Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Ecología austral
versión On-line ISSN 1667-782X
Ecol. austral vol.29 no.3 Córdoba ago. 2019
ARTÍCULO
Seasonal succession of gelatinous zooplankton (medusae and ctenophores) from Mar del Plata Harbor, Argentina (SW Atlantic Ocean)
F. Alejandro Puente Tapia1, 2,* & Genzano Gabriel1, 2
1 Departamento de Ciencias Marinas, Instituto de Investigaciones Marinas y Costeras (IIMyC), Universidad Nacional de Mar del Plata (UNMdP), Mar del Plata, Buenos Aires, Argentina.
2 Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Mar del Plata, Argentina
Recibido: 8 de Febrero de 2019
Aceptado: 28 de Agosto de 2019
Editor asociado: Pedro Daleo
ABSTRACT
Temporal variation of the population dynamics of the medusae and ctenophores (gelatinous zooplankton) is described over an annual cycle in the Mar del Plata Harbor, Argentina. A total of 18 species were identified, 3 of which were ctenophores (2 of Class Tentaculata and 1 of Nuda) and 15 medusae (14 of Class Hydrozoa and 1 of Scyphozoa). A species of hydromedusae, Annatiara affinis, was observed for the first time in the Argentine Sea. In both groups, species richness and abundances showed the lowest values in the cold period (austral autumn-winter) and the highest values in the warm period (spring-summer). The meroplanktonic medusae Obelia sp. and Eucheilota ventricularis as well as the holoplanktonic Liriope tetraphylla represented 94.2% of the total abundances of this group (classified as dominant). In the ctenophores, the dominant species were Pleurobrachia pileus and Mnemiopsis leidyi, which accounted for 99.3% of the ctenophores. Monthly medusae succession indicated that holoplanktonic species were dominant over practically all the annual cycle in terms of abundance, while considering species richness values, meroplanktonic species showed highest values. Ctenophores (considering abundance values) was displayed alternating periods of approximately three months of dominance between P. pileus and M. leidyi. The Bray-Curtis similarity index performed on medusae identified two groups of seasons with faunal affinity, the 1) cold and 2) warm periods, with the water temperature and non-gelatinous zooplankton abundances as the environmental factors that best explained this variability (BIO-ENV analysis), while for ctenophores, temporal faunal homogeneity was observed and a single group with faunal affinity was identified.
Keywords: Zooplankton abundance; Species richness; Faunal similarity; Obelia sp.; Liriope tetraphylla; Hydrozoa; Scyphozoa; Nuda; Tentaculata; Mar del Plata coast.
RESUMEN
Sucesión estacional de zooplancton gelatinoso (medusae y ctenophores) del puerto de Mar del Plata, Argentina (Océano Atlántico sudoccidental).
Se describe la variación estacional de la dinámica poblacional de las medusas y ctenóforos (zooplancton gelatinoso) a lo largo de un ciclo anual en el puerto de Mar del Plata, Argentina. Se identificaron un total de 18 especies, de las cuales 3 fueron ctenóforos (2 de la clase Tentaculata y 1 de Nuda) y 15 medusas (14 de la clase Hydrozoa y 1 de Scyphozoa). La hidromedusa Annatiara affinis representó un nuevo registro para el Mar Argentino. En ambas agrupaciones, la riqueza específica y las abundancias mostraron los valores más bajos en el período frío (otoño-invierno austral) y los más altos en el período cálido (primavera-verano). Las medusas meroplanctónicas Obelia sp. y Eucheilota ventricularis, así como la holoplactónica Liriope tetraphylla, representaron el 94.2% del total de las abundancias de este grupo (clasificadas como dominantes). En los ctenóforos, las especies dominantes fueron Pleurobrachia pileus y Mnemiopsis leidyi, que representaron el 99.3%. La sucesión mensual en las medusas indicó que las especies holoplanctónicas fueron dominantes casi en todo el ciclo anual en términos de abundancia, mientras que, sobre la base del número de especies, las meroplanctónicas presentaron valores mayores. En los ctenóforos (considerando sus abundancias), se observaron períodos intercalados de ~3 meses de dominancia entre M. leidyi y P. pileus. El índice de Bray-Curtis aplicado a las medusas identificó la presencia de dos agrupaciones de estaciones climáticas con afinidad faunística: 1) frío y 2) cálido; los factores ambientales que mejor explicaron dicha variabilidad fueron la temperatura del agua y las abundancias del zooplancton no gelatinoso (análisis BIO-ENV). En el caso de los ctenóforos, se observó una homogeneidad faunística temporal y se identificó un solo grupo con afinidad de especies.
Palabras clave: Abundancias del zooplancton; Riqueza específica; Similitud faunística; Obelia sp.; Liriope tetraphylla; Hydrozoa; Scyphozoa; Nuda; Tentaculata; Costas de Mar del Plata.
INTRODUCTION
Medusae and ctenophores have been categorized as part of the gelatinous zooplankton (GZ). GZ represent a functional group composed of different phyla (e.g., Chaetognatha, Ctenophora, Cnidaria, Chordata, etc.) that contain a high percentage of body water (>95%), which gives them their peculiar gelatinous aspect and some degree of transparency and fragility (Haddock 2004; Daly et al. 2007). Particularly, medusae and ctenophores are noticeable due to their abundance and species richness (Raskoff et al. 2003; Haddock 2004). In the present study, the broad term GZ encompasses the planktonic medusa phase of the classes Hydrozoa and Scyphozoa, as well as the ctenophores.
Although medusae and ctenophores being different phyla, having physiological characteristics that distinguish them and differentiate from other marine invertebrates (e.g., the presence of cnidocysts and colloblasts, respectively), both share certain life-history characteristics allowing them coincide temporal and spatially (Purcell and Mills 1988). However, despite that, they can exhibit different levels of responses in feeding, reproduction, growth and presence/absence to the variation of the physicochemical and biological conditions of the environment (Purcell and Mills 1988).
The seasonal variability of zooplankton abundance, including medusae and ctenophores, is associated with dynamic processes that modify the relative contributions of taxonomic groups or species, principally hydrodynamic interactions, intraspecific biological processes, interspecific ecological interactions (Mackas and Beaugrand 2010) and the life cycles (Graham et al. 2001). However, these influences vary according to the species because each one displays different affinities for particular hydrobiological characteristics, making the composition of this functional group temporally and spatially dynamic (Goy 1997).
