Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Ecología austral
versão On-line ISSN 1667-782X
Ecol. austral vol.27 no.2 Córdoba set. 2017
ARTÍCULOS
Factors affecting the home range size of felids (Mammalia, Carnivora) with emphasis on three American species
Renata F. Machado1,3,*; Felipe O. Cerezer2; Carla D. Hendges1 & Nilton C. Cáceres3
¹ Programa de Pós-Graduação em Biodiversidade Animal, CCNE, Universidade Federal de Santa Maria. Santa Maria, RS, 97110-970, Brasil.
2 Curso de Ciências Biológicas, Departamento de Ecologia e Evolução, CCNE, Universidade Federal de Santa Maria. Santa Maria, RS, 97110-970, Brasil.
3 Departamento de Ecologia e Evolução, CCNE, Universidade Federal de Santa Maria. Santa Maria, RS, 97110-970, Brasil.
* refghi@gmail.com
Editor asociado: Ricardo Grau
Recibido: 16 de agosto de 2016
Aceptado: 28 de enero de 2017
ABSTRACT
We evaluated several factors that might be related to the home-range size of felids at both inter and intraspecific levels. At the interspecific level, we tested the influence of body mass on home range size of 19 felid species, while controlling for phylogeny. At the species level, we evaluated the effect of sex and habitat type (open vs. closed) on the home range size of three species of felids occurring in America, Panthera onca, Leopardus pardalis and Puma concolor, which are among the most studied species concerning home ranges. Body mass, sex, and home range data were extracted from the Pantheria database (for 19 species, for interspecific comparisons) and from 48 studies for intraspecifc comparisons. We assessed the influence of body mass on the home range size of felids using phylogenetic generalized least squares analysis. We evaluated the existence of sexual dimorphism on both home range size and body mass using paired t-tests. Finally, we investigated the influence of habitat type (open vs. closed) on home range size using ANOVA. Our results show that home range size is positively influenced by body mass in felids. At the intraspecific level, we confirmed that both the body mass and home range are larger for males than for the females in P. onca, L. pardalis and P. concolor. Moreover, the average home range size of P. onca is larger in open (i.e., grasslands, deserts and shrublands) than in closed (i.e., forests) habitats. Overall, our results confirm that larger home ranges are associated with larger body sizes in animals that need a large amount of food resources or specific resources (such as the specific prey requirements of felids) to maintain their metabolic rates. Furthermore, home range size of these three felids seems to be strongly infuenced by reproductive attributes as well as by habitat quality, suggesting a connection with the spatial distribution of both food (prey) and mates.
KEYWORDS: Pantera onca; Leopardus pardalis; Puma concolor; Sexual dimorphism; Body size; Environment; Territory
Factores que afectan el tamaño del ámbito hogareño de felinos (Mammalia, Carnívora), con énfasis en tres especies americanas.
RESUMEN
El objetivo de este estudio fue evaluar los factores que influyen en el tamaño del ámbito hogareño de felinos en los niveles inter- e intraespecífico. En nivel interespecífico, evaluamos la influencia de la masa corporal en el ámbito hogareño de 19 especies de felinos, controlando la filogenia. A nivel de especies, evaluamos el efecto del sexo y el hábitat (abierto vs. cerrado) sobre el ámbito hogareño de tres especies de felinos americanos, Panthera onca, Leopardus pardalis y Puma concolor, para los cuales existe una buena cantidad de información. Los datos de masa corporal, sexo y ámbito hogareño fueron extraídos de la base de datos Pantheria (para 19 especies, para comparación interespecífca) y 48 estudios para la variación intraespecífica. Evaluamos la influencia de la masa corporal en el tamaño del ámbito hogareño de felinos utilizando un análisis filogenético de los mínimos cuadrados generalizados. Evaluamos la existencia de dimorfismo sexual en el ámbito hogareño y la masa corporal usando una prueba t pareada. Finalmente, investigamos la influencia del hábitat en el ámbito hogareño utilizando un modelo ANOVA. Nuestros resultados mostraron que el tamaño del ámbito hogareño está asociado positivamente con la masa corporal en felinos. A nivel intraespecífico, confirmamos que la masa corporal y el ámbito hogareño de los machos son mayores que los de las hembras en P. onca y L. pardalis y P. concolor. Además, el ámbito hogareño de P. onca aumenta en hábitats abiertos (i.e., pastizales, desiertos y matorrales), tal como se esperaba. En general, nuestros resultados confirman que los mayores ámbitos hogareños están asociados con un tamaño corporal más grande en los animales que necesitan más recursos alimentarios o recursos específicos (como presas para felinos) para satisfacer las tasas metabólicas. Además, el ámbito hogareño de los tres felinos parece estar muy influenciado por los atributos de reproducción, así como por la calidad del hábitat. Esto sugiere una conexión con la distribución espacial de alimentos (presas) y las oportunidades de apareamiento.
