Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Ecología austral
versão On-line ISSN 1667-782X
Ecol. austral vol.29 no.3 Córdoba ago. 2019
ARTÍCULO
Effects of leaf litter traits on alpha and beta diversities of invertebrate assemblages in a tropical watershed
Renan de Souza Rezende1; Cristiane Biasi2,*; Luiz Ubiratan Hepp2; Mauricio Mello Petrucio3 & José F. Gonçalvez Junior4
1 Programa de Pós-Graduação em Ciências Ambientais, Universidade Comunitária Regional de Chapecó, Chapecó, Santa Catarina, Brasil.
2 Programa de Pós-Graduação em Ecologia, Universidade Regional Integrada do Alto Uruguai e das Missões, Campus de Erechim, Erechim, RS, Brasil.
3 Departamento de Ecologia e Zoologia, Instituto de Ciências Biológicas, Universidade Federal de Santa Catarina, Florianópolis, Santa Catarina, Brasil.
4 AquaRiparia/ Lab. de Limnologia. Departamento de Ecologia, Instituto de Biologia, Universidade de Brasília, Brasília, Distrito Federal, Brasil
Recibido: 19 de Abril de 2018
Aceptado: 30 de Septiembre de 2019
Editora asociada: Natalia Pérez Harguindeguy
ABSTRACT
The aims of this study were to: a) investigate the importance of environmental and/or spatial factors on the composition of the invertebrate community, and b) examine the alpha (α) and beta (β) diversities of the invertebrate community associated to the leaf breakdown process at three different spatial scales (stream reach, stream order and sub-basin). The abiotic variables and colonization of invertebrate community in two detritus (Eucalyptus cloeziana and Inga laurina) were evaluated in 14 sampling sites. For both detritus types, the communities were influenced by environmental matrix. High invertebrate α diversity was related to an increase in water flow velocity (outflow) and orthophosphate levels. High canopy openness and, consequently, high temperatures, showed negative effects on invertebrate α diversity. The α diversity in high-quality litter (E. cloeziana) was mainly influenced by stream order, while β diversity was mainly influenced by sub-basins. However, in low-quality litter (I. laurina), α diversity was mainly influenced by sub-basins, and the β diversity by sampling sites. These findings indicated that changes in detritus quality of the riparian vegetation resulted in significant changes of invertebrate α and β diversity in these communities.
Keywords: Local diversity; Regional diversity; Assemblage dissimilarity; Litter decomposition; Diversity partitioning.
RESUMEN
Efectos de las características de la hojarasca sobre la diversidad alfa y beta de ensambles de invertebrados en una cuenca hidrográfica tropical.
El presente estudio tuvo por objetivos a) investigar la importancia de los factores ambientales o espaciales en la comunidad de invertebrados, y b) examinar las diversidades alfa (α) y beta (β) de la comunidad de invertebrados asociada al proceso de descomposición de hojas, en tres escalas espaciales (segmento de arroyo, orden del arroyo y sub-cuenca). Se evaluaron variables abióticas y la colonización de la comunidad de invertebrados en dos tipos de detritos (Eucalyptus cloeziana y Inga laurina) en 14 sitios de muestreo. En ambos detritos, las comunidades fueron influenciadas por la matriz ambiental. La mayor diversidad α de invertebrados se relacionó con un aumento en la velocidad del flujo de agua (flujo de salida) y con los niveles de ortofosfato. Mayores aperturas de dosel y, en consecuencia, altas temperaturas mostraron efectos negativos sobre la diversidad α de invertebrados. La diversidad α en detritos de mayor calidad química (E. cloeziana) fue influenciada principalmente por el orden del arroyo, mientras que la diversidad beta resultó mayormente influenciada por la sub-cuenca. Sin embargo, en detritos de menor calidad (I. laurina), la diversidad α fue más influenciada por la sub-cuenca, mientras que la diversidad beta, por los sitios de muestreo. Estos hallazgos indicaron que cambios en la calidad de detrito proveniente de la vegetación ribereña resulta en una significativa modificación de la diversidad α y β de invertebrados.
Palabras clave: Diversidad local; Diversidad regional; Disimilaridad de asambleas; Descomposición de hojarasca; Partición de la diversidad.
