Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Ecología austral
versão On-line ISSN 1667-782X
Ecol. austral vol.28 no.1 Córdoba abr. 2018
ARTÍCULO
Invertebrate composition in submerged macrophyte debris: habitat and degradation time effects
Edélti Faria Albertoni1,3,*; Luiz Ubiratan Hepp1,2; Cristiane Carvalho1 & Cleber Palma-Silva1,3
1 Programa de Pós-Graduação em Biologia de Ambientes Aquáticos Continentais, Universidade Federal do Rio Grande - FURG, Rio Grande, RS, Brasil.
2 Programa de Pós-Graduação em Ecologia, Universidade Regional Integrada do Alto Uruguai e das Missões, Campus de Erechim, Erechim, RS, Brasil.
3 Laboratório de Limnologia, Instituto de Ciências Biológicas - ICB, Universidade Federal do Rio Grande - FURG, Rio Grande, RS, Brasil.
Recibido: 25 de octubre de 2016
Aceptado: 11 de octubre de 2017
Editora asociada: Roxana Aragón
ABSTRACT
The aim of this study was to determine if the rate of degradation and the habitat type associated with two submersed macrophytes affect the structure of the invertebrate communities in a shallow subtropical lake. We evaluated debris decomposition rate with a litter bags approach, assigned to four treatments: Potamogeton pectinatus decomposing inside their own stand (PP) and inside a Chara zeylanica stand (PC), and C. zeylanica decomposing inside their own stand (CC) and inside a P. pectinatus stand (CP). During the degradation experiment (20 days), we evaluated the fauna associated both with debris and at live macrophytes. The debris were washed, dried and the coefficient of degradation was determined. We estimated the richness and abundance of taxa of associated invertebrates, and they were classified into functional feeding groups. We evaluated preference of invertebrate communities comparing fauna at live macrophytes and their debris. We observed differences in mass loss between the treatments. Chara zeylanica showed a mass loss four times faster than P. pectinatus. The highest invertebrate abundance occurred in treatment PP and highest invertebrate richness in treatments CC and PP. Collectors and predators showed the highest abundances. The PP treatment had the greatest number of collectors and PC an equitable distribution of all functional groups. Five taxa showed preference for P. pectinatus debris instead of C. zeylanica or P. pectinatus alive, and debris of charophyte was preferred by six taxa instead of alive plant, and by two taxa when the debris were incubated in the stand of P. pectinatus. Our study demonstrated the interference of the incubation site on the decomposition coefficient and on the structuring of the invertebrate communities, and that the abundance of invertebrate at detritus is mainly due to abundance at live plants. Also, the absence of shredders suggests the use of debris mainly as shelter instead of food resource.
Keywords: Functional feeding groups; Microhabitat structure; Decomposition; Shallow lake.
RESUMEN
Composición funcional de invertebrados en los detritos de macrófitas sumergidas: efectos del hábitat y del tiempo de degradación.
El objetivo de este estudio fue determinar si el tiempo de degradación y el hábitat provisto por dos macrófitas sumergidas afectan la estructura de la comunidad de invertebrados en un lago somero subtropical. Se evaluó la descomposición de detrito en 60 bolsas. Los detritos fueron asignados al azar a cuatro tratamientos: Potamogeton pectinatus en descomposición dentro de su propio stand (PP) y en el interior de un stand de Chara zeylanica (PC), y C. zeylanica dentro de su propio stand (CC) y en el interior de un stand de P. pectinatus (CP). Durante el experimento de degradación (20 días) se evaluó la fauna asociada tanto a los detritos como a las macrófitas vivas. Las bolsas de detrito se lavaron, se secaron y luego se determinó el coeficiente de degradación. Estimamos la riqueza y la abundancia de los taxa de invertebrados asociados y evaluamos la preferencia de las comunidades de invertebrados comparando la fauna de las macrófitas vivas y de sus detritos. Los invertebrados asociados se clasificaron en grupos funcionales de alimentación. Chara zeylanica mostró una pérdida de peso cuatro veces más rápida que P. pectinatus. La mayor abundancia de invertebrados se produjo en el tratamiento PP y la mayor riqueza de invertebrados en los tratamientos de CC y PP. Los colectores y depredadores presentaron las mayores abundancias. El tratamiento PP tuvo el mayor número de colectores y PC una distribución más equitativa de los grupos funcionales. Cinco taxones mostraron preferencia por detritos de P. pectinatus en lugar de C. zeylanica o P. pectinatus vivos, y los detritos de la carófita fueron preferidos por seis taxones dentro de las plantas vivas, y por dos taxones cuando se incubaron en el interior del stand de P. pectinatus. Nuestro estudio demostró el efecto del sitio de incubación en el coeficiente de descomposición y en la estructura de las comunidades de invertebrados, y reveló que la abundancia de invertebrados en detritus se debe principalmente a su abundancia en las plantas vivas. Además, la ausencia de trituradores sugiere el uso de detritos principalmente como refugio en lugar de como recurso alimentario.
Palabras clave: Grupos funcionales de alimentación; Estructura de microhabitat; Descomposición; Lagos someros.
INTRODUCTION
Spatial distribution of aquatic organisms is fundamentally influenced by habitat (Barreto 1999). Among the characteristics of habitats, stability is an important element which defines the distribution of organisms; less stable habitats offer less protection from predators (Hannigan and Quinn 2012). Moreover, in the structure of the communities, habitat heterogeneity is one factor that contributes to an increase in the abundance of organisms (Hepp et al. 2012).
