Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Ecología austral
On-line version ISSN 1667-782X
Ecol. austral vol.26 no.3 Córdoba Dec. 2016
ARTÍCULOS
Relationship between phytoplankton and bacterioplankton production in vegetated humic shallow lakes
Alex Aguilar Zurita1, 3 & Patricia Rodríguez2, 3, *
1 Defense against desertification and land conservation office (DSyLCD), Ministry of Environment and Sustainable Development (MayDS). Ciudad de Buenos Aires, Argentina (current address).
2 Austral Centre for Scientific Research (CADIC-CONICET). Ushuaia, Tierra del Fuego, Argentina (current address).
3 Departamento de Ecología, Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires.
* patricia.rodriguez@cadic-conicet.gob.ar
Editora asociada: María Dieguez
Recibido: 11 de diciembre de 2015 Aceptado: 5 de abril de 2016
ABSTRACT
In this study, we show that depth-integrated pelagic primary production (PP) can exceed bacterioplankton production (BP) in vegetated humic shallow lakes, giving as a result an autotrophic water column, despite light restrictions and availability of organic carbon for lake bacteria. Intuitively, these conditions should favor the development of a heterotrophic water column. Instead, during our survey, BP represented between 1.3 to 5% of PP most of the time. Only once, during late summer, BP was ~71% of PP. Although we cannot conclude about the mechanisms behind the observed results, previous surveys and experimentation in the wetland allow us to hypothesize that autotrophic conditions were favored by: i) the shallow nature of the lakes, which compensates for light attenuation by organic matter when integrating production in the water column, ii) the presence of anaerobic anoxygenic photosynthetic bacteria below the macrophyte cover, and iii) high predation rates on bacterioplankton by heterotrophic flagellates below the floating plants.
Keywords: Primary production; floating plants
RESUMEN
Relación entre la producción primaria y bacteriana pelágicas en dos lagunas húmicas vegetadas. En este trabajo se muestra que la producción primaria del fitoplancton (PP) puede exceder la producción bacteriana (BP) en cuerpos de agua húmicos vegetados, dando como resultado una columna de agua autotrófica, a pesar de ser ambientes restringidos lumínicamente y con alta concentración de carbono orgánico disuelto, sustrato de la producción bacteriana. Intuitivamente, estas condiciones favorecerían el desarrollo de una columna de agua heterótrofa. Por el contrario, en este estudio la BP representó entre 1.3 y 5% de la PP durante la mayor parte de los muestreos.Sólo una vez, durante el verano tardío, BP llegó al 71% de la PP. Si bien no podemos determinar el mecanismo detrás de estos resultados, sí podemos hipotetizar acerca de ellos basados en experimentación y estudios previos en el mismo humedal. De este modo, las condiciones de autotrofa se ven favorecidas principalmente por: i) la naturaleza somera de las lagunas, que amortigua el efecto de la atenuación lumínica al considerar tasas integradas de producción en toda la columna de agua, ii) la presencia de bacterias fotosintéticas anaeróbicas y anoxigénicas bajo la cobertura macrofítica, y iii) tasas elevadas de depredación sobre el bacterioplancton por parte de nanoflagelados heterotróficos.
Palabras clave: Producción fitoplanctónica; Plantas flotantes; Lagunas someras
INTRODUCTION
At the global scale, shallow lakes are among the most abundant type of aquatic ecosystems (Downing et al. 2006) and are recognized as important sources of carbon dioxide (CO2) to the atmosphere (Battin et al. 2009). The magnitude by which a lake behaves as a source or sink of greenhouse gases to the atmosphere depends on several factors. One of most significant processes that consume CO2 is primary production (PP), which, in turn, is counteracted by respiration (R) of organic substrata in the entire water body (Falkowski and Raven 2007). When the CO2 respired in the lake equals to the amount of CO2 fixed in the process of photosynthesis, the ecosystem is in metabolic balance (i.e., PP=R). In net autotrophic lakes (PP>R), the non-respired autochthonous organic matter is either available for storage, export or non-biological oxidation (Lovett et al. 2006). Hence, there is a net CO2 consumption in the water body and photosynthesis constitutes the main pathway by which the energy is mobilized to higher trophic levels (Jansson et al. 2000). When the CO2 respired in the aquatic system exceeds the primary production (net CO2 production), the respiration of organic substrata must be somehow subsidized with organic matter that is imported from the catchment (i. e., produced outside the lake ecosystem). This is generally the case for nutrient-poor ecosystems, which are net sources of CO2 to the atmosphere since respiration is higher than the production of autochthonous organic matter (R>PP) (del Giorgio et al. 1997; Cole et al. 2000). Therefore, heterotrophic bacteria mobilize energy from the base of the food web to higher trophic levels (Jansson et al. 2000).