INTRODUCTION
Invertebrate diversity in streams is subjected to variations of the environment, space and biological interactions that ultimately determine the abundances of species in a given area (Heino et al. 2015a). Specifically, in low-order streams, invertebrate communities are also affected by an increase of litter inputs (Rezende et al. 2014a), canopy cover (De Nadaï-Monoury et al. 2014), pebbles, gravel and stones in substrate composition (Rezende et al. 2014b), and chemical composition of the leaves (Graça et al. 2015), since they depend on the allochthonous material as a source of energy (Vannote et al. 1980). Generally, leaves with a lower toughness, high nitrogen and phosphorus contents (N and P, respectively) and low levels of structural compounds (i.e., cellulose and lignin) are considered highquality litter because these characteristics represent a resource of higher palatability for decomposers and detritivores (Graça et al. 2001). This high-quality litter may also support a higher diversity of detritivores than low-quality litter because, generally, the detritivores select food items that promote higher growth rate, survivorship, reproductive output and thus enhance the fitness (Graça et al. 2001). Thus, changes in riparian zones, such as the introduction of exotic plant species with different attributes than the species being replaced, can affect nutrient cycling and the biodiversity and structure of aquatic communities, mainly of invertebrate communities (Hieber and Gessner 2002).
Biodiversity in streams can be measured in three spatial scales: alfa (α), beta (β) and gama (γ) diversities. Alpha diversity can be measured by local species richness as well as by diversity indices (Whittaker 1960). The β diversity shows a variation in species composition among sites in the geographic area of interest by: a) species turnover (Whittaker 1960; Anderson et al. 2011), b) nestedness (Baselga 2012), and c) the ratio of total number to mean number of species per sample (Anderson et al. 2011; Whittaker 1960). Finally, the γ diversity is a product of α diversity of communities and the degree of β diversity differentiation among them (Whittaker 1960; Whittaker 1972).
Special attention should be given to β diversity when investigating the species richness patterns, especially when concerning aquatic invertebrates. β diversity represents the changes in species composition as a function of distance, and may reflect deterministic processes, such as the adaptation of species to different climates or substrates, or the result of dispersion (Heino et al. 2013; Heino et al. 2015a). Legendre et al. (2005) listed the three main current hypotheses about the origin of β diversity: 1) uniformity of species composition over large areas (null model), emphasizing the role of biological interactions (Anderson et al. 2011), 2) species composition fluctuating in a random, auto-correlated way (dispersal history), but with all species demographically and competitively equal (Hubbell 2001), and 3) species distributions related to environmental conditions, emphasizing the environmental control (Whittaker 1956; Hutchinson 1957). Most studies of aquatic invertebrate diversity seem to corroborate the third hypothesis, that is the importance of local environmental characteristics on determining species distributions (Heino et al. 2013; Rezende et al. 2014b; Heino et al. 2015a).
Due to the high environmental influence on the β diversity of aquatic invertebrate communities, a specific conceptual framework for lotic environments assumes that: a) local environmental traits among localities are more important in small spatial scales, and b) the relative importance of mechanisms influencing variability in species composition among localities is affected by the spatial level of a “region unit”, which may be a stream, a drainage basin or an ecoregion (Heino et al. 2015a). Therefore, for aquatic invertebrates, it is also important to understand the influence of the environment in β diversity levels at different scales in order to better elucidate diversity patterns (Ferreira et al. 2017; Tonin et al. 2017).
The effect of scale on diversity can exhibit different patterns and structures depending on which scale is analyzed (Wiens 1989; Heino et al. 2015b). For example, we can expect an increase in β diversity at higher spatial scales, due to 1) high environmental heterogeneity (Hutchinson 1957), 2) effects of dispersal limitation (differences in species composition among sites by spatial extent increase; Cottenie 2005), 3) the sampling of different regional species pools (Wiens 1989), and 4) a negative relationship between the pairwise similarity in assemblage composition and geographic distance (Hubbell 2001). Thus, it is important that spatial and temporal variation among and within stream systems is represented by hierarchical frameworks, such as pools and riffles inside stream reaches that shape the rivers and hydrographic basins. This allows studies performed across large areas to show a variation of measures at different resolutions (both spatially and temporally) and to test if changes on small scales are maintained at large scales (Schneider 2001).