The complexity of habitats allows efficient use of resources such as food and shelter, providing greater resistance to disturbances (Kovalenko et al. 2012). For aquatic invertebrates, submerged macrophytes play an important role (Hansen et al. 2011) as they increase habitat complexity, providing more ecological niches due to increased microhabitat availability (Thomaz et al. 2008; Kovalenko et al. 2012). Even after senescence, macrophyte debris contribute to the increase of potential sites for invertebrate colonisation (Janke and Trivinho-Strixino 2007).
The organic deposits in the sediment in shallow lakes are a mixture from macrophytes detritus that accumulate heterogeneously in the sediment (Rossi et al. 2010). In such environments, organic deposits from macrophytes provide a large number of ecological niches for a wide variety of animal species (Wetzel 1993; Esteves 2011). This condition allows increased environmental variability (microhabitat structure available), interfering with composition patterns and trophic structures of communities (Wardle and Yeates 1993; Bellisario et al. 2012). The functional characterisation of aquatic invertebrates is important for organisms which participate in the processing of organic matter and energy transfer to other ecosystem levels (Callisto et al. 2004; Cummins et al. 2005).
Stands of macrophytes are characterised by high invertebrate biomass compared to non-vegetated sites (Casagranda et al. 2006). In shallow lakes, these stands are the main habitat for invertebrates (Van den Berg et al. 1997, 1998; Casagranda et al. 2006; Albertoni et al. 2007), providing microhabitat that favours the establishment and colonisation of many invertebrates (Ali et al. 2007). In addition, the decomposition of macrophyte biomass is a key process in energy processing and nutrient cycling in shallow lakes (Casagranda et al. 2006). Thus, weeds are key factors in the establishment of the invertebrate community when alive and provide habitat that favours richness increases in the community of invertebrates that predominantly use them as a refuge (Silva et al. 2010; Poi de Neiff et al. 2009; Telöken et al. 2011; Carvalho et al. 2015). In addition, Baptista et al. (2001) showed that litter substrates are preferred by many taxa because they offer best shelter and feeding conditions due to their higher habitat heterogeneity and a higher richness of periphytic flora.
Thus, our objectives were based on two approaches: 1) to determine the composition and trophic structure of the invertebrate community in the stands of two aquatic macrophytes, and 2) to evaluate the decomposition rates of these plant species when incubated in their own stand and in stands dominated by another species. The questions that guided the study were: a) Are the communities of invertebrates in living plants more diverse than in debris?; b) Do some taxa prefer debris instead of living plants?, and c) Do plants with lower decomposition rates harbour more diverse and abundant fauna? Our main hypothesis was that macrophyte debris incubated in stands of a different macrophyte species constitutes a more heterogeneous habitat for invertebrates than debris incubated in their own stand, and that this may affect invertebrate abundance or richness by providing different food or shelter resources. We expected higher species richness and higher abundances in more heterogeneous habitats. Also, we expected that macrophyte debris with low decomposition rate (more rigid structural characteristics) constitutes a more favourable habitat for invertebrates.
MATERIALS AND METHODS
Study area
The study was conducted in January 2012 in a shallow lake located in the coastal plain of Rio Grande do Sul (central coordinates: 32°01’40’’ S – 52°01’40’’ W). The lake features alternate between clear water and turbid states (Silva et al. 2015) and, during the trial period, showed clear water characteristics (concentration of chlorophyll-a 12±7 μg/L and suspended material in the column of water with values of 3±1.8 mg/L). Air temperature was 27±0.5 °C and water temperature 26.6±0.5 °C, with a slightly basic pH (7.8±1.0) and a conductivity of 334±2.5 μS/cm. The concentrations of dissolved oxygen, nitrogen and total phosphorus were 6.6±1.7 mg/L, 1.6±0.5 mg/L and 0.04±0.05 mg/L, respectively, characterising the environment as eutrophic corroborating previous researches in this ecosystem (Pereira et al. 2012; Albertoni et al. 2014).
Field and laboratory procedures
In the field, two approaches were implemented. Firstly, the submersed macrophytes Chara zeylanica Willdenow and Potamogeton pectinatus L. were sampled in the shallow lake, with a manual sampler. During January 2012, we collected six samples from each macrophyte at an interval of ~10 days (two samples in each sampling date), coinciding with the decomposition experiment sampling dates (see below). We chose the sampling points randomly inside the stands of each macrophyte. The collected organisms were separated by washing in a sieve (250 μm mesh size). Plants were ovendried until constant weight to obtain dry mass in an analytical balance. We counted fauna in stereomicroscopic and identified at the lowest possible taxonomic level. Data from the invertebrate community on live plants are presented as total abundance and density (organisms/g). Secondly, to study the decomposition and associated fauna, the macrophytes C. zeylanica and P. pectinatus were collected and air-dried at 20 °C for two weeks. We used litter bags of 20x30 cm, with aperture of 1 cm2 on the upper face (in contact with the water column) and 0.1 cm2 on the face in contact with the sediment (adapted from Bedford 2004). Aliquots of detritus of both plant species were weighed (6.0±0.1 g dry weight), totalling 60 litter bags (30 containing P. pectinatus and 30 containing C. zeylanica). The bags were arranged in four treatments: 15 litter bags with P. pectinatus debris decomposing inside their own stand (called “PP” treatment), 15 litter bags with P. pectinatus debris decomposing amid C. zeylanica stands (called “PC”), 15 litter bags with C. zeylanica debris decomposing inside their own stand (called “CC”), 15 litter bags with C. zeylanica debris decomposing amid P. pectinatus stands (called “CP”). All bags were incubated over the sediment, at an average depth of 1.4 m, and with a distance of 10 m from each other. After 5, 10 and 20 days of decomposition, we removed five litter bags of each treatment, totalling 20 litter bags per collection. The litter bags were supported with a mesh network (250 μm mesh size), packed in plastic bags and stored in a cooler with ice until washing.