Bacterial production (BP) is related to bacterial respiration (BR) through bacterial growth efficiency (BGE=BP/[BR+BP]), which is in general lower than 50% (del Giorgio and Cole 1998). Therefore, the degree to which a water body will behave as net autotrophic or heterotrophic may be assessed from the comparison among primary production and bacterial production if BGE is known or assumed to be of a certain magnitude. Hence, a PP/BP ratio lower than 1 indicates that the carbon fixed by photosynthesis is insufficient to provide organic substrata for bacteria.
In humic lakes, light absorption by organic matter itself reduces its availability for autotrophic organisms in the water column (Jones 1992). Thus, it could be expected that bacterioplankton production (BP) will exceed phytoplankton production (PP) in humic lakes (Ask et al. 2009). Little is known about carbon metabolism in the water column of vegetated humic lakes. Therefore, in this study, we analyze the metabolic quotient PP/BP in two humic shallow lakes covered with free floating plants, which worsen light limitation (de Tezanos Pinto and O'Farrell 2014). As pelagic production is ca. 20 times higher than periphyton production in these lakes (Rodríguez and Pizarro 2015), we focused our investigations in the water column and hypothesized the water column of these systems to be net heterotrophic (PP/BP<1).
MATERIALS AND METHODS
The study was performed in two humic shallow lakes (maximum depth <1.5 m) from the Paraná river floodplain, in the Otamendi Natural Reserve, Buenos Aires province, Argentina (34°14' S - 58°50' W). The distance between the lakes is ~1 km. Grande Lake (GL) is 156 ha, with a maximum depth of 0.7 m and mean depth of 0.5 m. The oxbow lake (OL), much smaller, is 17 ha, has a maximum depth of 0.5 m and a mean depth of 0.3 m. Both water bodies were almost completely covered with free floating plants during the study period. In GL, the most abundant floating plant was Pistia stratiotes, while in the OL there was a mixed assemblage of Ricciocarpus natans, Azolla filiculoides, Wolffiella oblonga and Lemna spp. Three samplings were carried out in 2010 during late summer (February 23nd, March 2nd and 8th) and three in late winter (September 9th, 14th and 21th).
Physical and chemical variables
Temperature, pH and conductivity were measured with a HANNA HI 991301 portable meter and dissolved oxygen with a HANNA HI 9146 probe. Photosynthetic available radiation (PAR, 400-700 nm) was measured every 5 cm in the water column with a spherical quantum sensor (LI-193SA, Li-Cor, Lincoln, USA) to calculate the vertical attenuation coefficient (kd) as the slope of the regression between the natural logarithm of irradiance versus depth (Kirk 2011).
Integrated water samples were taken with a PVC tube sampler (1 m length, 5 cm inner diameter) and transported refrigerated to the laboratory for further analysis. Dissolved inorganic carbon (DIC) concentration was estimated from alkalinity, pH and temperature through a titration with 0.1 N HCl (Stumm and Morgan 1996). Samples were filtered through GF/F filters for dissolved nutrient analyses, dissolved organic carbon (DOC) and water absorbance measurements. The following analytical methods were used for dissolved nutrient analyses: the phenate method for ammonium (N-NH4), the cadmium reduction method for nitrate (N-NO3) and the stannous chloride method for dissolved phosphorus (P-PO4). To estimate the total fractions of nitrogen (TN) and phosphorous (TP), the same methodologies as described for nitrate and dissolved phosphorus were employed, with the previous step of digesting the unfiltered sample (APHA 2005). For DOC determination, water samples were filtered through pre-ignited GF/F filters and acidified for later analysis in a TOC Shimadzu analyzer (Columbia, USA). Absorbance was measured at 250, 254, 365 and 440 nm in the filtered sample in a Beckman 6500 spectrophotometer (Beckman Coulter, Fullerton, CA, USA). The SUVA index (Abs 254 /DOC) was employed as an indicator of dissolved organic matter (DOM) aromaticity, and the ratio between absorbance at 250 and 365 nm (E2/E3) was used as an inverse measure of the relative weight DOM (Roehm et al. 2009). The absorption coefficient at 440 nm (g440) was also calculated as a water colour estimator (Kirk 2011).