The life cycles (meroplanktonic and holoplanktonic strategy) plays an essential role in an organism´s presence or absence, the frequency of occurrence, and abundances (Graham et al. 2001). These life cycles involve, in most cases, rapid population growth, reaching high abundances (Mills 2001) and becoming dominants, at least seasonality, in the pelagic environment of different regions (Pagés et al. 1996). For these reasons, some species may be abundant in a particular period, followed by an extended period of absence in the water column and then reappear (Benovic et al. 1987), perhaps due to qualitative changes at individual levels (variation in the life cycle) or quantitative changes in population levels (variation in the life history) (Giangrande et al. 1994). In the first case, a species persists locally at different times during different life cycle stages, while with variation dependent on life history, a species may experience alternating peaks and seasonal scarcity in its adult populations (Boero et al. 2008).
The GZ species shown both strategies: meroplanktonic species with benthic stages adopt life cycle adjustments, whereas holoplanktonic species tend to be associated with life history variation. In both cases, massive occurrences can be regularly spaced in time, with alternating periods of abundance and scarcity, but these occurrences are usually irregular with variable lags between successive peaks (Boero et al. 2008 and references therein).
A few abundant species often dominate plankton communities, but these species may change with time, giving rise to rapid temporal successions (Boero 1994). Ecological succession is an orderly and directional process of community development; is selfcontrolled, discontinuous, and shaped by the physical variability of the environment and leads to a stable ecosystem (Odum 1969). In dynamic systems such as coastal areas, it has been shown that plankton assemblages are likely to evolve over short periods (i.e., among seasons or even within seasons). The development of the zooplankton population follows the spring phytoplankton bloom, with successive presence of grazers and predators. The combined effects of autumn water-column destabilization and reduction in day length bring the annual succession to its end (Romagnan et al. 2015).
Several studies of GZ have been conducted during the last 25 years, which allows for a complete knowledge of the species richness in the temperate waters of the Southwestern Atlantic Ocean (SWAO) (e.g., Mianzan 1999; Genzano et al. 2008a; Rodríguez et al. 2017); however, none of these studies have described the succession of species during the different seasons. The analysis of these regional topics is of great importance because GZ have several ecological roles, such as a considerable effect on the plankton community through direct predation and competition for food (Alldredge 1984), and trophic cascading effects (Schneider and Behrends 1998), and diverse non-trophic interactions with other biological groups (e.g., see Schiariti et al. 2018). However, due to the highly seasonal occurrence of many gelatinous species, their structuring effect is often temporary (Behrends and Schneider 1995). To quantify the role of GZ in an ecosystem, knowing their seasonality is paramount. For these reasons, we analyze the seasonal changes in the GZ in the Mar del Plata Harbor over an annual cycle with the aim to describe, for the first time, the seasonal succession of species, and their relationship with environmental factors.
MATERIALS AND METHODS
The zooplankton samples used in the present study were collected weekly-biweekly during the warm period (austral spring-summer) and monthly in the cold period (austral autumnwinter) over an annual cycle (March 2014 to March 2015) in the Mar del Plata Harbor, Argentina (38°08´17” S - 57°31´18” W) (Figure 1) with a standard zooplankton net (75 cm mouth diameter, 500 μm mesh size) by means of oblique tows from the surface to the bottom. All GZ specimens were classified into the lowest taxonomic level and their temporal distribution of their abundances was analyzed. For the purpose of the present study, we calculated the mean monthly and seasonal abundances data, as well as the frequency of occurrence (%FO, as the percentage of samples in which a given species occurred from the total), and relative abundance (%RA, as the percentage of specimens of a given species among of the total specimens).

Figure 1. Map of the study area (Mar del Plata Harbor: 38°08´17” S - 57°3´18” W). Black stars indicate the sampling stations.
Figura 1. Mapa del área de estudio (Puerto de Mar del Plata: 38°08´17” S - 57°3´18” W). Las estrellas negras indican las estaciones de muestreo.
The same plankton samples were used to count and identify the non-gelatinous zooplankton groups (non-GZ; biological parameter ) to a higher taxonomic level (Copepoda, Amphipoda, Mollusca, etc.), to have an idea of the prevailing zooplankton community. These non-GZ are considered a proxy of the food availability for GZ. A flowmeter attached to the net mouth allowed calculating the volume of water filtered to estimate the numerical abundance of the GZ and the non-GZ groups, which were standardized as ind/100 m3. With a multiparameter water quality instruments (HORIBA®), monthly average surface temperature (°C) and salinity were measured to hydrologically characterize the study area (physicochemical data). To identify if the respective abundances (GZ and non-GZ), as well as the hydrological data significantly differed between seasons, one-way ANOVA test was conducted. When significant differences were present, a post hoc multiple comparison Tukey test was performed to identify between which seasons the differences could be observed (Zar 1996). The data were previously transformed into the form log(x+1) to fulfill the assumption of homogeneity of variances (Levene test).
A number of univariate diversity indices were calculated with the PRIMER module DIVERSE to describe the temporal dynamics of the medusae community and subsequently of the ctenophores community: species richness (S, total number of species), Pielou´s evenness (J´), Shannon-Wiener diversity (H´ ind/bits), and Simpson dominance (ʎ) (Clarke and Warwick 2001). Possible significant differences (P<0.05) for S and H´ between seasons were tested using a nonparametric Kruskal-Wallis test (K-W). When significant differences were present, several Mann-Whitney U tests were conducted to determine between which seasons the difference could be observed. A Bonferroni correction was performed for multiple pairwise tests.
An Olmstead-Tukey analysis (using the relative FO and RA) was performed to classify the species into four different categories (dominant, casual, constant, and rare) (Sokal and Rohlf 1995). For the medusae group, a monthly succession analysis was described as a function of the number of species and the mean abundance values comparing meroplanktonic and holoplanktonic species. For the ctenophore group, because all species are holoplanktonic, this analysis was carried out using only the mean monthly abundances of each species.