Palabras clave: Pantera onca; Leopardus pardalis; Puma concolor; Dimorfismo sexual; Tamaño corporal; Ambiente; Territorio
Introduction
Understanding the factors that influence the variation in home range attributes of animals has long been a subject of ecological interest (Tufto et al. 1996; Perry and Garland 2002). Besides the phylogeny, several ecological and physiological factors are thought to influence the size of home ranges (McLoughlin and Ferguson 2000). In general, home range may be related to factors such as the body sizedependent metabolic rate, abundance and distribution of food, social organization, age, and habitat type (McNab 1963; Damuth 1981; Lindsted et al. 1986; Akbar and Gorman 1993; Perry and Garland 2002). However, biological characteristics such as body mass and the home range may have some degree of associated phylogenetic signal, and thus phylogenetically related species may be more similar to each other than less related ones (Felsenstein 1988; Blomberg and Garland 2002).
Terrestrial mammals exhibit a positive association of home range size with body mass (Lindsted et al. 1986; Kelt and Van Vuren 2001). Moreover, home range size may also vary according to feeding habits. For instance, carnivores tend to have larger home ranges than omnivores of the same body mass, which in turn have larger home ranges than herbivores (Kelt and Van Vuren 2001). In this regard, food availability, habitat productivity and even seasonality have been important predictors of home range size in mammals (Harestad and Bunnel 1979; Tufto et al. 1996; Ferguson et al. 1999; Grigione et al. 2002; Nilsen et al. 2005). Much of the variation in home range size of carnivores is due to these same factors (Grigione et al. 2002; Nilsen et al. 2005). However, Grigione et al. (2002) revealed that sex might be a strong determinant of home range size in some carnivores. These researchers showed that both young and adult males of Puma concolor had larger home ranges than adult females. Interestingly, this pattern was not positively correlated with differences of body mass, contrary to what is generally expected for mammals. However, carnivores have high species diversity that exhibit a wide range of ecological traits (Van Valkenburgh 1999) and, thus, these trends may vary among species. Nevertheless, to our knowledge, no other study has yet explored if this relationship between home range size and both body mass and sex, as suggested by Grigione et al. (2002), is prevalent in felids.
The Felidae family comprises a monophyletic group with about 38 species from the relatively recent (<11 million years ago) divergence and speciation events that produced successful predatory carnivores worldwide (Nowak 1999; Wozencraft 2005; Johnson et al. 2006). Felids live in a variety of habitats that varies according to the distribution and availability of resources and territoriality determines access to these resources (Rabinowitz and Nottingham 1986). They usually depend on relatively large home ranges for survival (Macdonald and Loveridge 2010), but habitat selection varies across species. Panthera onca (jaguars) use different habitat types in the same proportion as available within their home ranges (Scognamillo et al. 2003). In this species, open habitats could be associated with large home ranges (Silveira 2004). Leopardus pardalis (ocelots) require dense vegetation cover or forested portions when occurring in open habitats (Sunquist and Ludlow 1987). The mating system of solitary felids is flexible, and there is interspecific variation regarding the degree of sociability (Sunquist 1981). With the exception of Panthera leo (lions) and Acinonyx jubatus (cheetahs), most adult felids are intolerant to others adults of the same sex (Bekoff et al. 1984). Adult males keep exclusive territories that show little overlap with those of other males, but they may cover the home ranges of several females (Sunquist 1981; Manfredi et al. 2006). Males usually disperse farther than females, whereas females have smaller home ranges (Greenwood 1980; Sweanor et al. 2000). Thus, it seems that the home range attributes of felids could be strongly influenced by differences between the sexes, and even by habitat type.
The objective of this study was to evaluate the factors driving the home-range size of felids at both inter- and intraspecific levels. At the interspecific level, we tested the influence of body mass on the home range size of 19 species of felids while controlling for phylogeny. At the species level, we evaluated the effect of sex and habitat (open vs. closed) on the home range size of three species of American felids. Panthera onca (Linnaeus 1758) belongs to the Panthera lineage; L. pardalis (Linnaeus 1758) belongs to the Ocelot lineage; and P. concolor (Linnaeus 1771) belongs to the Puma lineage, which are among the most well studied in terms of home ranges. We predict that felids with large body masses will have also large home ranges according to the general pattern expected for terrestrial mammals (Lindsted et al. 1986; Kelt and Van Vuren 2001). We also expect that sexual differences in the body mass and home range size will occur because these trends have been previously reported for some felids species (Sweanor et al. 2000; Grigione et al. 2002; Manfredi et al. 2006). Moreover, phylogeny could eventually have a weak influence on the home range size of felids, as sex and habitat could play a more important role on it (Grigione et al. 2002).
Materials and Methods
Data acquisition
We extracted both mean body mass and mean home range data for 19 felid species from the Pantheria database (Jones et al. 2009; Supplementary material 1). We also compiled a database of 48 studies containing information about the home range size, sex, body mass and biome of P. onca, L. pardalis and P. concolor, plus 20 studies with information regarding body mass of the same three species. The studies included in both databases were performed between 1986 and 2010 (Table 1). We used SCOPUS (www.scopus.com) and ISI (www.isiwebofknowledge.com)search tools with the keywords: “Panthera onca”, “Leopardus pardalis”, “Puma concolor”, “mating system”, “social system”, “home range”, “territory”, “use of space”, “space use” and “felids”. We discarded articles resulting from the investigation of captive animals. The data compiled in this study represent a large part of their geographic distribution of these species according to the IUCN (Figure 1).