If the stream structure systems represent an important environmental effect (positive by increase of litter input, canopy cover and sediment coarse) on the invertebrate community structure, we expect that higher diversity occurs in sites with high canopy cover and low water velocities (due to the accumulation of POM and higher sediment variability). On the other hand, if the invertebrate diversity differs from this pattern, we propose a scale-based analysis according to the conceptual framework of Heino et al. (2015a). Following this, the α and β diversity is expected to be influenced to spatial scale, but the leaf quality can influence the diversity patterns (both α and β). Finally, our goals were to: a) quantify and describe α and β diversity of invertebrate communities associated with the leaf breakdown of Eucalyptus cloeziana F. Muell and Inga laurina (Sw.) Willd at three spatial scales (reach, order and sub-basin), and b) assess the importance of environmental and/or spatial factors on the structuring of the invertebrate community.
MATERIALS AND METHODS
Study sites
The study region included 14 sampling sites distributed along the Gama-Cabeça de Veado watershed in the “Cerrado” tropical savannah (altitudes between 1025 m and 1150 m a. s. l.) of Central-West Brazil, in the Federal District (Figure 1). The experiment was performed only during the dry season, allowing for a greater temporal homogeneity of physico-chemical traits of the environment, thus decreasing the effect of washing on the invertebrate community. The sampling sites have a high diversity of plants species, with ~146 native species, especially representatives of the families Fabaceae, Myrtaceae, Rubiaceae, Melastomataceae, Lauraceae, Annonaceae, Euphorbiaceae, Meliaceae, Primulaceae, Sapotaceae and Vochysiaceae (Bambi et al. 2016). As a representative of exotic species, Eucalyptus, has been well established throughout the region studied (Rezende et al. 2014a).

Figure 1. Geographic location of study sampling sites on the Gama-Cabeça de Veado watershed, Federal District (Brazil).
Figura 1. Ubicación geográfica de los sitios de muestreo del estudio en la cuenca Gama-Cabeça de Veado, Distrito Federal (Brasil).
Procedures
We chose two plant species that are common in the riparian zones of the region. The senescent leaves of E. cloeziana and I. laurina were collected, air-dried and separated for physical and chemical analyzes (see below). The Eucalyptus plantations (an exotic monoculture) have significantly expanded within Brazilian hydrographic basins due to an increased demand of coal for steelmakers, pulp for papermaking and other uses, allowing us to study the potential consequences of the replacement of native vegetation by substitute monocultures as a source of allochthonous material in lotic systems. On the contrary, I. laurina is abundant in native riparian vegetation, and is a good representative of typical plants of the riparian vegetation in the Cerrado (Brazilian Savanna the native).
The leaf traits of the species were analyzed prior to their use in the experiments (n=4, with the exception of nitrogen and phosphorusn= 1). The leaf toughness was determined with a penetrometer (Graça et al. 2005). Lignin and cellulose were determined through the fiber acid-detergent method (Goering and Van Soest 1970), total polyphenols by Folin-Denis method (Graça et al. 2005) and tannic acids by radial diffusion (Graça et al. 2005). The nitrogen content (N) was determined by Kjeldahl method and phosphorus concentrations (P) following APHA 1995.
Leaf samples were incubated in 252 litter bags (15×15 cm, 10 mm mesh size) and placed in shallow water (~0.3 m depth) in streams at 4 sub-basins (Figure 1, supplementary material). The sampling times were calculated by dividing the initial weight (W0) by the estimated value of k (for more details, see Rezende et al. 2014a). This calculation yields the time for the total duration of leaf processing (TLP, days). From the equation W0/k=TLP, we can calculate how many days will be required to reach a desired percentage of the initial weight (Wt). The first sample was collected after 10 days of incubation for both species, so that TLP for 10 days/0.25=day on which Wt=75%. The next sample was collected after 40 days for E. cloeziana and after 75 days for I. laurina, so that TLP 40/75 days/0.5=day for which Wt=50%. The above procedure was performed for each sampling site (based on the mean value) and type of detritus. However, it was not possible to determine the final value for I. laurina because the dry season ended after 120 days, before 50% of the mass was lost. Measurements after the end of the dry season would not have been meaningful because the variations in rainfall and associated variations in other physico-chemical parameters would have influenced the results. The final k value was calculated using the negative exponential model of percentage of mass lost over time (Wt =W0e-kt; Wt=remaining weight; W0=initial weight; -k=decay rate; t=time).
