean, June 1993. Deep-Sea Research 42(I):1965-1987. [ Links ]
10. Dolan, J. R., and M. T. Pérez. 2000. Costs, benefts and characteristics of mixotrophy in marine oligotrichs. Freshwater Biology 45:227-238. [ Links ]
11. Dutz, J., and J. Peters. 2008. Importance and nutritional value of large ciliates for the Reproduction of Acartia clausi during the post spring-bloom period in the North Sea. Aquat Microb Ecol 50:261-277. [ Links ]
12. Fessenden, L., and T. J. Cowles. 1994. Copepod predation on phagotrophic ciliates in Oregon coastal waters. Marine Ecology Progress Series 107:103-111. [ Links ]
13. Figueiredo, G. M., R. D. M. Nash, and D. J. S. Montagnes. 2007. Do protozoa contribute signifcantly to the diet of larval fsh in the Irish Sea? J Mar Biol Assoc UK 87:843-850. [ Links ]
14. Flynn, K. J., D. K. Stoecker, A. Mitra, J. A. Cuervo, P. M. Glibert, P. J. Hansen, E. Graneli, and J. M. Burkholder. 2012. Misuse of the phytoplankton-zooplankton dichotomy: the need to assign organisms as mixotrophs within plankton functional types. Journal Plankton Research 35:3-11. [ Links ]
15. Freije, R. H. & J. E. Marcovecchio. 2004. Oceanografía química. Pp. 69-78 in: M. C. Piccolo and M. Hoffmeyer (eds.). El ecosistema del estuario de Bahía Blanca. Instituto Argentino de Oceanografía, Bahía Blanca. Argentina. [ Links ]
16. Gismervik, I., Y. Olsen, and O. Vadstein. 2002. Micro- and mesozooplankton response to enhanced nutrient input-a mesocosm study. Hydrobiologia 484:75-87. [ Links ]
17. Grattepanche, J.D., E. Breton, J. M. Brylinski, E. Lecuyer, and U. Christaki. 2011. Succession of primary producers and micrograzers in a coastal ecosystem dominated by Phaeocystis globosa blooms. Journal of Plankton Research 33: 37-50. [ Links ]
18. Guinder, V.A., C. A. Popovich, and G. Perillo. 2012. Phytoplankton and Physicochemical Analysis on the Water System of the Temperate Estuary in South America: Bahía Blanca Estuary, Argentina. International Journal of Environmental Research 6:547-556. [ Links ]
19. Guinder, V. A., C. A. Popovich, and G. M. E. Perillo. 2009. Particulate suspended matter concentrations in the Bahía Blanca Estuary, Argentina: Implication for the development of phytoplankton blooms. Estuarine Coastal and Shelf Science 85:157-165. [ Links ]
20. Hammer, A. C. & J. W. Pitchford. 2005. The role of mixotrophy in plankton bloom dynamics, and the consequences for productivity. Journal of Marine Science 62:833-840. [ Links ]
21. Hasle, G. R. 1978. Using the inverted microscope. Pp: 191-196 in: A. Sournia (ed.). Phytoplankton manual. UNESCO, Paris. [ Links ]
22. Hoffmeyer, M. S. 2004. Mesozooplancton. Pp. 133-141 in: M. C. Piccolo and M. S. Hoffmeyer. (eds.). El ecosistema del estuario de Bahía Blanca. Instituto Argentino de Oceanografía. Bahía Blanca, Argentina. [ Links ]
23 . Johansson, M., E. Gorokhova, and U. Larsson. 2004. Annual variability in ciliate community structure, potential prey and predators in the open northern Baltic Sea proper. Journal Plankton Research 26:67-80. [ Links ]
24. Johnson, M.D. 2011a. Acquired phototrophy in ciliates: a review of cellular interactions and structural adaptations. Journal Eukaryotic Microbiology 58:185-195. [ Links ]
25. Johnson, M. D. 2011b. The acquisition of phototrophy: adaptive strategies of hosting endosymbionts and organelles. Photosynthesis Research 107:117-132. [ Links ]
26. Kocum, E., G. J. C. Underwood, and D. B. Nedwell. 2002. Simultaneous measurement of phytoplanktonic primary production, nutrient and light availability along a turbid, eutrophic UK east coast estuary (the Colne Estuary). Marine Ecology Progress Series 231:1-12. [ Links ]