The Bray-Curtis similarity index was calculated to identify the faunal affinity between seasons (using species composition and abundance values) (Krebs 1999), the mean abundance data were transformed into the fourth root form to minimize the effect of high abundances. A CLUSTER analysis was performed with the groupaverage method (SIMPROF test). A SIMPER routine (similarity percentages) was used to identify the medusae/ctenophore species that contributed the most to the (dis)similarities among and within groups. The biotic (i.e., medusae and ctenophores data) and environmental matrices (i.e., physicochemical data and non-GZ abundances) were analyzed using the BIO-ENV routine, based on Bray- Curtis dissimilarity measures and Euclidian distances, respectively, to find the best match for the environmental variables using the Spearman rank correlation method that measures agreement between the two matrices (Clarke and Warwick 2001).
RESULTS
Hydrological and biological temporal variability
The physicochemical and biological parameters recorded from the study area reflect the characteristic environmental heterogeneity, typical of the region, between the different months and seasons. Monthly surface temperature oscillated between 9.8 and 22.3 °C, with the highest and lowest values observed in the months of the austral summer and winter, respectively. According to the ANOVA, significant differences in the surface temperature between the seasons were observed (F(3,9)=10.7, P<0.01), identified between winter-spring (Tukey=-0.2, P=0.03) and winter-summer periods (Tukey=- 0.3, P<0.01). Salinity showed consistent homogeneity for all months sampled (F(3,9)=1.7, P>0.05) ranging from 33.7 to 36.0. The non-GZ abundances showed two well-defined periods of temporal distribution: 1) the period from March to October 2014 (autumn-winter) registering the lowest values of the annual cycle (960.2 to 7649.2 ind/100 m3) and 2) from November 2014 to March 2015 (springsummer), which recorded the highest values (11987.6 to 123995.6 ind/100 m3). Significant differences between seasons (F(3,9)=4.6, df=12, P=0.03) were observed in the autumn-summer comparison (Tukey test=-1.2, P=0.04).
This non-GZ community include seven phyla (Foraminifera, Polychaeta, Chaetognatha, Echinodermata, Arthropoda, Mollusca, and Chordata). Arthropods were the most numerous with nine groups (zoea larvae, decapods, copepods, amphipods, ostracods, nauplii barnacles, isopods, cladocerans, and euphausiids), followed by Chordata (fish eggs and larvae, tunicate larvae, and Appendicularia) and Mollusca (gastropod larvae and bivalve larvae), while the other phyla had a single group. Six of these groups (polychaetes, chaetognaths, zoea larvae, decapods, copepods, and nauplii barnacles) were observed in all months sampled. Zoea larvae accounted for 33.8% of the total abundance, followed by chaetognaths (18.3%) and nauplii barnacles (12.2%). Seasonally, chaetognaths were the most abundant group during the autumn and winter with a RA of 65.3 and 42.6%, respectively, while the zoea larvae dominated during the spring (68%) and summer (33.7%). During the midwinter (August), a slight peak in abundance was observed, mainly due to Arthropoda, while during January and February, a major peak in abundances was observed due to the high values of several groups of arthropods, as well as fish eggs and larvae (Figure 2).

Figure 2. Monthly abundances oscillation of the non-gelatinous zooplankton groups at Mar del Plata Harbor, Argentina during an annual cycle. 1) Foraminifera; 2) Polichaetes; 3) Chaetognathes; 4) Echinoderms (larvae); 5) Zoea larvae; 6) Decapoda (larvae); 7) Copepoda; 8) Amphipoda; 9) Ostracoda; 10) Barnacle nauplii; 11) Isopoda; 12) Cladocera; 13) Euphasiids; 14) Gastropod larvae; 15) Bivalve larvae; 16) Fish eggs; 17) Fish larvae; 18) Tunichata (larvae); 19) Appendicularia.
Figura 2. Oscilación mensual de las abundancias de los grupos del zooplancton no gelatinoso del Puerto de Mar del Plata, Argentina durante un ciclo anual. 1) Foraminiferos; 2) Poliquetos; 3) Quetognatos; 4) Equinodermos (larvas); 5) Larvas zoeas; 6) Decápodos (larvas); 7) Copépodos; 8) Anfípodos; 9) Ostrácodos; 10) Nauplio cirripedios; 11) Isópodos; 12) Cladóceros; 13) Eufasidos; 14) Larvas de gasterópodos; 15) Larva de bivalvos; 16) Huevos de peces; 17) Larvas de peces; 18) Larvas de tunicados; 19) Apendicularia.
Gelatinous zooplankton community
Over the annual cycle, a total of 18 species were identified, 15 of which were medusae (one Scyphozoa and 14 Hydrozoa) and 3 of which were ctenophores (two Tentaculata and one Nuda). Five species were holoplanktonic organisms (3 ctenophores and 2 hydromedusae) and 13 taxa were meroplanktonic (12 hydromedusae and 1 scyphomedusae).
Medusae
According to the K-W analysis, no significant differences were observed between seasons in S (P=0.11) and H´(P=0.10). The low Diversity observed during the cold period (mainly late autumn to early winter) is due to the strong dominance of few species, while the rich diversity in the warm season is due to the low dominance. In general (see below), the abundance values were distributed among many species in the warm season. Equity values oscillated between 0.6 and 1.0 (Table 1).
Table 1. Temporal oscillation of the ecological indices and total-mean abundances by medusae and ctenophores groups at Mar del Plata Harbor, Argentina, during an annual cycle.
Tabla 1. Oscilación temporal de los índices ecológicos y de las abundancias total-media de los grupos de las medusas y ctenóforos en el Puerto de Mar del Plata, Argentina, durante un ciclo anual.
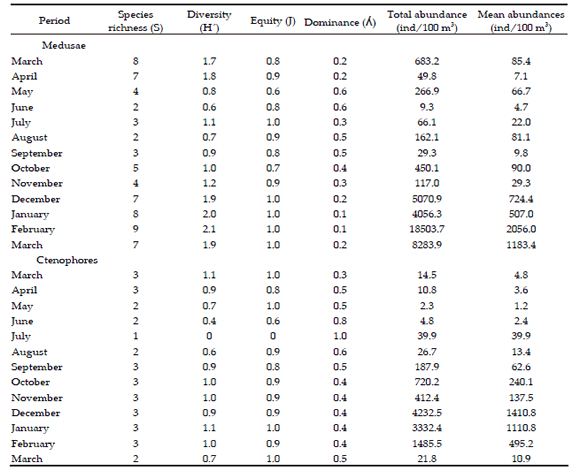
Species of medusae were observed in all the months of the annual cycle. They displayed two marked periods of mean abundances distributions: the lowest values from March to November (autumn-mid-spring), and the highest values from December 2014 to March 2015 (spring-summer). These values ranging from 4.7 ind/100 m3 (June) to 2056.0 ind/100 m3 (February) (Table 1). Significant differences were observed between the climate seasons (ANOVA, F(3,9)=6.0, P<0.02), after comparing autumn-summer (Tukey=-1.8, P=0.03) and winter-summer (Tukey=-1.8, P=0.03).