In the laboratory, the material was rinsed under running water on a sieve (250 μm mesh size). The plant material was oven-dried at 35 °C for 72 h to determine leaf degradation coefficients (k). Invertebrates retained on the sieve were placed in glass vials with 80% alcohol and identified to the lowest possible taxonomic level. We determined the richness (number of taxa) and abundance (number of organisms per taxon) of invertebrates associated to the litter bags. Although we did not analyse the stomach contents of the invertebrates, they were classified into functional feeding groups (FFG) according to the current literature, Domínguez and Fernández (2009), Cummins et al. (2005), Wantzen and Wagner (2006) and Merritt et al. (2008).
Data analysis
We determined the leaf degradation coefficients from the debris remaining in the bags over the time of incubation using the exponential decay model Wt = W0.e-kt, where Wt is the remaining weight at time t (days), W0 is the initial weight, “e” is the exponential coefficient and “k” is the leaf degradation coefficient (Bärlocher 2005).
With the data of the invertebrate communities on plants in the debris and in the living stands, habitat’s preference indices were calculated. The following arrangement was used: a) a set of organisms that inhabit P. pectinatus live in relation to their detritus incubated in its stand (PP) or in the C. zeylanica stand (PC), and b) a second arrangement, where organisms that inhabit C. zeylanica live in relation to organisms in its debris (CC), when incubated in its stand or P. pectinatus stand (CP). The Chesson index of electivity modified by Manly (Krebs 2014) was used to measure the habitat preference according the formula αi=(ri/pi)(1/Σ(ri/pi), where αi=electivity (=preference) index; ri=proportion of “i” taxa organisms in the detritus; pi=proportion of “i” taxa organisms in the live plant. αi is compared with the factor “1/m”, where “m” is total richness. If αi>(1/m), then detritus is preferred as habitat by “i” taxa.
We used a PerMANOVA (Bray-Curtis distance, Bonferroni correction of abundances). To test for differences in invertebrate composition between live plants and their detritus, incubated in their own stand and in the other macrophyte stand. Also, a PerMANOVA was applied to test the differences between FFGs of invertebrates at detritus of the different treatments.
To test for differences in the leaf degradation coefficients we used an ANCOVA, considering the model: remaining weight ~ treatments (4 categorical levels) + time (covariable quantitative). To evaluate differences between abundance and richness of associated invertebrates, we applied ANOVA one way with Tukey post-test. All analyses were conducted using the “vegan” package (Oksanem et al. 2012) in the R software (R Core Team 2013).
RESULTS
Mass loss
We observed significant differences in mass loss between the treatments (F3,57=8.4, P=0.0001) over the time of incubation (F1,57=150.6, P<0.0001). The difference between the decomposition rates between the treatments was evident between the C. zeylanica and P. pectinatus debris, regardless of the incubation site. Decomposition rates for C. zeylanica were four times higher than for P. pectinatus, and at the end of the experiment (20th day), only debris of P. pectinatus were present. After 10 days of incubation, C. zeylanica showed 14.8% (k=-0.20 1/d, R2=0.86) and 15.9% (k=-0.19 1/d, R2=0.88) remaining mass in the CC and CP treatments, respectively (Figure 1). At the end of the experiment (after 20 days), P. pectinatus debris showed a remaining mass of 33.1% (k=-0.05 1/d, R2=0.93) and 43.7% (k=-0.04 1/d; R2=0.91) in PP and PC treatments, respectively (Figure 1).
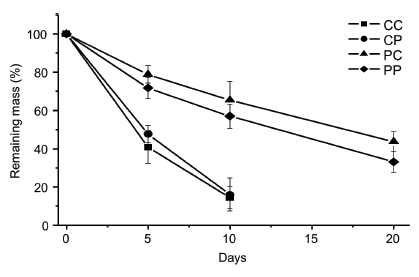
Figure 1. Percentage of remaining weight (±SD) Chara zeylanica and Potamogeton pectinatus during decomposition in incubation treatments. CC: C. zeylanica amid C. zeylanica; CP: C. zeylanica amid P. pectinatus; PC: P. pectinatus amid C. zeylanica; PP: P. pectinatus amid P. pectinatus.
Figura 1. Porcentaje de peso remanente de Chara zeylanica y Potamogeton pectinatus durante la descomposición en los tratamientos de incubación. CC: C zeylanica en medio C. zeylanica; CP: C. zeylanica en medio P. pectinatus; PC: P. pectinatus en medio C. zeylanica; PP: P. pectinatus en medio P. pectinatus.