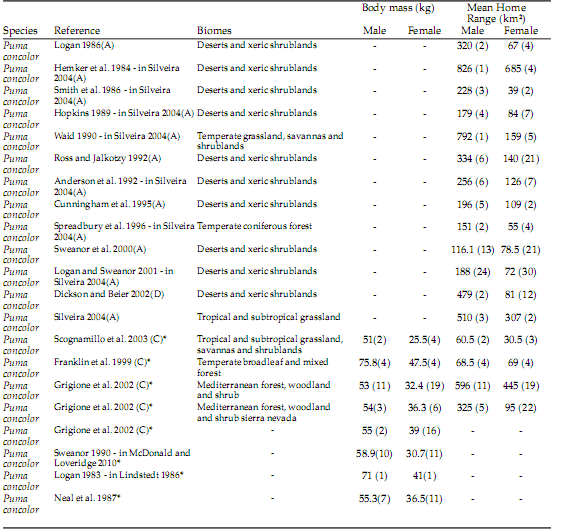
Table 1. Home range size and mean body mass of the studied felid species based on data from the literature. Asterisks indicate studies of body mass used to test for sexual dimorphism, and numbers in parentheses indicate the number of individuals.
Tabla 1. Tamaño del ámbito hogareño y media de masa corporal de las especies de felinos estudiados basada en datos de la literatura. Los asteriscos indican los estudios de masa corporal utilizados para probar dimorfismo sexual; los números entre paréntesis indican el número de individuos.

*A) Minimum Convex Polygon (MCP) at 100% of locations; B) Minimum Convex Polygon (MCP) at 98% of locations; C) Minimum Convex Polygon (MCP) at 95% of locations; D) Minimum Convex Polygon (MCP) at 85% of locations; E) Minimum Convex Polygon (MCP) at 80% of locations; F) Fixed Kernel at 95% of locations; G) Fixed Kernel at 85% of locations.
* Biomes according to Olson et al. 2001

Figure 1. Distribution maps of Panthera onca, Leopardus pardalis and Puma concolor, according to the IUCN (2008).
Figura 1. Mapa de distribución de Panthera onca, Leopardus pardalis y Puma concolor, de acuerdo con la IUCN (2008).
Statistical analyses
Initially, we analyzed the phylogenetic signal for both body mass and home range across the 19 felid species for which we found enough data. For this, we used the Pagel's λ method; Pagel's λ has a natural scale between zero (no correlation between species) and one (correlation between species equal to the Brownian expectation). The value of λ itself is not a correlation, but a scaling factor for a correlation (Pagel 1999). Then, we tested the influence of body mass on home range size, controlling for phylogeny by using a phylogenetic generalized least squares analysis (PGLS). The PGLS is a phylogenetic regression method in which the covariance among specimens as result of phylogeny is expressed in the regression error term (Martins and Hansen 1997; Grafen 1989). For these phylogenetic analyses, we reconstructed the phylogenic tree of the Felidae according to the most recent phylogeny by Li et al. (2015), using the Mesquite 2.75 software (Maddison and Maddison 2005) (Supplementary material 2). We used the mean home range size and mean body mass data available from Pantheria database.
At the species level, we analyzed the differences in body mass and home range size between males and females using a paired ttest separately for each of the three species. We used paired t-tests because male and female individuals, that were compared, were present in the same local environments, and thus they were affecting each other (not independent). In order to test for differences in home range size of the three species in relation to habitat, we applied a two-way Anova, including sex, habitat, and their interaction as predictor variables. We categorized the habitat data as (0) open habitat (i.e., grasslands, dessert and shrubland), and (1) closed habitat (i.e., forests). We did not include P. concolor in this analysis due to lack of information in closed habitats.
We used log-transformed variables in all analyses to meet the assumptions of parametric tests. Values of P<0.05 were considered significant. All analyses were conducted using the R environment, version 3.1.3 (09-03-2015) (R Development Core Team 2015). The phylogenetic signal and PGLS analyses were performed using the R package picante (Kembel et al. 2010), ape (Paradis et al. 2004), adephylo (Jombart and Dray 2008), ade4 (Dray and Dufour 2007), geiger (Harmon et al. 2008), phytools (Revell 2012), and nlme (Pinheiro et al. 2016).
Results
Phylogenetic signal on home range size and body mass in felids
We detected a weak and non-significant phylogenetic signal in both home range
(Pagel's λ<0.001, P=1.00) and body mass (Pagel's λ=0.274, P=0.115) of Felidae, suggesting ecological or other factors affecting them. The PGLS corroborates the Pagel's λ method, showing that body mass had an influence on home range size of Felidae, even when controlling for the weak phylogenetic signal (β=-0.965, t=2.527, df1 =19, df2 =17, P=0.02). The home range size increased according to the increase in body mass of Felidae (Figure 2).