According to the Olmstead-Tukey test, three species were dominant: Obelia sp., Liriope tetraphylla (Chamisso and Eysenhardt), and Eucheilota ventricularis McCrady, representing 94.2% of the total mean abundance. Obelia sp. accounted for 63.7%, L. tetraphylla for 19.8%, and E. ventricularis for 10.8%. Cunina octonaria McCrady and Bougainvillia pagesi Nogueira et al. were classified as constant, while the remaining species were grouped as rare. Only L. tetraphylla was considered dominant during all four seasons (Table 2). Each species showed a particular time of occurrence, and a unique temporal pattern of minimum and maximum abundances; however, in general, all these species showed a clear seasonal pattern with the highest and lowest values during the warm and cold periods, respectively (Table 3). The three dominant species exhibited significant differences between seasons: Obelia sp. (ANOVA, F(3,9)=4.1, P<0.05) differed between winter and summer (Tukey=-3.1, P=0.04); L. tetraphylla (ANOVA, F(3,9)=5.7, P<0.05) differed between autumn and spring (Tukey=-1.6, P=0.03) and autumn and summer (Tukey=1.8, P=0.04); E. ventricularis (ANOVA, F(3,9)=12.1, P<0.05) differed in the autumnspring (Tukey=-1.8, P= 0.02), autumn-summer (Tukey=-2.3, P=0.02), winter-spring (Tukey=- 2.3, P=0.01), and winter-summer comparisons (Tukey=-2.7, P=0.01).
Table 2. Annual values of frequency of occurrence (%FO) and relative abundance (%RA), Olmstead-Tukey test, hydrological intervals (temperature-salinity) during the period of occurrence of the medusae and ctenophores species at Mar del Plata Harbor. D=Dominant, C=Constant, R=Rare.
Tabla 2. Valores anuales de la frecuencia de ocurrencia (%FO) y abundancia relativa (%RA), análisis de Olmstead- Tukey, intervalos hidrológicos (temperatura-salinidad) durante el período de ocurrencia de las especies de medusas y ctenóforos del Puerto de Mar del Plata. D=Dominante, C=Constante, R=Rara.

Table 3. Monthly abundances values of the medusae and ctenophores species at Mar del Plata Harbor, Argentina, during an annual cycle. 1=meroplanktonic, 2=holoplanktonic.
Tabla 3. Valores de abundancia mensual de las especies de medusas y ctenóforos del Puerto de Mar del Plata, Argentina durante un ciclo anual. 1=meroplanctónica, 2=holoplanctónica.

Only Obelia sp. and L. tetraphylla, were observed throughout the annual cycle in high abundances and showed a peak of abundance from late spring to early summer; Bougainvillia pagesi and C. octonaria were present in all seasons, but with low abundances. Certain species were observed only during one season at low abundances: A. affinis, Amphinema dinema Perón and Lesueur, Bougainvillia muscus Allman, Proboscidactyla mutabilis (Browne), and Aequorea coerulescens (Brant) during the summer, and Halitiaria formosa Fewkes, Gossea brachymera Bigelow, and the scyphomedusae Chrysaora lactea Eschscholtz during the early and mid-autumn (see Table 3 for abundances).
Using the number of species and then the mean abundances, we analyzed the monthly succession of meroplanktonic and holoplanktonic species, which showed that, during practically the entire annual cycle, the meroplanktonic species showed highest number of species, except between June and August when both showed similar numbers. In contrast, based on the abundances, the holoplanktonic species were more abundant in almost all months, except from December to March.
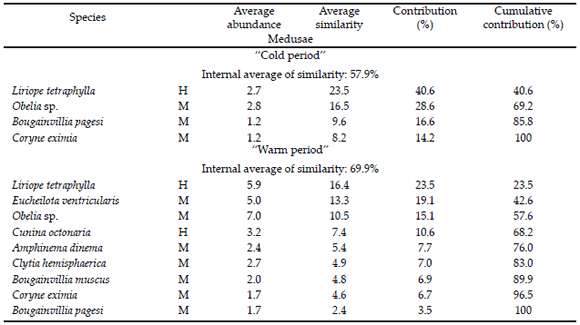
The application of the Bray-Curtis index to the abundance matrix in the different seasons defined two faunal affinity groups: “cold period” and “warm period”. The first group was composed of autumn and winter, recording a 57.9% average group value of similarity (SIMPER). It consisted of four species (three meroplanktonic and one holoplanktonic), of which L. tetraphylla were the most common (40.6%), followed by Obelia sp. (28.5%). The “warm period” was composed of the springsummer seasons (SIMPER=69.9%). This group included nine taxa (two holoplanktonic and seven meroplanktonic). Liriope tetraphylla was the most numerous (23.5%), followed by E. ventricularis (19.1%) and Obelia sp. (15.1%) (Table 4). The BIO-ENV procedure indicated that the association of environmental parameters that best grouped the different seasons in agreement with the biological patterns was the one integrated by the combination of the surface water temperature and non-GZ abundances (ρ=0.94).
Table 4. SIMPER analysis of the medusae species for the determination of the climate seasons with faunal affinity. H=holoplanktonic, M=meroplanktonic. Tabla 4. Análisis de SIMPER en las especies de medusas para la determinación de las épocas climáticas con afinidad faunística. H=holoplanctónicos, M=meroplanctónicos.