A multianalyzer was used to measure water temperature, conductivity, pH, dissolved oxygen and turbidity in situ. The water velocity associated with depth was measured at three points in each stream using a flow meter for the calculation of stream discharge. Additionally, water was collected for analyses of the nitrate (Golterman et al. 1978), nitrite (Koroleff 1976) and orthophosphate (Strickland and Parsons 1960), which were analyzed by absorption spectrometry. Canopy openness was quantified using hemispherical photographs taken using a digital camera coupled to a fish-eye lens.
Litter bags were transported to the laboratory in a cooler and the leaves were washed with distilled water in a sieve (120 μm mesh). The invertebrates retained on the sieve (after the washing) were fixed in 70% ethanol for later sorting and identification to the family level (Hamada et al. 2014; Merritt and Cummins 1996). The family level is enough to observe community patterns, with a loss of explanation of only ~6% (Marshall et al. 2006) compared to genus taxonomic level. The number of taxa (taxonomic richness) and density (individual per dry mass of leaves [DM]) was calculated based on the benthic invertebrate inventory.
Measuring α and β diversities We calculated the Shannon-Wiener diversity index in the two litter types at all sampling sites, hydrological orders, and sub-basins, and used as a proxy of as α diversity at each spatial level. We estimated the β diversity of all of the sampling sites, hydrological orders, and sub-basins with the multivariate dispersion method (Anderson et al. 2006). Multivariate dispersion estimated the β diversity as a given site’s average dissimilarity (i.e., distance) from their group centroid in a multivariate space. The comparison among the sampling sites was based on the Whittaker index (βw) (Whittaker 1960; Magurran 2001), which measures the change or rate of substitution in species composition from one site to another, and the gradient comparison among hydrological orders and sub-basins were based on Lennon (βgl) (Lennon et al. 2001), as proposed by Koleff et al. (2003). The βw is the most widely used β diversity measure in ecology, and the values show a simple relationship to the variation, in which all variations scale negatively with increases in the matching component (Koleff et al. 2003). The βgl values depend on the difference in the number of taxa between the two samples under consideration, and were employed to test whether the other β diversity measures are able to recover patterns regarding the local number of taxa gradients (Lennon et al. 2001; Koleff et al. 2003).
Statistical analyses
The leaf traits of plant species were compared with t test (P<0.05), with the exception of N and P that were compared with chi-square test (P<0.05). The geographical coordinates (latitude and longitude in the UTM) of each sampling site (Figure 2, supplementary material) were used with the principal coordinates of the neighbor matrices (PCNM) method described by Borcard and Legendre (2002). The relative importance of spatial (geographical distance) and environmental variables (temperature, conductivity, pH, dissolved oxygen, turbidity, discharge, water velocity, nitrate, nitrite and orthophosphate concentrations, and canopy openness of riparian vegetation) for the invertebrate community structure was evaluated using a partial redundancy analysis in both detritus types (pRDA; varpart function, vegan package for R) (Oksanen et al. 2013).

Figure 2. Partial redundancy analysis (pRDA) of the invertebrate communities based on the spatial and environmental matrices (percentage-explanation values) of the sampling sites in Eucalyptus cloeziana (A) and Inga laurina (B) detritus.
Figura 2. Análisis de redundancia parcial (pRDA) de la comunidad de invertebrados basados en las matrices espacial y ambiental (valores de porcentaje de explicación) de los sitios de muestreo en Eucalyptus cloeziana (A) y Inga laurina (B) detritos.