Figure 2. Phylogenetic generalized least squares analysis between the home range (log km2) of felid species and their body mass (log g).
Figura 2. Análisis filogenético de los mínimos cuadrados generalizados entre el ámbito hogareño de las especies (log km2) y la masa corporal (log g).
Effects of sex on body mass and home range size
Males of the three species have larger body mass than females (P. onca t=8.544, df=14, P<0.001; L. pardalis t=7.868, df=6, P<0.004; P. concolor t=11.524, df=14, P<0.001) (Figure 3). Male home ranges are also larger than those of females for the three felid species (P. onca t=4.064, df=22, P<0.001; L. pardalis t=4.612, df=26, P<0.001; P. concolor t=6.409, df=32, P<0.001) (Figure 4).

Figure 3. Body mass of males and females of Panthera onca, Leopardus pardalis and Puma concolor. Boxplot indicates mean, standard deviation (box), and maximum and minimum values (vertical bar). Data used to generate the boxplots were included without log transformation as a merely illustrative case, but the main analyses in the text were done with log-transformed data.
Figura 3. Dimorfismo sexual entre especies. Los boxplots indican los valores de media, desviación estándar (caja), máximos y mínimos (barra vertical). Los datos usados para generar el boxplot se hicieron sin transformación logarítmica como un caso meramente ilustrativo, pero los principales análisis en el texto se realizaron con datos log transformados.

Figure 4. Home range sizes of males and females of Panthera onca, Leopardus pardalis and Puma concolor. Boxplot indicates mean, standard deviation (box), and maximum and minimum values (vertical bar). Data used to generate the boxplots were included without log transformation as a merely illustrative case, but the main analyses in the text were done with log-transformed data.
Figura 4. Ámbito hogareño entre machos y hembras de las especies estudiadas. Los boxplots indican los valores de media, desviación estándar (caja), máximos y mínimos (barra vertical). Los datos usados para generar el boxplot se hicieron sin transformación logarítmica como un caso meramente ilustrativo, pero los principales análisis en el texto se realizaron con los datos long transformados.
Effects of habitat type on home range size
The ANOVA revealed significant differences in the home range size between open and closed habitats for P. onca (F=5.337, df=22, P=0.031). Thus, the home range of this species is larger in open habitats than in closed habitats (Figure 5). However, the interaction between sex and habitat was not significant (F=0.291, df=22, P=0.595). There was no difference in home range size between habitat types for L. pardalis (F=0.885, df=26, P=0.356) neither there was any interaction between sex and habitat (F=0.031, df=26, P=0.861).

Figure 5. Home range size of P. onca in open and closed habitats. Boxplot indicates mean, standard deviation (box), and maximum and minimum values (vertical bar). Data used to generate the boxplot were included without log transformation as a merely illustrative case, but the main analyzes in the text were done with log-transformed data.
Figura 5. Ámbito hogareño de la especie P. onca en ambiente abierto y cerrado. Los boxplots indican los valores de la media, desviación estándar (caja) y los valores máximos y mínimos (barra vertical). Los datos usados para generar el boxplot se hicieron sin transformación logarítmica como un caso meramente ilustrativo, pero los principales análisis en el texto se realizaron con los datos log transformados.
Discussion
The home-range size of Felidae showed a positive correlation with body mass, and this remains true after accounting for phylogeny, meaning that other environmental (e.g., habitat, food supply) and intrinsic factors (i.e., sex, body mass) are likely to be affecting this home-range size variation. As expected, our results support the general pattern of positive association between home range size and body mass for terrestrial mammals (Lindsted et al. 1986; Kelt and Van Vuren 2001). This relationship between home range size and body mass may be a consequence of energetic requirements (McNab 1963; Gittleman and Harvey 1981). Particularly, large-bodied animals need to increase their home ranges to access more resources to meet their metabolic rates and ensure their survival, especially during critical biological periods (Lindsted et al. 1986; Ferguson et al. 1999). Moreover, carnivorous species that include large proportions of meat in their diets also tend to have larger home ranges than those ones with a more generalist diet (Gittleman and Harvey 1981). Felids are predominantly carnivorous (Emmons 1987; González and Miller 2002; Novack et al. 2005), and thus, many of them need to adjust their home ranges in function of the abundances and spatial distributions of their main prey (Lindsted et al. 1986; Crawshaw and Quigley 1991; Grigione et al. 2002; Dillon and Kelly 2008). Therefore, these differences in the felid home-range sizes may reflect both their differences regarding body size and the necessary movements to find their prey.