Associated invertebrates
In live macrophytes, a total of 1290 organisms were recorded for C. zeylanica and 18608 for P. pectinatus. Abundance of macroinvertebrates in live macrophytes was higher than their abundance in the detritus. Total richness was 23 taxa for C. zeylanica, with predominance of Oligochaeta (205±189.6 organisms; average±SD) and Chironomidae (143±94.7 organisms), while P. pectinatus presented a total richness of 21 taxa, with predominance of Nematoda (1422.2±1238.1 organisms) and Chironomidae (976.5±933.3 organisms) (Table 1). Potamogeton pectinatus had a mean richness of 14.5±1.23 taxa and mean density of 28.3±2.97 organisms/g, while C. zeylanica presented mean richness of 20.2±2.9 taxa, and mean density of 5.24±0.45 organisms/g.
Table 1. Mean abundance (±SD) of invertebrates (n=6) associated with live Potamogeton pectinatus and live Chara zeylanica during degradation experiment at a shallow lake in southern Brazil. FFG: functional feeding group; Cg: collectorgathered; Pr: predator; Sc: scraper; Fi: filterer.
Tabla 1. Abundancia media (±DS) de invertebrados (n=6) asociados con Potamogeton pectinatus vivo y Chara zeylanica vivo durante el experimento de degradación en un lago somero en el sur de Brasil. FFG: grupo de alimentación funcional; Cg: recolector; Pr: depredador; Sc: raspador; Fi: filtrador.

At detritus, a total of 7048 individuals in 25 taxa were recorded throughout the degradation experiment in the four treatments (Table 2). Mean abundance was higher in the treatment PP (395.7±106.9 individuals, F(3,28)=7.24, P=0.0009). Tukey post tests showed differences of PP treatment with all treatments, PP vs. PC P=0.0087, PP vs. CC P=0.031, PP vs. CP, P=0.001. Comparisons between PC vs. CC were also different (P=0.012), and PC and CP and CC and CP did not differ (P=0.96 and P=0.63, respectively) (Figure 2). Greater taxonomic mean richness was observed in the treatments CC and PP (11.3±1.4 and 10.1±2.2 taxa, respectively) and lowest in the treatment CP (8.3±0.5 taxa) (F3,31=11.43, P<0.0001). Tukey post-doc tests showed that CC was different from PP (P=0.012) and from PC (P=0.0009), and CP vs PP were also different (P=0.001) (Figure 2). These results are opposite to our initial hypothesis, since we expected that higher environmental heterogeneity would be associated with higher richness.
Table 2. Mean abundance (±SD) of invertebrate community associated with debris of macrophytes in a shallow lake in southern Brazil. FFG: functional feeding group; PP: P. pectinatus debris amid P. pectinatus stand; PC: P. pectinatus debris amid C. zeylanica stand; CC: C. zeylanica debris amid C. zeylanica stand; CP: C. zeylanica debris amid P. pectinatus stand. Day 5, 10, 20 = time of degradation evaluation.
Tabla 2. Abundancia media (±DS) de la comunidad de invertebrados asociados con detritos de macrófitas en un lago somero en el sur de Brasil. FFG: grupo de alimentación funcional; PP: detritos de P. pectinatus en medio P. pectinatus; PC: Detritos de P. pectinatus en medio C. zeylanica; CC: detritos de C. zeylanica en medio C. zeylanica; CP: detritos de C. zeylanica en medio P. pectinatus. Día 5, 10, 20 = tiempo de evaluación de la degradación.
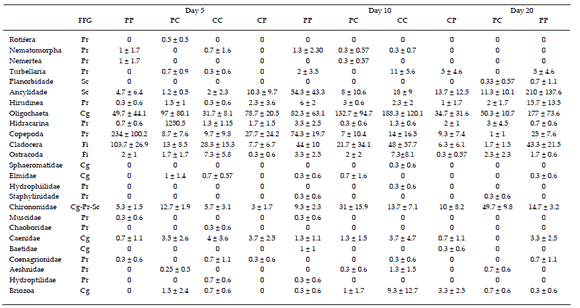

Figure 2. Mean richness and abundance (±SD) of benthic invertebrates in each treatment during decomposition of Chara zeylanica and Potamogeton pectinatus in a shallow lake. Treatments are CC: C. zeylanica amid C. zeylanica; CP: C. zeylanica amid P. pectinatus; PC: P. pectinatus amid C. zeylanica; PP: P. pectinatus amid P. pectinatus. Different letters show statistical differences, capital letters for abundance and lower case for richness.
Figura 2. Media de riqueza y abundancia de invertebrados bentónicos en cada tratamiento durante la descomposición de Chara zeylanica y Potamogeton pectinatus en un lago somero. Los tratamientos son CC: C zeylanica en medio C. zeylanica; CP: C. zeylanica en medio P. pectinatus; PC: P. pectinatus en medio C. zeylanica; PP: P. pectinatus en medio P. pectinatus. Diferentes letras muestran diferencias estadísticas, letras mayúsculas para la abundancia y minúsculas para la riqueza.
The invertebrates showed different preferences when evaluated at different incubation sites and the different debris, and all treatments of detritus showed a significant difference in their composition when compared to the composition of the community of the living plants. For the P. pectinatus debris, total richness (detritus+live) was 31 taxa, and 1/m Mainly factor=0.0326. Four taxa showed preference for their debris, as well as incubated in the middle of their own stand (F2,30=8.8, P=0.002) and when incubated in the C. zeylanica stand (F2,30=10.3, P=0.001). Ancylidae, Hirudinea, Ostracoda e Tardigrada showed higher densities in the debris in the PP treatment when compared to the community in the plant stand. When debris of P. pectinatus was incubated at the C. zeylanica stand (PC), in addition to the taxa mentioned above, Copepoda also showed preference for detritus. However, in the community associated with C. zeylanica, total richness (detritus+live) was 32, and Maily factor 1/m=0.0321. When comparing the abundances of the organisms that were identified in their bank and their detritus, Oligochaeta, Copepoda, Cladocera, Ostracoda and Caenidae showed preference for their detritus when they were incubated in the medium of its own stand (CC treatment, F2,31=8.1, P=0.002). Only two taxa, Copepoda and Hirudinea preferred the detritus when it degrades in the middle of the P. pectinatus (CP treatment, F2,31=12.4, P=0.001) stand.