27. López Abbate, M. C., J. C. Molinero, V. A. Guinder, M. S. Dutto, S. M. Barria De Cao, L. A. Ruiz Etcheverry, R. E. Pettigrosso, M. C. Carcedo, and M. S. Hoffmeyer. 2015. Microplankton dynamics under heavy anthropogenic pressure. The case of the Bahía Blanca Estuary, southwestern Atlantic Ocean Mar. Marine Pollution Bulletin 95:305-314. [ Links ]
28. Lorenzen, C. J., and S. W. Jeffrey. 1980. Determination of chlorophyll in seawater. UNESCO Technical Papers in Marine Science 35:1-20. [ Links ]
29. Lynn, D. H., D. J. S. Montagnes, T. Dale, G. L. Gilron, and S. L. Strom. 1991. A reassessment of the genus Strombidinopsis (Ciliophora, Choreotrichida) with descriptions of four new planktonic species and remarks on its taxonomy and phylogeny. Journal of the Marine Biological Association UK 71:597-602. [ Links ]
30. Maeda, M., and P. Carey. 1985. An illustrated guide to the species of the family Strombiidae (Oligotrichida, Ciliophora), free swimming protozoa common in the aquatic environment. Bulletin of the Ocean Research Institute, University of Tokyo 19:1-68. [ Links ]
31. Maeda, M. 1986. An illustrated guide to the species of the families Halteriidae and Strobilidiidae (Oligotrichida, Ciliophora), free swimming protozoa common in the aquatic environment. Bulletin of the Ocean Research Institute, University of Tokyo 21:1-67. [ Links ]
32. Mclusky, D. S., and M. Elliott (eds.). 2004. The Estuarine Ecosystem: Ecology, Threats and Management, 3rd ed. University Press, Oxford. Pp. 214. [ Links ]
33. Mironova, E., I. Telesh, and S. Skarlato. 2011. Diversity and seasonality in structure of ciliate communities in the Neva Estuary (Baltic Sea). Journal Plankton Research 34:208-220. [ Links ]
34. Mironova, E., I. Telesh & S. Skarlato. 2013. Planktonic Ciliates of the Neva Estuary (Baltic Sea): Community Structure and Spatial Distribution. Acta Protozoology 52:13-23. [ Links ]
35. Mironova, E., I. Telesh, and S. Skarlato. 2014. Ciliates in plankton of the Baltic Sea. Protistology 8:81-124. [ Links ]
36. Modigh, M. 2001. Seasonal variations of photosynthetic ciliates at a Mediterranean coastal site. Aquatic Microbiol Ecology 23:163-175. [ Links ]
37. Montagnes, D. J. S., and D. H. Lynn. 1991. Taxonomy of the major groups of marine planktonic ciliates, with emphasis on the aloricate forms. Marine Microbial Food Webs 5:59-74. [ Links ]
38. Montagnes, D. J. S., and F. J. R. Taylor. 1994. The salient features of fve marine ciliates in the class Spirotrichea (Oligotrichia), with notes on their culturing and behaviour. Journal of Eukaryotic Microbiology 41:569-586. [ Links ]
39. Ota, T., and A. Taniguchi. 2003. Standing crop of planktonic ciliates in the East China Sea and their potential grazing impact and contribution to nutrient regeneration. Deep-Sea Research 50:423-442. [ Links ]
40. Pérez, M. T., J. R. Dolan, and E. Fukai. 1997. Planktonic oligotrich ciliates in the NW Mediterranean: growth rates and consumption by copepods. Marine Ecology Progress Series 155:89-101. [ Links ]
41. Pérez, M. T., J. R. Dolan, F. Vidussi, and E. Fukai. 2000. Diel vertical distribution of planktonic ciliates within the surface layer of the NW Mediterranean (May 1995). Deep-Sea Research 47:479-503. [ Links ]
42 .Perillo, G. M. E., M. C. Piccolo, E. R. Parodi, and R. H. Freije. 2001. The Bahía Blanca Estuary ecosystem: a review. Pp. 205-217 in: U. Seelinger, L. Lacerda, and B. Kjerve (eds.). Coastal Marine Ecosystems of Latin America. Springer Verlag, Heidelberg, Germany. [ Links ]
43. Perillo, G. M. E., and M. C. Piccolo. 1991. Tidal response in the Bahía Blanca estuary, Argentina. Journal of Coastal Research 7:437-449. [ Links ]