A redundancy analysis was used to select among the distance and environmental matrix to assess the most important variables. First, a global test was performed, including all explanatory variables and adjusting for R2 according to Ezekiel´s correction (Peres-Neto et al. 2006). Second, the R2 adj of the global test was calculated as a second criterion (in addition to an α-value of 0.05) to select the variables that would be retained in the subsequent analyses. A forward model-selection procedure was performed, starting with the selection of the explanatory variable that maximized the fit of the model, and then computing an F-ratio and P-value by permuting the residuals using the full-model approach (Legendre and Legendre 1998). Whenever P≤0.05 was obtained, R2 adj was computed for the forward test. If R2 adj was smaller for the forward test than for the global test, another variable was added to the analysis, and the permutation test was repeated. Otherwise, the procedure was stopped. All of the analyses were performed using the average values of the environmental variables measured during all of the sampling periods at each site. Therefore, we calculated the following: the amount of variation due to the environmental variables; the amount of spatially structured variables; the amount of variation due to both the environmental and spatially structured variables (shared); and the amount of variation that remained unexplained (Legendre et al. 2005).
The α diversity in the invertebrate community was examined with a nested analysis of variance (Bailey 1992) to detect the importance at three nested spatial scales: sub-basin, stream order (nested into sub-basin) and sampling site (nested into stream order). The β diversity was calculated from betadiver function, Vegan package for R version 2.0.8 -Oksanen et al. (2013). A Permutational Multivariate Analysis of Variance (PerMANOVA) for two detritus was used (distance matrix of Bray-Curtis, 10000 permutation and with pseudo-F; Adonis function, vegan package for R) (Oksanen et al. 2008) to estimate the difference for β diversity (different axes related to the distance from the centroid) among the scales. In this analysis, we tested the dispersion differences and not the location differences in a multivariate space (Heino et al. 2013). The sum of squares percentage in the nested ANOVA and PerMANOVA analyses was used to determine which accounted for the highest variance among the different scales (Anderson 2001).
RESULTS
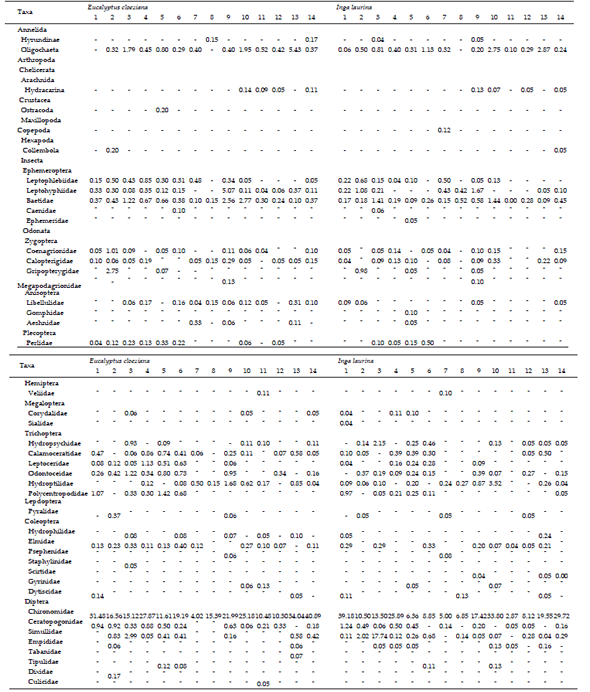
Invertebrate community We found a total of 44 taxa in leaf litter (E. cloeziana and I. laurina; Table 1). In leaf litter of E. cloeziana, the invertebrates with highest abundance was Chironomidae (20.3 ind./g DM) followed by Oligochaeta (0.9 ind./g DM) and Baetidae (0.7 ind./g DM). Leptohyphiidae, Simullidae, Ceratopogonidae, Odontoceidae, Hydroptilidae, Polycentropodidae and Calamoceratidae complete family lists of the most abundant (ranging from 0.5 to 0.3 ind./g DM). The most abundant groups in I. laurina was Chironomidae (16.2 ind./g DM) followed by Simullidae (1.6 ind./g DM) and Oligochaeta (0.7 ind./g DM). Baetidae, Hydroptilidae, Leptohyphiidae, Ceratopogonidae, Hydropsychidae, Odontoceidae and Leptophlebiidae complete lists of the most abundant (ranging from 0.4 to 0.1 ind./g DM).