Functional feeding group composition differed between treatments and incubation habitats depending on the decomposition time (Table 3). Collectors were the most abundant (49.3%), followed by predators (20.3%) (Figure 3). The largest number of collectors was observed in the treatment PP (36%). The treatment PC showed an equitable distribution of all functional feeding groups (Figure 3).
Table 3. Summary PerMANOVA for functional invertebrate composition between treatments and incubation time. df: degrees of freedom; SS: sum of squares; MS: mean square.
Tabla 3. Resumen del PerMANOVA para la composición funcional de invertebrados entre los tratamientos y tiempo de incubación. df: grados de libertad; SS: suma de cuadrados; MS: cuadrado medio.

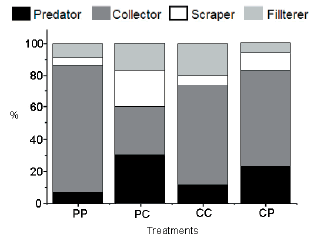
Figure 3. Percentage of functional feeding groups in treatments: CC: C. zeylanica amid C. zeylanica; CP: C. zeylanica amid P. pectinatus; PC: P. pectinatus amid C. zeylanica; PP: P. pectinatus amid P. pectinatus.
Figura 3. Porcentaje de grupos funcionales de alimentación en los tratamientos: CC: C zeylanica en medio de C. zeylanica; CP: C. zeylanica en medio de P. pectinatus; PC: P. pectinatus en medio de C. zeylanica; PP: P. pectinatus en medio de P. pectinatus.
DISCUSSION
In our study, macrophytes decomposed more quickly in stands of their own species than in stands of the other species. Thus, the differences in mass loss of both species were influenced by both degradation time and site of incubation. The surrounding vegetation is one of the factors that can interfere with the decomposition of submerged macrophytes (Vandel 2010). Our results show that the site of detritus storage can influence degradation rates of the studied macrophytes.
The site of debris decomposition also influenced the structure and composition of the community of associated invertebrates. We observed a higher taxonomic richness in the debris of macrophytes that have the highest richness also as live plant, and in the treatment in the middle of their own stand (CC). In the treatment where P. pectinatus decomposed in the midst of a stand of its own species (PP), we found the highest organism abundance. This probably reflects the more abundant community that inhabits this macrophyte that is ~14 times higher than C. zeylanica. These results contradict our initial hypothesis, given that the heterogeneity promoted by debris amid stands of a different species did not contribute to a greater invertebrate richness or abundance.
Our results may reflect better shelter conditions and/or availability of food in P. pectinatus debris and at stands of living plant. This assumption is supported by the lower decay coefficients observed for this treatment (PP). According to Wallace and Webster (1996), the physical environment and available food resources can influence the local abundance of invertebrates. Furthermore, the slow decomposition of debris positively affects the invertebrate community, allowing greater abundance of organisms (Gonçalves et al. 2004) by providing a relatively stable substrate.
The greatest abundance of invertebrates in detritus was found in the treatment PP. Potamogeton pectinatus stands had the higher abundance, and their detritus decomposed slower, factors that may contribute to greater habitat resource availability to detritivorous invertebrates. Potamogeton pectinatus is a submerged macrophyte with a complex morphological structure due to the formation of dense stands that may occupy the entire water column (Van den Berg et al. 1998). Plants with a more complex architecture favour the establishment of a greater diversity of organisms because, according to Barreto (1999) and Kovalenko et al. (2012), increased biomass area increases spatial niches and the number of individuals. Therefore, this factor, coupled with slower mass loss, makes P. pectinatus a more favourable habitat for colonisation by invertebrates and for the invertebrate community structure, as they use the debris as a place for foraging and shelter. Stands of macrophytes in shallow waters provide a high diversity of microhabitats, favouring the coexistence of many species of different functional divisions of the FFG (Padisák and Reynolds 2003). Such biomass degradation, with the consequent release of the compounds into the water column, is one of the most important factors in the functioning of shallow lakes (Asaeda et al. 2000). Therefore, our results clearly prove the important role of submersed aquatic macrophytes in maintaining invertebrate biodiversity and abundance in shallow lakes, even in detritivorous chains.