Phytoplankton chlorophyll a (chl a)was determined from the filters used for nutrient analysis. The filters were stored at -20 °C and pigments were extracted with ethanol (60-70 °C) and left overnight under dark and cold (4 °C) conditions. The absorbance at 665 and 750 nm was measured in a Beckman 6500 spectrophotometer before and after acidification with 0.1 N HCl (Jespersen and Christoffersen 1987).
Primary production (PP)
We employed in situ the 14C assimilation technique as described in Holm-Hansen and Helbling (1995). For incubations, we used an experimental device that allowed the acrylic tubes (67.5 mL) to be held horizontally through the depth profile. Duplicate incubations with 2 µCi of NaHCO3 were carried out for 2-3 h around noon at the depth where the samples were obtained. In GL, 5-9 points along the depth profile were used whereas in the OL, 4 points were taken. A pair of dark tubes was also incubated in each lake, and the 14C incorporation in the dark tubes was subtracted from the clear ones. The tubes were transported to the laboratory in cold and dark conditions for further analysis.
Samples were filtered through GF/F filters, these were placed in scintillation vials and left overnight in an atmosphere impregnated with HCl. Scintillation cocktail was added (Opti-phase Hi Safe 3, Perkin Elmer, Life Sciences, Inc., USA) and the radioactivity incorporated by the algae was measured in a Beckman LS 6500 scintillation counter (Beckman Coulter, Fullerton, CA, USA). To assess the radioactivity added to the sample, 1 mL of the incubated water was pipetted into a vial containing three drops of 0.1 N NaOH. The radioactivity in each sample was measured after adding the scintillation cocktail and counted as described above.
Bacterial production (BP)
The 3H-Leucine method was employed (Smith and Azam 1992). Water samples were taken at the same depths where the phytoplankton 14C incubations were performed. By triplicate, 1.2 mL of water from each depth was pipetted into an eppendorf tube. To each eppendorf, 120 and 40 nM 3H-Leucine were added for the GL and the OL water, respectively. The 3H-Leucine activity added in each lake was previously determined from saturation curves. For each depth, one killed control was employed (120 µL of 50% trichloracetic acid [TCA] was added before the isotope addition). The 3H activity of the killed control was subtracted from the sample activity. Incubations were performed in a cooling box containing lake water as thermal bath and lasted one hour. Incubations finished with the addition of 120µL 50% TCA.
In the laboratory, the samples were vortexed, centrifuged for 10 minutes at 12000 rpm and the supernatant was discarded. Samples were washed twice with the addition of 1.2 mL of TCA 5% and with 1.2 mL of 80% ethanol. Each time the samples were vortexed, centrifuged, and the supernatant discarded. Finally, 1.2 mL of scintillation cocktail (Opti-phase HiSafe 3, Perkin Elmer, Inc.) was added and the 3H radioactivity from the samples was measured in a Beckman LS 6500 scintillation counter. The 3H-Leucine incorporation was converted to carbon units according to Simon and Azam (1989).
Data analysis
Hourly rates of PP and BP obtained for each depth (µg C.L-1.h-1) were integrated by calculating the area below the curve of production versus depth (µg C.m-2 .h-1). Daily rates of PP were estimated as described in Rodríguez et al. (2012), and for BP hourly rates were multiplied by 24. Parametric correlations and tests were used to explore the data and compare the results between water bodies. A P level lower than 0.05 was regarded as statistically significant. Data were Log10 transformed to fulfill the assumptions of the method, when needed.