At intraspecific level, we found that the home range size of males is larger than that of females for P. onca, L. pardalis and P. concolor. In addition to larger home ranges, males of these three felids species have also larger body masses than females. Sexual dimorphism in mammals is often associated with intense competition among males for the access to estrous females (Weckerly 1998). Moreover, sexual dimorphism also tends to increase in species with polygynous mating system as occurs in felids (Plavcan 2000; Cavalcanti and Gese 2009). Thus, these results may be related to the reproductive aspects of these solitary felids. In general, home range sizes of females are strongly influenced by their own metabolic demands (e.g., during pregnancy), while home range sizes of males are determined by the availability of food and mates (females) distribution (Sandell 1989; Silveira 2004; Astete et al. 2008). In this regard, adult males of felids with large home ranges could increase the likelihood of overlapping with more females with which they potentially can mate (Greenwood 1980; Lindsted et al. 1986; Sweanor et al. 2000). Furthermore, males generally exhibit intense competition for territories that are actively marked and patrolled by single dominant males that expel other males, if necessary (VanValkenburgh and Sacco 2002). Therefore, both the larger male body masses and home ranges of these felids may represent responses not only in relation to their metabolic requirements (as in females) but also in terms of intraspecific competition (Heske and Ostfeld 1990; Silveira 2004).
We also found that home range size of P. onca is larger in open habitats than in closed, forested habitats. Since jaguars are opportunistic feeders, their spatial dynamics likely reflect those of their prey (Crawshaw and Quigley 1991). In general, jaguars tend to kill larger prey in open habitats when compared to prey kill in closed habitats (Rabinowitz and Nottingham 1986; Iriarte et al. 1990; Astete et al. 2008). For instance, Crawshaw and Quigley (1991) showed that jaguars living in Pantanal wetland (i.e., open habitat) prey mostly on large and highly mobile species. Meanwhile, jaguars in forests of Belize prey on small and less mobile species (Rabinowitzand and Nottingham 1986). Still, in closed habitats, some prey of jaguars are vertically distributed in the arboreal stratum (e.g., monkeys, sloths, birds) or associated to water bodies like caimans, as occurs in flooded forest of the Amazon basin (Garla et al. 2001; Nunez et al. 2002; Astete et al. 2008). Meanwhile, in open habitats, the prey of jaguars consist mainly of cursorial species such as deer, tapirs and capybaras (Rabinowitz and Nottingham 1986; Silveira 2004; Astete et al. 2008). Moreover, the body mass itself could be another factor influencing these differences in home range size between habitats because jaguars also tend to be larger in open areas, in regard to body mass, than in forested areas (Silveira 2004). This implies that jaguars living in open habitats should require more food, and their large home ranges are a consequence of their foraging movements.
In resume, we observed that the home range size of felids was positively correlated with body mass, regardless of shared ancestry, meaning that the environment (e.g., habitat) or intrinsic (e.g., sex, body mass) factors could play a major role in determining such variation in this family. Overall, our results support previous findings that larger home ranges are associated with larger body sizes in animals that require large amounts of resources, or specific resources (e.g., prey for felids) to meet their metabolic demands. At the intraspecific level, we confirmed that both the body mass and home range of males are larger than those of females for P. onca, P. concolor, and L. pardalis. These results suggest that large body masses and home ranges can represent strong advantages related to sexual selection in male felids. Moreover, our results also show that the home range size of P. onca increases in open habitats (i.e., grasslands, deserts and shrublands), likely as a response to the sparse spatial distribution of prey as well as to its higher metabolic requirements linked to its proportionally larger body size in such habitats.
ACKNOWLEDGEMENTS. Our thanks to Ismari Ramírez from the Programa de Pósgraduação em Ecologia e Conservação da Universidade Federal do Mato Grosso do Sul for her help in translating the abstract to Spanish. Renata F. Machado and Carla D. Hendges were supported by CAPES with a scholarship. Nilton Cáceres is a researcher fellow in ecology, granted by the Brazilian Agency for Scientific Research (CNPq).