The classification of functional feeding groups is a classic tool to describe the function of invertebrates in the processes of decomposition of plant material in aquatic ecosystems and use both morphological and behavioural characters of organisms to acquire food resources (Cummins et al. 2005; Zilli et al. 2008; Poi de Neiff et al. 2009; Carvalho et al. 2015; among others). More recently, the knowing of feeding habits of organisms has been done through in situ observation studies, laboratory experiments and stomach content analyses, and the latter approach has been considered the most accurate (Ramírez and Gutiérrez-Fonseca 2014). In a recent review, Ramírez and Gutiérrez-Fonseca (2014) made a discussion about the needs of analysis of gut content to assign the correct FFGs, and pointed out that, even with limitations, it is not strictly necessary. Thus, even with the inherent limitations, we used the literaturebased classification in our study, since it is still a valid tool to indicate the FFGs of invertebrates. The treatment with slower weight loss (PP treatment) favoured collectors, since they found a greater availability of food and/or shelter for a longer period. During macrophyte decomposition, the generation of fine particulate organic matter (FPOM) favours the development of collectors which use it as a food resource (Gimenes et al. 2010). Also, intermediates periods of degradation are associated with the time where microorganisms start to decompose the detritus, making it suitable for invertebrates (Biasi et al. 2013). Thus, large numbers of these organisms indicate high amounts of debris particles on the surface (Stripari and Henry 2002). However, according to Zilli et al. (2008), the number of collectors is not necessarily positively related to the amount of food. Although we found high numbers of predators and collectors during early incubation of debris, in these initial stages there is little production of FPOM (Stripari and Henry 2002). Thus, the high abundances of collectors might have been due to the use of resources by these organisms primarily as habitat. These results corroborate the findings of Telöken et al. (2011, 2014), who observed a similar pattern in the decomposition of tree species in a subtropical shallow lake and in a stream, near the area of our study. In tropical and subtropical environments, particularly in lakes, collectors appear to be dominant in detritivorous communities (Stripari and Henry 2002; Gonçalves et al. 2003; Telöken et al. 2011; 2014). Thus, the results on the trophic structure of the associated invertebrate community with the debris of the two studied macrophytes reinforce the hypothesis that invertebrates are using the debris as habitat.
When we evaluated the invertebrate community of living plants, the virtual absence of shredders is another factor that allows suggesting the use of detritus as habitat more than food resource. Although with some controversy, most of the studies related the weak relation of invertebrates with direct process of degradation (e.g., shredders) in lentic subtropical systems (Wantzen and Wagner 2006; Silva et al. 2010; Telöken et al. 2011; 2014; Carvalho et al. 2015). When we looked the results of richness of invertebrate community, at both plant species the mean richness was lower at detritus than at live plants. The species with faster degradation time (C. zeylanica) presented higher richness both when as plant material and in its detritus. One factor pointed out by Srivastava et al. (2009) is that breakdown of detritus is affected strongly and positively by the topdown effects of detritivore diversity. This corroborates with the biodiversity-ecosystem function theory. So, even with the gap of shredders, which used directly the detritus, the diversity of detritivores invertebrates presented high diversity when compared with similar systems (Titus and Pagano 2002; Nelson 2011; Carvalho et al. 2015, among others). This could be a factor that contribuyes to the time of degradation of macrophytes at shallow lakes system, more than habitat or the kind of detritus.
The results of our study suggest that the submerged vegetation causes changes in the structure of the aquatic community, which was also mentioned by Van den Berg et al. (1997), who studied invertebrates in lakes dominated by Chara sp. Other studies have highlighted the importance of macrophyte complexity in terms of habitat diversity (Lopes et al. 2011) and species richness (Thomaz et al. 2008). However, our study is the first to address habitat complexity in terms of debris decomposition. Here, habitat complexity can be understood as diversity of structural elements which positively impact the diversity and abundance of species, mainly by increasing spatial niches (see also Kovalenko et al. 2012). According to the same author, the greater habitat variability allows the coexistence of organisms with a wider range of resource uses.
In conclusion, the trophic structure of invertebrate community at debris of both macrophytes at all four treatments, presented four functional feeding groups, and absence of shredders, suggesting that detritus was used mainly as shelter more than food resource in this shallow lake. The species P. pectinatus, with a low decomposition rate, had the highest abundance of invertebrates when debris was decomposing amid its own stand, probably reflecting the highest abundance of invertebrates at live plant stand. Chara zeylanica, even with high decomposition rate, presented highest richness both at debris and at live plant, even with lower density. Therefore, our study shows that the place of debris decomposition interferes with the decomposition coefficients and the structuring of the invertebrate community.
ACKNOWLEDGEMENTS
We thank Leonardo Furlanetto for help in field, CAPES for the scholarship to CC, Norma Catarina Bueno for helping with the identification of the Chara zeylanica. LUH received financial support from CNPq (Process #305203/2017-1). The authors are grateful to anonymous reviewers for suggestions and criticisms.