RESULTS
Water level was similar between seasons and was generally higher in GL (Table 1). Water temperature was lower during winter and the pH ranged from acidic to neutral in both shallow lakes (Table 1). Dissolved oxygen (DO) fluctuated between anoxic conditions to low concentrations, being lower in GL. The conductivity increased in winter as well as the vertical attenuation coefficient (kd) and the dissolved inorganic carbon concentration (DIC) (Table 1). The absorbance indexes indicated that DOM aromaticity (SUVA), colour (g440) and molecular weight (E2/E3) decreased from summer to winter. Nonetheless, the DOC concentration increased in the two lakes in winter (DOC-temperature, P<0.05; r=-0.91 and -0.97 in the OL and GL, respectively). Dissolved inorganic nitrogen concentration was similar between lakes and increased towards winter; contrarily, total nitrogen (TN) was higher during summer (Table 1). In summer, phytoplankton chl a concentration ranged between 9 and 152 µg/Overall depth-integrated PP was similar between lakes (t=-1.84, P=0.09) (Table 2). In GL, PP was inversely correlated with water temperature (P<0.05, r=-0.84) whilst in OL, PP increased along with water temperature, (P<0.05; r=0.91) (Table 2). Although no statistical differences were detected, BP tended to be higher in GL than in OL (t=-1.55, P=0.15) (Table 2). In OL, BP was positively correlated to water temperature and PP (P<0.05; r=0.9 and 0.85, respectively). PP was higher than BP; in summer, the ratio PP/BP was in average 22 at OL and 1.4 at GL. During winter, PP/BP was 77 at OL and 34 at GL. Hence, BP represented a relatively small fraction of PP in the OL, between 1.3 (winter) and 5% (summer). In GL, BP was a larger proportion of PP during summer, 71%, but was ~3% in winter.
Table 1. Ranks of physical and chemical variables measured during the study period in the oxbow lake (OL) and Grande lake (GL). DO=dissolved oxygen, kd=light extinction coefficient, g440=absorption coefficient at 440 nm, SUVA=absorbance at 254 nm/dissolved organic carbon (DOC), E2/E3=quotient between absorbance at 250 and 365 nm, DIC=dissolved inorganic carbon, DIN=dissolved inorganic nitrogen, TN=total nitrogen, P-PO4 =dissolved phosphorus, TP=total phosphorus, nd=non-detectable.
Tabla 1. Rangos de variación de las variables físicas y químicas registradas durante el período de estudio en el meandro abandonado (OL) y la Laguna Grande (GL). DO=oxígeno disuelto, kd=coeficiente de atenuación vertical de la luz, g440=coeficiente de absorción a 440 nm, SUVA=absorbancia a 254 nm/carbono orgánico disuelto (DOC), E2/E3=cociente entre la absorbancia a 250 y 365 nm, DIC=carbono inorgánico disuelto, DIN=nitrógeno inorgánico disuelto, TN=nitrógeno total, P-PO4 =fósforo disuelto, TP=fósforo total, nd=no detectable.
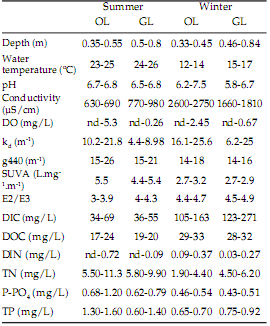
L in the OL and between 13 and 94 µg/L in GL. In winter, chl a concentration was lower than in summer in both lakes (overall t-test, t=2.33, P=0.042), ranging from non-detectable to 5 µg/Lin the OL and 2-71 µg/Lin GL.
Table 2. Ranks and average (in parenthesis) of depth-integrated pelagic primary production (PP) and bacterioplankton production (BP) in the oxbow lake (OL) and Grande lake (GL) during the study period.
Tabla 2. Rangos y media (entre paréntesis) de la producción primaria integrada en la columna de agua (PP) y la producción bacteriana (BP) en el meandro abandonado (OL) y la Laguna Grande (GL) durante el período.