Ctenophores
Three species were observed over the annual cycle: Pleurobrachia pileus (O.F. Müller ), Mnemiopsis leidyi A. Agassiz, and Beroe ovata Bruguière. Due to the low number of species, the analysis of diversity and equity were omitted for this group. The ctenophores were observed throughout the annual cycle. It showed monthly mean abundances between 1.2 (May) and 1410.8 ind/100 m3 (December). In general, one period had the lowest abundances (March to September), and another had the highest abundances (October to February). After the maximum peak of abundances, a clear decrease of the mean values during the warm period was observed. At the end of the annual cycles, abundances were as low as those observed in the cold period (Table 1). Significant differences were observed between the climate seasons (ANOVA, F(3,9)=11.4, P<0.01), identified in the autumn-spring comparison (Tukey=-2.1, P<0.01).
The Olmstead-Tukey analysis showed P. pileus and M. leidyi as the dominant species (annual cycle data). They accounted for 59.1% and 40.2% of the total abundance, respectively, while B. ovata was classified as a rare species (0.7%). Mnemiopsis leidyi was a dominant species in all seasons except summer, P. pileus oscillated between dominant and constant depending on the period. Beroe ovata was classified as rare, except in the spring (constant) (Table 2). Mnemiopsis leidyi (except in July) and P. pileus were observed during all months sampled with the lowest abundances in the months of the autumn-winter period; they increased their abundances in October, and maximum values in December (late spring). From this month on and throughout the summer, abundances decreased and, by the end of the annual cycle, reaches values as low as those observed in early autumn. Beroe ovata showed low abundances throughout the annual cycle and reached its maximum value during the summer (Table 3). Both P. pileus (ANOVA, F(3,9)=9.3, P<0.01) and M. leidyi (ANOVA, F(3,9)=9.1, P<0.01) showed significant differences between seasons, especially in the autumn-spring comparison (Tukey=-2.3, P<0.01) for the former, and in the autumnspring (Tukey=-2.3, P<0.01) and winterspring (Tukey=-2.0, P=0.02) comparisons for the latter.
Since ctenophores are holoplanktonic species, the succession analysis was performed by comparing the monthly abundances. This analysis allowed identifying short periods of dominance between P. pileus and M. leidyi, in which each species dominated for approximately three months and was then succeeded by the other species.
The Bray-Curtis index revealed one group with faunal affinities, composed of the four seasons, which indicated that ctenophore species showed a temporary faunal homogeneity throughout the annual cycle. The most important species was P. pileus (SIMPER=42.0%), followed by M. leidyi (39.4%). According to the BIO-ENV procedure, this faunal homogeneity can be explained with two variables (ρ=0.94): surface water temperature and non-GZ abundances.
DISCUSSION
Hydrologically, the variation of the surface temperature and salinity coincided in magnitude with the patterns observed previously in the study area, with lowest and highest temperatures recorded in the cold and warm periods, respectively (Bastida 1971), while the salinity showed the characteristic seasonal homogeneity (Bastida 1971; Guerrero and Piola 1997). Individual values of abundances of the non-GZ groups recorded in different studies cannot always be compared because of differences in the methods used. However, considering our data, it may be stated that the Mar del Plata Harbor is an area of great secondary productivity. This productivity has also been observed in adjacent zones of the study (e.g., Ramírez 1981; Di Mauro et al. 2009; Viñas et al. 2013).
There are no previous studies describing the seasonal variation of all groups of zooplankton in this port area. However, an annual cycle analysis of the zooplankton at a permanent coastal station (EPEA) located ~27 nautical miles south from the study area (Viñas et al. 2013) showed certain differences from our observations in terms of the dominant group. It allowed us to identify similar patterns in the temporal abundance, with various peaks of abundances throughout the year, mainly in the spring and summer, and variation only in the group represented. In this comparative study, the eggs and nauplii of copepods dominated in summer, followed by larvaceans, but groups such as chaetognaths and cladocerans showed the highest abundances during that season. Other components, including the larvae of gastropods, polychaetes, and decapods, had their maximum abundance in autumn, with a secondary peak in summer. According to several studies, some of these non-GZ groups represent prey items in the GZ diet (Purcell 1997; Mianzan 1999 and references therein). The diet of GZ species depends upon prey availability, and can be affected by the predominance of alternative prey (Purcell 1997). The importance of a seasonal analysis of these non-GZ groups lies in the fact that food availability is one of the factors that can determine the spatiotemporal distribution of the presence/absence of GZ species and their respective abundances (Ramírez and Zamponi 1981; Purcell 1997). The fact that the abundances of the dominant GZ species varied temporally following the variation of the non-GZ abundances suggests that the food availability determines the distribution of the GZ abundances, at least for these dominant medusae and ctenophores.
The effects of temperature and salinity on GZ have been well documented, including patterns in occurrence, abundance, geographic distribution (Graham et al. 2001; Richardson et al. 2009), and phenology (Blackett et al. 2015), which in turn are correlated with a variety of other changes, such as decreased zooplankton production (Pitt et al. 2014). In the present study, GZ species showed a correlation with water temperature. These organisms and practically all zooplankton are poikilothermic, unable to regulate their internal temperature. Hence, temperature changes in the marine environment directly affect their physiological processes, such as ingestion, respiration, reproductive development (Richardson 2008), and subsequently their abundances. They cause in some species the “disappearance” or decrease in abundance during the cold period. However, Obelia sp., L. tetraphylla, M. leidyi, and P. pileus have been categorized as eurytherm-euryhaline organisms (see Oliveira et al. 2016 for references), which allows them to live in different habitats and/or seasons at high abundances. They are dominant in the study area and other regions of the Argentine Sea (e.g., Buenos Aires Province coast) (Mianzan 1999; Dutto et al. 2017). In to this, Boero (1994) indicated that some abundant species often dominate plankton communities, but these may change with time and cause rapid temporal succession.
A change in the seasonal period is accompanied by a regular succession of the zooplankton species and their abundances (Yoshida et al. 2001). Our analysis of the temporal succession showed a clear oscillation in dominance when comparing meroplanktonic with holoplanktonic medusae and the abundances of the two dominant ctenophores. Different GZ species appear in the plankton, to some extent, in a predictable succession (Mills 1993); they differ in terms the duration they stay in the water column (Hosia and Båmstedt 2007). This succession of species is similar year after year, with starting times varying widely depending on the species (Mills 1993). Some species appear shortly after the spring plankton bloom begins, and others follow as ecological conditions continue to change, but the terminations are almost as clearly marked as their arrival in the spring (Mills 1993). The succession of species in plankton communities reflects a shifting balance between resource availability and predation, as well as the constantly changing trophic arrangements these promote (Acevedo- Trejos et al. 2015). The timing of the presence of meroplanktonic species is influenced by factors that control the benthic phase and their medusae generations, with the following suggested order of importance: temperature, food availability, photoperiod, salinity, lunar cycles, and possible combinations of these factors (Arai 1992). While holoplanktonic organisms are influenced mainly by water temperature, food availability, and marine currents.