REFERENCES
1. Albertoni, E. F., L. J. Prellvitt, and C. Palma-Silva. 2007. Macroinvertebrate fauna associated with Pistia stratiotes and Nymphoides indica in subtropical lakes (south Brazil). Brazilian Journal of Biology 67:499-507. [ Links ]
2. Albertoni, E. F., C. Palma-Silva, C. R. T. Trindade, and L. M. Furlanetto. 2014. Field evidence of the influence of aquatic macrophytes on water quality in a shallow eutrophic lake over a 13-year period. Acta Limnologica Brasiliensia 26(2): 176-185 [ Links ]
3. Ali, M. M., A. A. Mageed, and M. Heikal. 2007. Importance of aquatic macrophyte for invertebrate diversity in large subtropical reservoir. Limnologica 37:155-169. [ Links ]
4. Asaeda, T., V. K. Trung, and J. Manatunge. 2000. Modelling the effects of macrophytes growth and decomposition on the nutrient budget in shallow lakes. Aquatic Botany 68:217-237. [ Links ]
5. Baptista, D. F., D. F. Buss, L. F. M. Dorvillé, and J. L. Nessimian. 2001. Diversity and habitat preference of aquatic insects along the longitudinal gradient of the Macaé river basin, Rio de Janeiro, Brazil. Revista Brasileira de Biologia 61(2):249-258. [ Links ]
6. Bärlocher, F. 2005. Leaching. Pp. 329 in M. A. S. Graça, F. Bärlocher and M. O. Gessner (eds.). Methods to study litter decomposition. Dordrecht: Springer. [ Links ]
7. Barreto, C. C. 1999. Heterogeneidade espacial do hábitat e diversidade específica: implicações ecológicas e métodos de mensuração. Oecologia Brasiliensis 7:121-153. [ Links ]
8. Bedford, A. P. 2004. A modified litter bag design for use in lentic habitat. Hydrobiologia 529:187-193. [ Links ]
9. Bellisario, B., F. Cerfolli, and G. Nascetti. 2012. The interplay between network structure and functioning of detritusbased communities in patchy aquatic environment. Aquatic Ecology 46:431-441. [ Links ]
10. Biasi, C., A. M. Tonin, R. M. Restello, and L. U. Hepp. 2013. The colonisation of leaf litter by Chironomidae (Diptera): the influence of chemical quality and exposure duration in a subtropical stream. Limnologica 43:427-433. [ Links ]
11. Callisto, M., M. Goulart, A. O. Medeiros, P. Moreno, and C. A. Rosa. 2004. Diversity assessment of benthic macroinvertebrates, yeasts, and microbiological indicators along a longitudinal gradient in Serra do Cipó, Brazil. Brazilian Journal of Biology 64:743-755. [ Links ]
12. Carvalho, C., L. U. Hepp, C. Palma-Silva, and E. F. Albertoni. 2015. Decomposition of macrophytes in a shallow subtropical lake. Limnologica 53:1-9. [ Links ]
13. Casagranda, C., M. S. Dridi, and C. F. Boudouresque. 2006. Abundance, population structure and production of macro-invertebrate shredders in a Mediterranean brackish lagoon, Lake Ichkeul, Tunisia. Estuarine, Coastal and Shelf Science 66:437-446. [ Links ]
14. Cummins, K. W., R. W. Merritt, and P. C. N. Andrade. 2005. The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil Studies on Neotropical Fauna and Environment 40:69-89. [ Links ]
15. Domínguez, E., and H. R. Fernández (ed.). 2009. Guía para la determinación de los artrópodos bentónicos sudamericanos. 2ª Ed. Tucumán: Editorial Universitaria de Tucumán. Pp. 654. [ Links ]
16. Esteves, F. A. 2011. Fundamentos de Limnologia. 3ª ed. Rio de Janeiro. Interciência. Pp. 790. [ Links ]
17. Gimenes, K. Z., M. B. Cunha-Santino, and I. Bianchini Jr. 2010. Decomposição de matéria orgânica alóctone e autóctone em ecossistemas aquáticos. Oecologia Australis 14:1075-1112. [ Links ]
18. Gonçalves Jr, J. F., F. A. Esteves, and M. Callisto. 2003. Chironomids colonization on Nymphaeae ampla L. detritus during a degradative ecological succession experiment in Brazilian coastal lagoon. Acta Limnologica Brasiliensia 15:21-27. [ Links ]
19. Gonçalves Jr, J. F., A. M. Santos, and F. A. Esteves. 2004. The influence of the chemical composition of Typha domingensis and Nymphaea ampla detritus on invertebrate colonization during decomposition in a Brazilian coastal lagoon. Hydrobiologia 527:125-137. [ Links ]
20. Hansen J. P., S. A. Wikström, H. Axemar, and L. Kautsky. 2011. Distribution differences and active habitat choices of invertebrates between macrophytes of different morphological complexity. Aquatic Ecology 45:11-22. [ Links ]
21. Hannigan, E., and K. M. Quinn. 2012. Composition and structure of macroinvertebrate communities in contrasting open-water habitat in Irish peatlands: implications for biodiversity conservation. Hydrobiologia 692:19-28 [ Links ]
22. Hepp, L. U., V. L. Landeiro, and A. S. Melo. 2012. Experimental assessment of the effects of environmental factors and longitudinal position on alpha and beta diversities of aquatic insects in a neotropical stream. International Review of Hydrobiologia 97:157-167. [ Links ]
23. Krebs, C. J. 2014. Ecological methodology. 3rd ed. URL: www.zoology.ubc.ca/~krebs/books.html. [ Links ]
24. Kovalenko, K. E., S. M. Thomaz, and D. M. Warfe. 2012. Habitat complexity: approaches and future directions. Hydrobiologia 685:1-17. [ Links ]
25. Janke, H., and S. Trivinho-Strixino. 2007. Colonization of leaf litter by aquatic macroinvertebrates: a study in a low order tropical stream. Acta Limnologica Brasiliensia 19:109-115. [ Links ]
26. Lopes, A., J. D. Paula, S. F. Mardegan, N. Hamada, and. M. T. F. Piedade. 2011. Influência do hábitat na estrutura da comunidade de macroinvertebrados aquáticos associados às raízes de Eichhornia crassipes na região do Lago Catalão, Amazonas, Brasil. Acta Amazonica 41:493-502. [ Links ]
27. Merritt, R. W., K. W. Cummins, and M. B. Berg. 2008. An Introduction to the Aquatic Insects of North America. Dubuque, Kendall/Hunt Publishing Co. Pp. 1158. [ Links ]
28. Nelson, M. 2011. Comparisons of macrophyte breakdown, associated plant chemistry, and macroinvertebrates in a wastewater dominated stream. International Review of Hydrobiologia 96:72-89. [ Links ]
29. Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, R. G. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, and H. Wagner. 2012. Vegan: Community Ecology Package. R package version 1.17-0. URL: CRAN.R-project.org/package=vegan.