DISCUSSION
In this study, we found the relationship between phytoplankton and bacterioplankton production to be net autotrophic in two humic shallow lakes almost completely covered with floating plants, regardless temperature seasonality. This result was unexpected considering the high concentration of DOC and the low irradiances that penetrated in the water column (high kd), which theoretically should promote BP and restrict PP.
Autotrophic systems rely on the carbon produced in the water body for the growth and metabolic demands of other organisms in the food web (Jansson et al. 2000). In heterotrophic systems, an additional external carbon source is required to support the bacterial respiration (BR), which exceeds the carbon fixation by photosynthesis in the lake (Cole et al. 2000). If we assume a bacterial growth efficiency (BGE=BP/[BR+BP]) of 50% (del Giorgio and Cole 1998), BR equals bacterial production and, in the case of this study, the same conclusion would be achieved (i.e., pelagic organic carbon production would be sufficient to provide substrata for pelagic BR). In this study, we are not considering the role of the macrophyte cover or the respiration in the sediments, both of which could affect the metabolic balance between the lake and the atmosphere. In this sense, a recent study showed that the presence of aquatic macrophytes can offset open waters CO2 emissions in tropical floodplain lake ecosystems (Peixoto et al. 2016). Conversely, respiration in the sediments could potentially counteract the high autochthonous pelagic production (Kortelainen et al. 2006). However, there is evidence from boreal and sub-arctic lakes that shows that respiration in the water column can exceed sediment respiration (Algesten et al. 2005). Hence, we will cautiously discuss about two possible mechanisms that would explain the observed patterns in the water column: high PP or low BP which lead to the observed net autotrophy.
PP rates recorded during this study are in the range of the previously registered in the same wetland but generally under circumstances of partial or no coverage of floating plants (Rodríguez and Pizarro 2007, 2015; Rodríguez et al. 2012). However, during this study, the estimated concentrations of dissolved inorganic carbon (DIC) were particularly higher than in previous studies which could partially compensate for the presence of floating macrophytes and consequent diminished PP rates because of light limitation. In this sense, a recent study has shown that carbon dioxide super-saturation promotes primary production (Jansson et al. 2012). In addition, previous experimentation and research in the water bodies studied here have shown that anaerobic anoxygenic photosynthetic bacteria are common below the floating plants when oxygen concentrations are very low (Izaguirre et al. 2010). Thus, their presence during this study could be explaining both the high PP rates observed particularly in winter at GL, and the autotrophic conditions despite the low O2 concentrations registered. Furthermore, the shallow nature of the lakes studied here plays also a role in terms of carbon balance. The water column is short but still photosynthetic thus compensating for the light attenuation effect when considering the contribution of each single depth to primary production in comparison to what happens in deeper lakes with even lower DOC concentrations.
The second proposed pathway to explain the net autotrophy in the lakes are low BP rates. Temperature is known to affect bacterioplankton metabolism (Kirchman 2012). Accordingly, BP fluctuation in both lakes was tightly coupled to temperature seasonality, which in turn was reflected in the absorbance indexes. During winter, when BP was lower, there was higher availability of organic matter of low molecular weight, less coloured and aromatic than in summer, when the consumption was higher. We compared our values with the literature and concluded that the BP estimated during this study are within the range of those registered for pelagic bacteria in a broad range of aquatic environments, from humic lakes to wetlands (e.g., Stanley et al. 2003; Andersson and Brunberg 2006; Jasser et al. 2009). Thus, decreased BP does not seem to be the best explanation for the autotrophic conditions, although higher rates could be potentially expected given the high organic carbon concentrations in the lakes. In this sense, predation by heterotrophic nano-flagellates (HNF) could be affecting bacterial abundance and consequent production. HNF are known to be active and abundant below the floating plants at GL (Izaguirre et al. 2012). Additionally, the floating plant coverage could be hindering the photo-oxidation/ degradation of high molecular weight organic matter (OM). The sun-induced rupture of the OM constitutes an important step for further bacterial incorporation (Reche et al. 1998), and in the studied lakes this process would be occurring to a lesser extent than in non-vegetated systems. Nevertheless, irrespective of the mechanisms behind the observed results, in this study we provide some evidence that vegetated humic shallow lakes might still have a net autotrophic water column even with low light conditions and high DOC concentrations.