In terms of species composition, the GZ identified from the Mar del Plata Harbor may be considered typical of the study area and neighboring regions. All identified species have been previously recorded for the Argentine Sea (Mianzan 1999; Rodríguez 2012; Dutto et al. 2017), except for the hydromedusae Annatiara affinis (Hartlaub), which has been formerly registered up to Brazilian and Uruguayan coast (23.8° S to 24.8° S, and 29° S to 35° S). However, additional sampling is necessary to confirm its presence in this region. The identified set of dominant species have been previously labeled as common and widely distributed in several regions of the Argentine Sea (see Oliveira et al. 2016 and references therein).
Almost simultaneous dominance between medusae L. tetraphylla and Obelia sp., and between ctenophores M. leidyi and P. pileus, can be understood as the result of different feeding mechanisms/strategy that favor the capture of a different fraction of the available plankton, which allows them to cooccur temporally and spatially (Costello and Coverdale 1998), with variations only in their abundances. These aspects could explain the identification of different groups of seasons with faunal affinity. These groups were influenced by the abundance variation of the dominant species, which suggests that the GZ community is dominated by few species with a great capacity to adapt to environmental changes, such as food availability (Graham et al. 2001).
Our sampling represents a relatively small volume in comparison with other plankton samplings. This limitation may also underestimate abundances of larger or sparse animals, such as Scyphozoan species, or not record their presence. In the present study, C. lactea was the only scyphomedusae identified; it was observed only during the early autumn. Certain environmental conditions, such as the predominant northeast winds and the passive transport of these organisms by the marine current to the shore, facilitate its capture in coastal samplings. The fact that we have observed a few specimens and only during early autumn may be because these conditions were not present in the sampling period. Thus, its absence in our sampling would not indicate precisely that this species was not present in the region. According to Schiariti et al. (2018), in the Argentine Sea, this species lives all over the Río de la Plata estuary at the Transitional Neritic Domain (TND), but specimens reach higher latitudes along the Buenos Aires Province coast up to Las Grutas (41° S - 65° W). This species has been sporadically found deeper waters on the continental shelf up to the shelf break front but always within the TND, and is one of the most common and abundant species in the region. Adult specimens have been observed mostly from December to May, with specimens found sporadically in August (late spring to mid-autumn) and accumulations observed between January and April (Schiariti et al. 2018).
This study covered just one annual cycle of zooplankton sampling. We cannot know whether the patterns we observed are representative of a typical year for GZ species. In general, GZ populations can exhibit significant interannual differences in terms of abundances (e.g., Ballard and Mayers 2000); however, these differences have shown certain annual regularity (Schneider and Behrends 1998). For example, the holoplanktonic hydromedusae L. tetraphylla and ctenophore M. leidyi are typical species of the GZ community, they have been observed in different periods and regions of the Bonaerense coasts with the highest abundances during the warm period or even in autumn-spring (see Mianzan and Sabatini 1985; Gaitán 2004; Dutto et al. 2017). In contrast, the strikingly different abundances of meroplanktonic hydromedusae Obelia sp. observed in early studies at adjacent areas from the Mar del Plata Harbor (see Genzano et al. 2008b), as well as our observations, suggest that interannual variations are significant at least in this medusa, but the observations coincide with a seasonal peak of its highest abundances. Comparisons of specific seasonality patterns among studies or regions should be made with caution due to possible regional and interannual variability. Long-term monitoring programs should be implemented to reach a better understanding of the temporal dynamics and interannual variability. To reach a better understanding of the temporal dynamics, long-term monitoring programmer should be implemented, to account for the interannual variability. Moreover, not only the natural species seasonality should be considered but studies of anthropogenic factors also should be conducted to evaluate their influence on long-term dynamics, such as faunistic or phenological changes, invasions, and extinctions (Nagata et al. 2014). Despite this limitation, the present study represents the basis of a general description of the seasonal succession in the study area in terms of the species richness, abundance values, and the correlations with the hydrobiological characteristics of the area.
ACKNOWLEDGEMENTS
We thank the collaboration of the Captain C. Brelles, L. Díaz Briz and the crew that participated in this investigation, as well as the anonymous reviewers for their comments, which greatly improved this manuscript. The authors thank Roberto Ascención for their help with the English grammar and style of this manuscript. This work was supported by the National University of Mar del Plata under grant EXA 829/17.
REFERENCES
1. Acevedo-Trejos, E., G. Brandt, J. Bruggeman, and A. Merico. 2015. Mechanisms shaping size structure and functional diversity of phytoplankton communities in the ocean. Scientific Reports 5:1-8. https://doi.org/10.1038/srep08918. [ Links ]
2. Alldredge, A. L. 1984. The quantitative significance of gelatinous zooplankton as pelagic consumers. Pp. 407-433 in M. J. R. Fasham (ed.). Flow of energy and materials in marine ecosystems: theory and practice. Plenum Press, London, UK. https://doi.org/10.1007/978-1-4757-0387-0_16. [ Links ]
3. Arai, M. N. 1992. Active and passive factors affecting aggregations of Hydromedusae: a review. Scientia Marina 56: 99-108. [ Links ]
4. Ballard, L., and A. Mayers. 2000. Observations on the seasonal occurrence and abundance of gelatinous zooplankton in Lough Hyne, Co. Cork, South-West Ireland. Biology and Environment Proceedings of the Royal Irish Academy B 100:75-83. [ Links ]
5. Bastida, R. 1971. Las incrustaciones biológicas en el Puerto de Mar del Plata, período 1966-67. Revista del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” e Instituto Nacional de Investigación de las Ciencias Naturales. Hidrobiología III 2:203-285.