44. Pettigrosso, R. E., and C. A. Popovich. 2009. Phytoplankton- aloricate ciliate community in the Bahía Blanca Estuary (Argentina): Seasonal patterns and trophic groups. Brazilian Journal of Oceanography 57:215-227. [ Links ]
45. Pettigrosso, R. E. 2003. Planktonic Ciliates Choreotrichida and Strombidiida from the Inner zone of the Bahía Blanca Estuary, Argentina. Iheringia Serie Zoologia 93:117-126. [ Links ]
46. Piccolo, M. C., and M. E. Perillo. 1990. Physical characteristics of the Bahía Blanca estuary Argentina. Estuarine, Coastal and Shelf Science 31:303-317. [ Links ]
47. Pierce, W. R., and J. T. Turner. 1992. Ecology of planktonic ciliates in marine food webs. Florida, Reviews in Aquatic Science 6(2):139-181. [ Links ]
48. Popovich, C. A., and J. E. Marcovecchio. 2008. Spatial and temporal variability of phytoplankton and environmental factors in a temperate estuary of South America (Atlantic coast, Argentina). Continental Shelf Research 28:236-244. [ Links ]
49. Popovich, C. A., C. V. Spetter, R. H. Freije, and J. E. Marcovecchio. 2008. Dissolved Nutrients Availability during winter diatom bloom in a turbid and shallow estuary (Bahía Blanca, Argentina). Journal of Coastal Research 24:95-102. [ Links ]
50. Putt, M., and D. Stoecker. 1989. An experimentally determined carbon:volume ratio for marine ‘oligotrichous' ciliates from estuarine coastal waters. Limnology Oceanography 34:1097-1103.
51. Putt, M. 1990. Abundance, chlorophyll content and photosynthetic rates of ciliates in the Nordic Seas during summer. Deep-Sea Res A 37:1713-1731. [ Links ]
52. Sanders, R. W. 1995. Seasonal distributions of the photosynthesizing ciliates Laboea strobila and Myrionecta rubra (=Mesodinium rubrum) in an estuary of the Gulf of Maine. Aquatic Microbial Ecology 9:237-242. [ Links ]
53. Smayda, T. J. 1983. The phytoplankton of estuaries. Pp. 65-102 in: B. H. Ketchum (ed.). Estuaries and Enclosed Seas. Elsevier, Amsterdam. [ Links ]
54. Sokal, R. R., and F. J. Rohlf (eds.). 1999. Introducción a la Bioestadística. Editorial Reverté SA, Barcelona, España. Pp. 362. [ Links ]
55. Stelfox-Widdicombe, C. E., S. D. Archera, P. H. Burkilla, and J. Stefelsc. 2004. Microzooplankton grazing in Phaeocystis and diatom-dominated waters in the southern North Sea in spring. Journal of Sea Research 51:37-51. [ Links ]
56. Stoecker, D. K., D. J. Gifford, and M. Putt. 1994. Preservation of marine planktonic ciliates: losses and cell shrinkage during fxation. Marine Ecology Progress Series 110:293-299. [ Links ]
57. Stoecker, D. K., D. E. Gustafson, and P. G. Verity. 1996. Micro- and mesoprotozooplankton at 140°W in the equatorial Pacifc: heterotrophs and mixotrophs. Aquatic Microbial Ecology 10:273-282. [ Links ]
58. Stoecker, D, K., M. D. Johnson, C. De Vargas & F. Not. 2009. Acquired Phototrophy in Aquatic Protists. Aquatic Microbial Ecology 57:279-310. [ Links ]
59. Stoecker, D.K., M. W. Silver, A. E. Michaels, and L. H. Davis. 1988-1989. Enslavement of algal chloroplasts by fourStrombidium spp. (Ciliophora, Oligotrichida). Marine Microbial Food Webs 3:79-100. [ Links ]
60. Stoecker, D. K., A. C. Weigel, D. A. Stockwell, and M. W. Lomas. 2014. Microzooplankton: Abundance, biomass and contribution To chlorophyll in the Eastern Bering Sea in summer. Deep-Sea Research II 109:134-144. [ Links ]
61. Urrutxurtu, I., E. Orive, and A. Sota. 2003. Seasonal dynamics of ciliated protozoa and their potential food in an eutrophic estuary (Bay of Biscay). Estuarine Coastal and Shelf Science 57:1169-1182 [ Links ]