30. Padisák, J., and C. S. Reynolds. 2003. Shallow lakes: the absolute, the relative, the functional and the pragmatic. Hydrobiologia 506-509:1-11. [ Links ]
31. Pereira, S. A., C. R. T. Trindade, E. F. Albertoni, and C. Palma-Silva. 2012. Aquatic macrophytes as indicators of water quality in subtropical shallow lakes, Southern Brazil Acta Limnologica Brasiliensia 24(1):52-63 [ Links ]
32. Poi de Neiff, A., M. E. Galassi, and M. C. Franceschini. 2009. Invertebrate assemblages associated with leaf litter in three floodplain wetlands of the Parana river. Wetlands 29:896-906 [ Links ]
33. Ramírez, A., and P. E. Gutiérrez-Fonseca. 2014. Functional feeding groups of aquatic insect families in Latin America: a critical analysis and review of existing literature. Revista de Biología Tropical 62(suppl. 2):155-167. [ Links ]
34. R Core Team. 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: www.R-project.org. [ Links ]
35. Rossi, L., M. L. Costantini, P. Carlino, A. Lascio, and D. Rossi. 2010. Autochthonous and allochthonous plant contributions to coastal benthic detritus deposits: a dual-stable isotope study in a volcanic lake. Aquatic Science 72:227-236. [ Links ]
36. Silva, J. S., W. T. Silveira, E. F. Albertoni, and C. Palma-Silva. 2010. Diversity of Chironomidae (Diptera) during the decomposition of Nymphoides indica (L.) Kuntte in two subtropical lakes with different trophic levels (south Brazil). Panamerican Journal of Aquatic Sciences 5:557-571. [ Links ]
37. Silva, J. S., E. F. Albertoni, and C. Palma-Silva. 2015. Temporal variation of phytophilous Chironomidae over a 11-year period in a shallow Neotropical lake in southern Brazil Hydrobiologia 742:129-140 [ Links ]
38. Stripari, N. de L., and R. Henry. 2002. The invertebrate colonization during decomposition of Eichhornia azurea kunth in a lateral lake in the mouth zone of Paranapanema river into Jurumirim reservoir (São Paulo, Brazil). Brazilian Journal of Biology 62:293-310. [ Links ]
39. Telöken F., E. F. Albertoni, and C. Palma-Silva. 2011. Leaf degradation of Salix humboldtiana Willd. (Salicaceae) and invertebrate colonization in a subtropical lake (Brazil). Acta Limnologica Brasiliensia 23:30-41. [ Links ]
40. Telöken, F., E. F. Albertoni, L. U. Hepp, and C. Palma-Silva. 2014. Invertebrados aquáticos associados a serapilheira de Salix humboldtiana em um riacho subtropical. Ecología Austral 24:220-228. [ Links ]
41. Titus, J. E., and A. M. Pagano. 2002. Decomposition of litter from submersed macrophytes: the indirect effects of high (CO2). Freshwater Biology 47:1367-1375. [ Links ]
42. Thomaz, S. M., E. D. Dibble, L. R. Evangelista, J. Higuti, and L. M. Bini. 2008. Influence of aquatic macrophyte habitat complexity on invertebrate abundance and richness in tropical lagoons. Freshwater Biology 53:358-367. [ Links ]
43. Wallace, J. B., and J. R. Webster. 1996. The role of macroinvertebrates in stream ecosystem function. Annual Review of Entomology 41:115-139. [ Links ]
44. Wantten, K. M., and R. Wagner. 2006. Detritus processing by invertebrate shredders: a neotropical-temperate comparison. Journal of the North American Benthological Society 25:216-232. [ Links ]
45. Wardle, D. A., and G. W. Yeates. 1993. The dual importance of competition and predation as regulatory forces in terrestrial ecosystems: evidence from decomposer food-webs. Oecologia 93:303-306. [ Links ]
46. Wettel, R. G. 1993. Limnologia. Lisboa: Fundação Calouste Gugelheim. Pp. 919. [ Links ]
47. Van Den Berg, M. S., H. Coops, R. Noordhuis., J. Van Schie, and J. Simons. 1997. Macroinvertebrate communities in relation to submerged vegetation in two Chara-dominated lakes. Hydrobiologia 342-343:143-150. [ Links ]
48. Van Den Berg, M. S., H. Coops, J. Simons, and A. Keizer. 1998. Competition between Chara aspera and Potamogeton pectinatus as a function of temperature and light. Aquatic Botany 60:241-250. [ Links ]
49. Vandel, E. 2010. Effect of vegetation on alpha cellulose decomposition in littoral lake sediments. Aquatic Botany 93: 179-184. [ Links ]
50. Zilli, F. L., L. Montalto, and M. R. Marchese. 2008. Benthic invertebrate assemblages and functional feeding groups in the Paraná River floodplain (Argentina). Limnologica 38:159-171. [ Links ]