ACKNOWLEDGEMENTS. The authors wish to thank to the colleagues from the laboratory of Limnology from the University of Buenos Aires and R. Rodríguez for their assistance in the field. We thank to Dr H. Pizarro for her help with the analysis of DIC and to Dr P. de Tezanos Pinto whose comments on the manuscript were much appreciated. This study was granted with funds from the Argentinean Agency of Scientific and Technological Promotion, ANPCyT (PICT 536), the University of Buenos Aires (X815) and the National Scientific and Technical Research Council from Argentina, CONICET (PIP 64).
REFERENCES
1. Algesten, G., S. Sobek, A K. Bergström, A. Jonsson, L. J. Tranvik, and M. Jansson. 2005. Contribution of sediment respiration to summer CO2 emission from low productive boreal and subarctic lakes. Microbial Ecology 50:529-535. [ Links ]
2. Andersson, E., and A-K. Brunberg. 2006. Net autotrophy in an oligotrophic lake rich in dissolved organic carbon and with high benthic primary production. Aquatic Microbial Ecology 43:1-10. [ Links ]
3. Ask, J, J Karlsson, L Persson, P Ask, P Byström, and M Jansson. 2009. Terrestrial organic matter and light penetration: Effects on bacterial and primary production in lakes. Limnology and Oceanography 54:2034-2040. [ Links ]
4. APHA (American Public Health Association). 2005. Standard Methods for the Examination of Water and Wastewaters. American Water Works Association, Water Environmental Federation, Washington, DC. Pp. 1656. [ Links ]
5. Battin, T. J., S. Luyssaert, L. A. Kaplan, A. K. Aufdenkampe, A. Richter, and L. J. Tranvik. 2009. The boundless carbon cycle. Nature Geosciences 2:598-600 [ Links ]
6. Cole, J. J., M. L. Pace, S. Carpenter, and J. F. Kitchell. 2000. Persistence of net heterotrophy in lakes during nutrient addition and food web manipulations. Limnology and Oceanography 45:1718-1730. [ Links ]
7. del Giorgio, P. A., J. J. Cole, and A. Cimbleris. 1997. Respiration rates in bacteria exceed phytoplankton production in unproductive aquatic systems. Nature 385:148-151. [ Links ]
8. del Giorgio, P. A., and J. J. Cole. 1998. Bacterial growth efficiency in natural aquatic systems. Annual Review of Ecology and Systematics 29:503-541. [ Links ]
9. de Tezanos Pinto, P., and I. O'Farrell. 2014. Regime shifts between free-foating plants and phytoplankton: a review. Hydrobiologia 740:13-24. [ Links ]
10. Downing, J. A., Y. T. Prairie, J. J. Cole, C. M. Duarte, L. J. Tranvik, et al. 2006. The global abundance and size distribution of lakes, ponds, and impoundments. Limnology and Oceanography 51:2388-2397. [ Links ]
11. Falkowski, P. G., and J. A. Raven. 2007. Aquatic photosynthesis. Princeton University Press, Pp. 484. [ Links ]
12. Holm-Hansen, O., and E. W. Helbling. 1995. Techniques for measurement of primary production in phytoplankton (in Spanish). In: K. Alveal, M. E. Ferrario, E. C. Oliveira and E. Sar (eds.). Phycological methods handbook (in Spanish). University of Concepción, Chile. Pp. 329-350. [ Links ]
13. Izaguirre, I., H. Pizarro, P. de Tezanos Pinto, P. Rodríguez, I. O'Farrell, F. Unrein, and J. M. Gasol. 2010. Macrophyte influence on the structure and productivity of photosynthetic picoplankton in wetlands. Journal of Plankton Research 32:221-238. [ Links ]
14. Izaguirre, I., R. Sinistro, M. R. Schiaffino, M. L. Sánchez, F. Unrein, and R. Massana. 2012. Grazing rates of protists in wetlands under contrasting light conditions due to floating plants. Aquatic Microbial Ecology 65:221-232. [ Links ]