6. Behrends, G., and G. Schneider. 1995. Impact of Aurelia aurita medusae (Cnidaria, Scyphozoa) on the standing stock and community composition of mesozooplankton in the Kiel-bight (Western Baltic-Sea). Marine Ecology Progress Series 127:39-45. https://doi.org/10.3354/meps127039. [ Links ]
7. Benovic, A., J. Dubravko, and A. Bender. 1987. Enigmatic changes in the hydromedusan fauna of the northern Adriatic Sea. Nature 326:597-600. https://doi.org/10.1038/326597a0. [ Links ]
8. Blackett, M., C. H. Lucas, R. Harmer, and P. Licandro. 2015. Population ecology of Muggiaea atlantica (Cnidaria, Siphonophora) in the Western English Channel. Marine Ecology Progress Series 535:129-144. https://doi.org/10.3354/meps11423. [ Links ]
9. Boero, F. 1994. Fluctuations and variations in coastal marine environments. Marine Ecology 15:3-25. https://doi.org/10.1111/j.1439-0485.1994.tb00038.x. [ Links ]
10. Boero, F., J. Bouillon, C. Gravili, M. P. Miglietta, T. R. Parsons, and S. Piraino. 2008. Gelatinous plankton: irregularities rule the world (sometimes). Marine Ecology Progress Series 356:299-310. https://doi.org/10.3354/meps07368. [ Links ]
11. Clarke, K. R., and R. M. Warwick. 2001. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation. Second edition. Plymouth Marine Laboratory, Plymouth, UK. [ Links ]
12. Costello, J. H., and R. Coverdale. 1998. Planktonic feeding and evolutionary significance of Lobate body plan within the ctenophora. Biological Bulletin 195:247-248. https://doi.org/10.2307/1542863. [ Links ]
13. Daly, M., M. R. Brugler, P. Cartwright, A. G. Collins, M. N. Dawson, D. G. Fautin, S. C. France, C. S. Mcfadden, D. M. Opresko, E. Rodríguez, S. L. Romano, and J. L. Stake. 2007. The Phylum Cnidaria: A review of phylogenetic patterns and diversity 300 years after Linnaeus. Zootaxa 1668:127-182. [ Links ]
14. Di Mauro, R., F. Capitanio, and M. D. Viñas. 2009. Capture efficiency for small dominant mesozooplankters (Copepoda, Appendicularia) off Buenos Aires Province (34°S-41°S9, Argentine Sea, using two plankton mesh sizes. Brazilian Journal of Oceanography 57:205-214. https://doi.org/10.1590/S1679-87592009000300004. [ Links ]
15. Dutto, M. S., G. N. Genzano, A. Schiariti, J. Lecanda, M. S. Hoffmeyer, and P. Pratolongo. 2017. Medusae and ctenophores from Bahía Blanca Estuary and neighboring inner shelf (Southwest Atlantic Ocean, Argentina). Marine Biodiversity Records 10:1-14. https://doi.org/10.1186/s41200-017-0114-1. [ Links ]
16. Gaitán, E. N. 2004. Distribución, abundancia y estacionalidad de Liriope tetraphylla (Hidromedusae) en el Océano Atlántico Sudoccidental y su rol ecológico en el estuario del Río de la Plata. Degree thesis. Universidad Nacional de Mar del Plata, Buenos Aires, Argentina. Pp. 42. [ Links ]
17. Genzano, G., H. Mianzan, and J. Bouillon. 2008a. Hydromedusae (Cnidaria: Hydrozoa) from the temperate southwestern Atlantic Ocean: a review. Zootaxa 1750:1-18. https://doi.org/10.11646/zootaxa.1750.1.1. [ Links ]
18. Genzano, G., H. Mianzan, L. Díaz-Briz, and C. Rodríguez. 2008b. On the occurrence of Obelia medusa blooms and empirical evidence of unusual massive accumulations of Obelia and Amphisbetia hydroids on the Argentina shoreline. Latin America Journal Aquatic Research 36:301-307. https://doi.org/10.3856/vol36-issue2-fulltext-11 [ Links ]
19. Giangrande, A., S. Geraci, and G. Belmonte. 1994. Life-cycle and life-history diversity in marine invertebrates and the implications in community dynamics. Oceanography and Marine Biology: An Annual Review 32:305-333. [ Links ]
20. Goy, J. 1997. The medusae (Cnidaria, Hydrozoa) and their trophic environment: an example in the north-western Mediterranean. Annales de l´lnstitut Océanographique, Paris 63:47-56. [ Links ]
21. Graham, W. M., F. Pagés, and W. M. Hamner. 2001. A physical context for gelatinous zooplankton aggregations: a review. Hydrobiologia 451:199-212. https://doi.org/10.1023/A:1011844208119. https://doi.org/10.1023/A:1011876004427. [ Links ]
22. Guerrero, R. A., and A. Piola. 1997. Masas de agua en la plataforma continental. El Mar Argentino y sus recursos pesqueros 1:107-118. [ Links ]
23. Haddock, H. D. 2004. A golden age of gelata: past and future research on planktonic ctenophores and cnidarians. Hydrobiologia 530/531:549-556. https://doi.org/10.1007/s10750-004-2653-9. [ Links ]
24. Krebs, C. J. 1999. Ecological methodology. Second edition. Benjamin Cummings, Menlo Park. [ Links ]
25. Mackas, D. L., and G. Beaugrand. 2010. Comparison of zooplankton time series. Journal of Marine Systems 79:286-304. https://doi.org/10.1016/j.jmarsys.2008.11.030. [ Links ]
26. Mianzan, H. W. 1999. Ctenophora. Pp. 561-573 in D. Boltovskoy (ed.). South Atlantic Zooplankton. Backhuys Publishers, Leiden, The Netherlands. [ Links ]
27. Mianzan, H. W., and M. E. Sabatini. 1985. Estudio preliminar sobre distribución y abundancia de Mnemiopsis maccradyi en el estuario de Bahía Blanca, Argentina (Ctenophora). Spheniscus 1:53-68. [ Links ]
28. Mills, C. E. 1993. Natural mortality in NE Pacific coastal hydromedusae: grazing predation, wound healing and senescence. Bulletin of Marine Science 53:194-203. [ Links ]