References
1. Akbar, Z., and M. L. Gorman. 1993.The effect of supplementary feeding upon the sizes of home ranges of woodmice Apodemus sylvaticus, living on a system of maritime sand-dunes. Journal of Zoology 231:233-237. [ Links ]
2. Animal Diversity Web (ADW). Disponível em: http://animaldiversity.org/. [ Links ]
3. Astete, S., R. Sollmann, and L. Silveira. 2008. Comparative ecology of jaguars in Brazil. Cat News Special 4:9-14. [ Links ]
4. Azevedo, F. C. C., and D. L. Murray. 2007. Spatial organization and food habits of jaguars (Panthera onca) in a foodplain forest. Biological Conservation 137:391-402. [ Links ]
5. Bekoff, M., T. J. Daniels, and J. L. Gittleman. 1984. Life history patterns and comparative social ecology of carnivores. Annual Review of Ecology and Systematics 15:191-232. [ Links ]
6. Blomberg, S. P., and T. Garland. 2002. Tempo and model in evolution: phylogenetic inertia, adaptation and comparative methods. Journal of Evolutionary Biology 15:899-910. [ Links ]
7. Cavalcanti, S. M., and E. M. Gese. 2009. Spatial ecology and social interactions of jaguars (Panthera onca) in the southern Pantanal, Brazil. Journal of Mammalogy 90:935-945. [ Links ]
8. Ceballos, G., C. Chávez, A. Rivera, C. Manterola, and B. Wall. 2002. Tamaño poblacional y conservación del jaguar en la reserva de la biosfera Calakmul, Campeche, México. Pp. 403-417 en R. A. Medellín: El jaguar en el nuevo milenio. [ Links ]
9. Crawshaw, P. G., and H. B. Quigley. 1991. Jaguar spacing, activity and habitat use in a seasonally fooded environment in Brazil. Journal of Zoology 223:357-370. [ Links ]
10. Crawshaw, P. G. 1995. Comparative ecology of ocelot (Felis pardalis) and jaguar (Panthera onca) in a protected subtropical forest in Brazil and Argentina. Dissertation. University of Florida. [ Links ]
11. Cullen Jr., L., K. C. Abreu, D. Sana, and A. F. D. Nava. 2005. Jaguars as landscape detectives for the upper Paraná River corridor, Brazil. Natureza e Conservação 3:124-146. [ Links ]
12. Cunningham, S. C., L. A. Haynes, C. Gustavson, and D. D. Haywood. 1995. Evaluation of the interaction between mountain lions and cattle in the Aravaipa-Klondyke area of southeast Arizona: a final report. Technical report/Arizona Game and Fish Dept., Research Branch (USA). [ Links ]
13. Damuth, J. 1981. Home range, home range overlap, and species energy use among herbivorous mammals. Biological Journal of the Linnaean Society 15:185-193. [ Links ]
14. Di Bitetti, M. S., A. Paviolo, and C. De Angelo. 2006. Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic Forest of Misiones, Argentina. Journal of Zoology 270:153-163. [ Links ]
15. Dickson, B. G., and P. Beier. 2002. Home-Range and Habitat Selection by Adult Cougars in Southern California. The Journal of Wildlife Management 66:1235-1245. [ Links ]
16. Dillon, A. 2005. Ocelot density and home range in Belize, Central America: camera-trapping and radio telemetry. Tesis doctoral. Virginia Polytechnic Institute and State University. [ Links ]
17. Dillon, A., and M. J. Kelly. 2008. Ocelot home range, overlap and density: comparing radio telemetry with camera trapping. Journal of Zoology 275:391-398. [ Links ]
18. Dray, S., and A. B. Dufour. 2007. The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software 22:1-20. [ Links ]
19. Emmons, L. H. 1987. Comparative feeding ecology of felids in a neotropical rainforest. Behavioral Ecology and Sociobiology 20:271-283. [ Links ]
20. Emmons, L. H. 1988. A feld study of ocelots (Felispardalis) in Peru. Revue d'Ecologie la Terre et la Vie 43:133-157 [ Links ]
21. Emmons, L. H., P. Sherman, D. Bolster, A. Goldizen, and J. Terborgh. 1989. Ocelot behavior in moonlight. Neotropical Mammal 1989:233-242. [ Links ]
22. Felsenstein, J. 1988. Phylogenenies and quantitative characters. Annual Review of Ecology and Systematics 19:445-471. [ Links ]
23. Ferguson, S., M. Taylor, E. Born, A. Rosing-Asvid, and F. Messier. 1999. Determinants of Home Range Size for Polar Bears (Ursus maritimus). Ecology Letters 2:311-318. [ Links ]
24. Franklin, W. L., W. E. Johnson, R. J. Sarno, and J. A. Iriarte. 1999. Ecology of the Patagonia puma Felisconcolorpatagonica in southern Chile. Biological Conservation 90:33-40. [ Links ]
25. Garla, R. C., E. Z. F. Setz, and N. Gobbi. 2001. Jaguar (Panthera onca) Food Habits in Atlantic Rain Forest of Southeastern Brazil1. Biotropica 33:691-696. [ Links ]
26. Gittleman, J. L., and P. H. Harvey. 1982. Carnivore home-range size, metabolic needs and ecology. Behavioral Ecology and Sociobiology 10:57-63. [ Links ]
27. Goulart, F., M. E. Graipel, M. Tortato, I. Ghizoni-Jr, L. G. Oliveira-Santos, and N. Cáceres. 2009. Ecology of the ocelot (Leoparduspardalis) in the Atlantic Forest of Southern Brazil. Neotropical Biology and Conservation 4:137-143. [ Links ]