15. Jansson, M., A. K. Bergström, P. Blomqvist, and S. Drakare. 2000. Allochtonous organic carbon and phytoplankton/bacterioplankton production relationships in lakes. Ecology 81:3250-3255 [ Links ]
16. Jansson, M., J. Karlsson, and A. Jonsson. 2012. Carbon dioxide supersaturation promotes primary production in lakes. Ecology Letters 15:527-532. [ Links ]
17. Jasser, I., I. Kostrzewska-Szlakowska, J. Ejsmont-Karabin, K. Kalinowska, and T. Węgleńska. 2009. Autotrophic versus heterotrophic production and components of trophic chain in humic lakes: the role of microbial communities. Polish Journal of Ecology 57:423-439. [ Links ]
18. Jespersen A. M., and K. Christoffersen. 1987. Measurements of chlorophyll-a from phytoplankton using ethanol as extraction solvent. ArchivfürHydrobiologie 109:445-454. [ Links ]
19. Jones, R. I. 1992. The influence of humic substances on lacustrine planktonic food chains. Hydrobiologia 229:73-91. [ Links ]
20. Kirchman, D. L. 2012. Processes in microbial ecology. Oxford University Press. Pp. 312. [ Links ]
21. Kirk, J. T. O. 2011. Light and Photosynthesis in Aquatic Ecosystems. University Press, Cambridge, UK. Pp. 649. [ Links ]
22. Kortelainen, P., M. Rantakari, J. T. Huttunen, T. Mattsson, J. Alm, et al. 2006. Sediment respiration and lake trophic state are important predictors of large CO2 evasion from small boreal lakes. Global Change Biology 12:1554-1567. [ Links ]
23. Lovett, G. M., J. J. Cole, and M. L. Pace. 2006. Is net ecosystem production equal to ecosystem carbon accumulation? Ecosystems 9:152-155. [ Links ]
24. Peixoto, R. B., H. Marotta, D. Bastviken, and A. Enrich-Prast. 2016. Floating aquatic macrophytes can substantially offset open water CO2 emissions from tropical foodplain lake ecosystems. Ecosystems doi:10.1007/s10021-016-9964-3. [ Links ]
25. Reche, I., M. L. Pace, and J. J. Cole. 1998. Interactions of photobleaching and inorganic nutrients in determining bacterial growth on colored dissolved organic carbon. Microbial Ecology 36:270-280. [ Links ]
26. Rodríguez, P., and H. Pizarro. 2007. Phytoplankton productivity in a highly colored shallow lake of a South American foodplain. Wetlands 27:1153-1160. [ Links ]
27. Rodríguez, P., H. Pizarro, and MS Vera. 2012. Size fractionated phytoplankton production in two humic shallow lakes with contrasting coverage of free floating plants. Hydrobiologia 691:285-298. [ Links ]
28. Rodríguez, P., and H. Pizarro. 2015. Phytoplankton and periphyton production and its relation to temperature in a humic lagoon. Limnologica 55:9-12. [ Links ]
29. Roehm, C., R. Giesler, and J. Karlsson. 2009. Bioavailability of terrestrial organic carbon to lake bacteria: The case of a degrading subarctic permafrost mire complex. Journal of Geophysical Research: Biogeosciences 114:G03006. [ Links ]
30. Simon, M., and F. Azam. 1989. Protein content and protein synthesis rates of planktonic marine bacteria. Marine Ecology Progress Series 51:201-213. [ Links ]
31. Smith, D. C., and F. Azam. 1992. A simple, economical method for measuring bacterial protein synthesis rates in sea water using 3H-Leucine. Marine Microbial Food Webs 6:107-109. [ Links ]
32. Stanley, E. H., M. D. Johnson, and A. K. Ward. 2003. Evaluating the infuence of macrophytes on algal and bacterial production in multiple habitats of a freshwater wetland. Limnology and Oceanography 48:1101-1111. [ Links ]
33. Stumm, W., and J. Morgan. 1996. Aquatic Chemistry. Wiley, New York. Pp. 1040. [ Links ]