29. Nagata, R. M., M. Nogueira Jr., and M. A. Haddad. 2014. Faunistic survey of Hydromedusae (Cnidaria, Medusozoa) from the coast of Paraná State, Southern Brazil. Zootaxa 3768:291-326. https://doi.org/10.11646/zootaxa.3768.3.3. [ Links ]
30. Odum, E. 1969. Strategy of ecosystem development. Science 164:262-270. https://doi.org/10.1126/science.164.3877.262. [ Links ]
31. Oliveira, O. M., T. P. Miranda, E. M. Araujo, P. Ayón, C. M. Cedeño-Poso, A. A. Cepeda-Mercado, P. Córdoba, A. F. Cunha, G. N. Genzano, M. A. Haddad, H. W. Mianzan, A. E. Migotto, L. S. Miranda, A. C. Morandini, R. M. Nagata, K. B. Nascimento, M. Nogueira Jr., S. Palma, J. Quiñones, C. S. Rodríguez, F. Scarabino, A. Schiariti, S. N. Stampar, V. B. Tronolone, and A. C. Marques. 2016. Census of Cnidaria (Medusozoan) and Ctenophora from South American marine waters. Zootaxa 4194:1-256. https://doi.org/10.11646/zootaxa.4194.1.1. [ Links ]
32. Pagés, F., M. G. White, and P. G. Roadhouse. 1996. Abundance of gelatinous carnivorous in the nekton community of the Antarctic Polar Frontal Zone in summer 1994. Marine Ecology Progress Series 141:139-147. https://doi.org/10.3354/meps141139. [ Links ]
33. Pitt, K. A., A. C. Budarf, J. G. Browne, and R. H. Condon. 2014. Bloom and Bust: Why do blooms of jellyfish collapse? Pp. 79-103 in K. Pitt and C. Lucas (eds.). Jellyfish Blooms. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7015-7_4. [ Links ]
34. Purcell, J. E. 1997. Pelagic cnidarians and ctenophores as predators: selective predation, feeding rates, and effects on prey populations. Annales de l´Institut océanographique, Paris 73:125-137. [ Links ]
35. Purcell, J. E., and C. E. Mills. 1988. The correlation between nematocyst types and diets in pelagic Hydrozoa. Pp. 463-485 in D. Hessinger and H. Lenhoff (eds.). The biology of nematocysts. Academic Press, San Diego, USA. https://doi.org/10.1016/B978-0-12-345320-4.50029-8. [ Links ]
36. Ramírez, F. C. 1981. Zooplancton y producción secundaria. Parte I. Distribución y variación estacional de los copépodos. Contribuciones del Instituto nacional de Investigación y Desarrollo Pesquero, Mar del Plata 383:202-212. [ Links ]
37. Ramírez, F. C., and M. O. Zamponi. 1981. Hydromedusae. Pp. 443-469 in D. Boltovskoy (ed.). Atlas del zooplankton del Atlántico sudoccidental y métodos de trabajo con el zooplancton marino. INIDEP, Mar del Plata, Argentina. [ Links ]
38. Raskoff, K. A., F. A. Sommer, W. M. Hamner, and K. M. Cross. 2003. Collection and culture techniques fir gelatinous zooplankton. Biological Bulletin 204:68-80. https://doi.org/10.2307/1543497. [ Links ]
39. Richardson, A. J. 2008. In hot water: zooplankton and climate change. ICES Journal of Marine Science 65:279-295. https://doi.org/10.1093/icesjms/fsn028. [ Links ]
40. Richardson, A. J., A. Bakun, G. C. Hays, and M. J. Gibbons. 2009. The jellyfish joyride: causes, consequences and management responses to a more gelatinous future. Trends in Ecology and Evolution 24:312-322. https://doi.org/10.1016/j.tree.2009.01.010. [ Links ]
41. Rodríguez, C. S. 2012. Hidromedusas del Atlántico sudoccidental: Biodiversidad y patrones de distribución. PhD Thesis. Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata, Buenos Aires, Argentina. Pp. 213. [ Links ]
42. Rodríguez, C. S., A. C. Marques, H. W. Mianzan, V. B. Tronolone, A. E. Migotto, and G. N. Genzano. 2017. Environment and life cycles influence distribution patterns of hydromedusae in austral South America. Marine Biology Research 13:659-670. https://doi.org/10.1080/17451000.2017.1280170. [ Links ]
43. Romagnan J.-B., L. Legendre, L. Guidi, J.-L. Jamet, D. Jamet, L. Mousseau, M.-L. Pedrotti, M. Picheral, G. Gorsky, C. Sardet, and L. Stemmann. 2015. Comprehensive model of annual plankton succession based on the whole plankton time series approach. PLoS ONE 10: e0119219. https://doi.org/10.1371/journal.pone.0119219. [ Links ]
44. Schneider, G., and G. Behrends. 1998. Top-down control in a neritic plankton system by Aurelia aurita medusae: a summary. Ophelia 48:71-82. https://doi.org/10.1080/00785236.1998.10428677. [ Links ]
45. Schiariti, A., M. S. Dutto, D. Y. Pereyra, G. Failla Siquier, and A. C. Morandini. 2018. Medusae (Scyphozoa and Cubozoa) from southwestern Atlantic and Subantarctic region (32-60°S, 34-70°W): species composition, spatial distribution and life history traits. Latin American Journal of Aquatic Research 46: 240-257. https://doi.org/10.3856/vol46-issue2-fulltext-1. [ Links ]
46. Sokal, R. R., and F. J. Rohlf. 1995. Biometry. The principles and practice of statistical in biological research. Third edition. W. H. Freeman, New York, USA. [ Links ]
47. Viñas, M. D., R. M. Negri, G. D. Cepeda, D. Hernández, R. Silva, M. C. Daponte, and F. L. Capitanio. 2013. Seasonal succession of zooplankton in coastal waters of the Argentine Sea (Southwest Atlantic Ocean): prevalence of classical or microbial food webs. Marine Biology Research 9:371-382. https://doi.org/10.1080/17451000.2012.745003. [ Links ]
48. Yoshida, T., M. Kagami, T. B. Gurung, and J. Urabe. 2001. Seasonal succession of zooplankton in the north basin of Lake Biwa. Aquatic Ecology 35:19-29. https://doi.org/10.1023/A:1011498202050. [ Links ]
49. Zar, J. H. 1996. Biostatistical analysis. Third edition. Prentice-Hall International Editions, New Jersey, USA. [ Links ]