28. Grafen, A. 1989. The phylogenetic regression. Philosophical Transactions of the Royal Society of London B. 326:119-157. [ Links ]
29. Greenwood, P. J. 1980. Mating systems, philopatry and dispersal in birds and mammals. Animal Behaviour 28:1140-1162. [ Links ]
30. Grigione, M. M., P. Beier, R. A. Hopkins, D. Neal, W. D. Padley, C. M. Schonewald, and M. L. Johnson. 2002. Ecological and allometric determinants of home-range size for mountain lions (Puma concolor). Animal Conservation 5:317-324. [ Links ]
31. Harestad, A. S., and F. L. Bunnel. 1979. Home Range and Body Weight-A Reevaluation. Ecology 60:389-402. [ Links ]
32. Harmon, L. J., J. T. Weir, C. D. Brock, R. E. Glor, and W. Challenger. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24:129-131. [ Links ]
33. Heske, E. J., and R. S. Ostfeld. 1990. Sexual Dimorphism in Size, Relative Size of Testes, and Mating Systems in North American Voles. Journal of Mammalogy 71:510-519. [ Links ]
34. Iriarte, J. A., L. F. William, E. J. Warren, and H. R. Kent. 1990. Biogeographic variation of food habits and body size of the America puma. Oecologia 85:185-190. [ Links ]
35. Jedrzejewski, W., M. Abarca, A. Viloria, H. Cerda, D. Lew, H. Takiff, E. Abada, P. Velozo, and K. Schmidt. 2011. Jaguar Conservation in Venezuela against the backdrop of current knowledge on its biology and evolution. Interciência 36:954-966. [ Links ]
36. Johnson, W. E. 2006. The Late Miocene Radiation of Modern Felidae: A Genetic Assessment. Science 311:73-77. [ Links ]
37. Jombart, T., and S. Dray. 2008. adephylo: exploratory analyses for the phylogenetic comparative method. [ Links ]
38. Jones, K. E., J. Bielby, M. Cardillo, et al. 2009. Pantheria: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90(9):2648. [ Links ]
39. Kelt, D. A., and D. H. V. Vuren. 2001. The Ecology and Macroecology of Mammalian Home Range Area. The American Naturalist 157:637-645. [ Links ]
40. Kembel, S. W., P. D. Cowan, M. R. Helmus, W. K. Cornwell, H. Morlon, D. D. Ackerly, S. P. Blomberg, and C. O. Webb. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463-1464. [ Links ]
41. Konecny, M. J. 1989. Movement patterns and food habits of four sympatric carnivore species in Belize, Central America. Advances in Neotropical Mammalogy 1989:243-264. [ Links ]
42. Li, G., B. Davis, E. Eizirik, and W. Murphy. 2015.Phylogenomic evidence for ancient hybridization in the genomes of living cats (Felidae). Genome research 26:1-11. [ Links ]
43. Lindstedt, S. L., B. J. Miller, and S. W. Buskirk. 1986. Home Range, Time, and Body Size in Mammals. Ecology 67: 413-418. [ Links ]
44. Logan, K. A., L. L. Irwin, and R. Skinner. 1986. Characteristics of a hunted mountain lion population in Wyoming. The Journal of Wildlife Management 50:648-654. [ Links ]
45. López González, C. A, and B. J. Miller. 2002. Do jaguars (Panthera onca) depend on large prey? Western North American Naturalist 62:218-222. [ Links ]
46. MacDonald, D. W., and A. J. Loveridge. 2010. The biology and conservation of wild felids. Vol. 2. Oxford University Press. [ Links ]
47. Maddison, W. P., and D. R. Maddison. 2015. Mesquite: a modular system for evolutionary analysis. Version 3.03. http://mesquiteproject.org/. [ Links ]
48. Manfredi, C., L. Soler, M. Lucherini, and E. B. Casanave. 2006. Home range and habitat use by Geoffroy's cat (Oncifelis geoffroyi) in a wet grassland in Argentina. Journal of Zoology 268:381-387. [ Links ]
49. Martins, E. P., and T. F. Hansen. 1997. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. American Naturalist 149:646-667. [ Links ]
50. McLoughlin, P. D., and S. H. Ferguson. 2000. A hierarchical pattern of limiting factors helps explain variation in home range size. Ecoscience 7:123-130. [ Links ]
51. McNab, B. K. 1963. Bioenergetics and the determination of home range size. American Naturalist 97:133-140. [ Links ]
52. Neal, D. L., G. N. Steger, and R. C. Bertram. 1987. Mountain lions: preliminary findings on home-range use and density in the central Sierra Nevada. Vol. 392. US Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station. [ Links ]
53. Nilsen, E. B., I. Herfndal, and J. D. C. Linnell. 2005. Can intra-specific variation in carnivore home-range size be explained using remote-sensing estimates of environmental productivity? Ecoscience 12:68-75. [ Links ]
54. Nowak, R. M. 1999. Walker's mammals of the world. Vol. 1. Page 313. Johns Hopkins University press. [ Links ]
55. Novack, A., B. M. Martin, M. E. Sunquist, and F. R. Labisky. 2005. Foraging ecology of jaguar (Panthera onca) and puma (Puma concolor) in hunted and non-hunted sites within the Maya Biosphere Reserve, Guatemala. Journal of Zoology 267:167-178. [ Links ]
56. Nunez, R., B. Miller, and F. Lindzey. 2002. Ecología del jaguar en la reserva de la biosfera Chamela-Cuixmala, Jalisco, México. Pp. 107-126 in Medellin, R. A., C. Chetkiewicz, A. Rabinowitz, K. H. Redford, J. G. Robinson, E. Sanderson and A. Taber (eds.). Jaguars in the new millennium. A status assessment, priority detection, and recommendations for the conservation of jaguars in the Americas. Mexico D. F., UNAM/WCS. [ Links ]
57. Pagel, M. 1999. Inferring the historical patterns of biological evolution. Nature 401:877-884. [ Links ]
58. Paradis E., J. Claude, and K. Strimmer. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289-290. [ Links ]
59. Perry, G., and T. Garland. 2002. Lizard Home Ranges Revisited: Effects of Sex, Body Size, Diet, Habitat, and Phylogeny. Ecology 83:1870. [ Links ]
60. Pinheiro J., D. Bates, S. Deb Roy, D. Sarkar, and R Core Team. 2016. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-128. URL: cran.r-project.org. [ Links ]
61. Plavcan, J. 2000. Inferring social behavior from sexual dimorphism in the fossil record. Journal of Human Evolution 39:327-344. [ Links ]
62. R Core Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.URL: www.r-project.org. [ Links ]
63. Rabinowitz, A. R., and B. G. Nottingham. 1986. Ecology and behavior of the jaguar (Panthera onca) in Belize, Central America. Journal of Zoology 210:149-159. [ Links ]
64. Revell, L. J. 2012. Phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3:217-223. [ Links ]
65. Ross, P. I., and M. G. Jalkotzy. 1992. Characteristics of a hunted population of cougars in southwestern Alberta. Journal of Wildlife Management 56:417-426. [ Links ]
66. Sandell, M. 1989. The mating tactics and spacing patterns of solitary carnivores. Pp. 164-182 in Carnivore Behavior, Ecology and Evolution. J. L. Gitleman (ed.). Cornell University Press, New York, USA. [ Links ]
67. Scognamillo, D. I. E., M. Maxit, M. E. Sunquist, and L. Farrell. 2002. Jaguar ecology and the problem of cattle predation in Hato Pinero, Venezuela. Pp. 139 in El jaguar en el Nuevo milenio. [ Links ]
68. Scognamillo, D., I. E. Maxit, M. Sunquist, and J. Polisar. 2003. Coexistence of jaguar (Panthera onca) and puma (Puma concolor) in a mosaic landscape in the Venezuelan llanos. Journal of Zoology 259:269-279. [ Links ]
69. Seymour, K. L. 1989. Panthera onca. Mammalian Species Archive 340:1-9. [ Links ]
70. Schaller, G. B., and J. M. C. Vasconcelos. 1978. Jaguar predation on capybara. Z. Säugetierk 43:296-301. [ Links ]
71. Schaller, G. B., and P. G. Crawshaw Jr. 1980. Movement Patterns of Jaguar. Biotropica 12:161-168. [ Links ]
72. Silveira, L. 2004. Ecologia comparada e conservação da onça-pintada (Pantheraonca) e onça-parda (Puma concolor), no Cerrado e Pantanal.Tese de doutorado.Programa de Pós-Graduação em Biologia Animal, Universidade de Brasilia. [ Links ]
73. Soisalo, M. K., and S. M. Cavalcanti. 2006. Estimating the density of a jaguar population in the Brazilian Pantanal using camera-traps and capture-recapture sampling in combination with GPS radio-telemetry. Biological Conservation 129:487-496. [ Links ]
74. Sunquist, M. E. 1981. The social organization of tigers (Panthera tigris) in Royal Chitawan National Park, Nepal. Smithsonian Contributions to Zoology.Smithsonian Institute Press, Washington, D.C. 336:1-98. [ Links ]
75. Sunquist, M. E., and M. E. Ludlow. 1987. Ecology and behavior of ocelots in Venezuela. National Geographic Research 3: 447-461. [ Links ]
76. Sweanor, L. L., K. A. Logan, and M. G. Hornocker. 2000. Cougar dispersal patterns, metapopulation dynamics, and conservation. Conservation Biology 14:798-808. [ Links ]
77. Tufto, J., R. Andersen, and J. Linnell. 1996. Habitat Use and Ecological Correlates of Home Range Size in a Small Cervid: The Roe Deer. Journal of Animal Ecology 65(6):715-724. [ Links ]
78. Van Valkenburgh, B. V. 1999. Major Patterns In The History Of Carnivorous Mammals. Annual Review of Earth and Planetary Sciences 27:463-493. [ Links ]
79. Van Valkenburgh, B. V., and T. Sacco. 2002. Sexual dimorphism, social behavior, and intrasexual competition in large Pleistocene carnivorans. Journal of Vertebrate Paleontology 22:164-169. [ Links ]
80. Weckerly, F. W. 1998. Sexual-Size Dimorphism: Influence of Mass and Mating Systems in the Most Dimorphic Mammals. Journal of Mammalogy 79:33-52. [ Links ]
81. Wozencraft, W. 2005. Order Carnivora. Pp. 2142 in Wilson, D. E. and D. M. Reeder (eds.). Mammal species of the world: a taxonomic and geographic reference. Vol. 1. Johns Hopkins University press. [ Links ]