Table 1. Average values of the density of invertebrates (individuals per leaf litter g) at 14 sampling sites of Brazilian savanna streams.
Tabla 1. Valores medios de la densidad de invertebrados (individuos por g de la hojarasca) en 14 sitios de arroyos de la sabana brasileña.
Leaf traits
Leaves of E. cloeziana and I. laurina showed similar mean values of total polyphenols, total tannic acids, nitrogen and phosphorus content (Table 1, supplementary material). On the other hand, I. laurina showed 25% more Lignin:N ratios when compared with E. cloeziana. The species I. laurina presented the highest values of lignin (~7% more), cellulose concentration (33% more) and leaf toughness (3-fold) when compared to E. cloeziana (Table 1, supplementary material). Leaves of E. cloeziana show higher mean values of k (- 0.0067 day-1, ranging from -0.0046 to -0.0131 day-1) than I. laurina (-0.0035 day-1, ranging from -0.0015 to -0.0071 day-1). We considered E. cloeziana as high-quality detritus due to the low concentrations of structural compounds (lignin, cellulose and leaf toughness) when compared to I. laurina (low-quality), although there was no difference in other leaf traits. In addition, the leaves of E. cloeziana presented a rapid decomposition rate when compared to the leaves of I. laurina.
Environmental and spatial influence
The environmental matrix explained 17% and 33% of community structure in E. cloeziana (high-quality litter) and I. laurina (low-quality litter), respectively (Figure 2). The simultaneous effect of both the environmental and spatial matrices (8% and 9%, respectively), and spatial matrix alone for E. cloeziana and I. laurina (7% and 0%, respectively) contributed little to the explanation of the observed variance. The unexplained variance for E. cloeziana and I. laurina was of 69% and 58%, respectively. The two detritus were applicable in the spatial matrix for only one vector (Vector 1; adjusted R2=0.14, F=2.26, P<0.01). In E. cloeziana, three environmental variables were selected: temperature, water velocity, and water orthophosphate concentration (adjusted R2>0.062, F>1.87, P<0.001) (Figure 2A). In I. laurina, four environmental variables were selected: temperature, water velocity, discharge, and canopy openness (adjusted R2>0.187, F>2.22, P<0.001) (Figure 2B).
The α and β diversities in spatial scales The most important scale for invertebrate α diversity in E. cloeziana was stream order, due to the higher variance explanation in this intermediate scale than in others. The next scales in importance were the subbasin (although with a similar explanation of the scale order), followed by sampling site (Table 2A; Figure 3). In I. laurina, the most important scale for α diversity was the sub-basin, followed by stream order (with a similar explanation), and sampling site (Table 2B; Figure 4). The β diversity in E. cloeziana showed a higher percentage of explanation by the sub-basin, followed by stream order and sampling site (Table 2B; Figure 3). The invertebrate β diversity in I. laurina was better explained by the sampling site, followed by the sub-basin and stream order, although the latter two with similar explanation (Table 2B; Figure 4).
Table 2. The values of sums of squares percentage (SS), degrees of freedom (DF), F test, P value and residuals of analysis by nested ANOVA (A; testing α diversity) and PerMANOVA (B; testing β diversity) among sub-basin, stream order and sampling site in Eucalyptus cloeziana and Inga laurina detritus.
Tabla 2. Valores de porcentajes de sumas de cuadrados (SS), grados de libertad (DF), prueba F, valor de P y residuales del ANOVA anidado (A; análisis de la diversidad α) y PerMANOVA (B; análisis de la diversidad β) entre la subcuenca, orden de arroyo y el sitio de muestreo en detritos de Eucalyptus cloeziana y Inga laurina.


Figure 3. α diversity estimated by Shannon-Weaver Index (A, C and E), β diversity (B, D and F) in the sampling sites (A and B), sub-basins (C and D), and hydrological order (E and F) in Eucalyptus cloeziana.
Figura 3. Diversidad α estimada por el Índice Shannon-Weaver (A, C y E), diversidad β (B, D y F) en los sitios de muestro (A y B), sub-cuenca (C y D), y orden hidrológico (E y F) en Eucalyptus cloeziana.

Figure 4. α diversity estimated by Shannon-Weaver Index (A, C and E), β diversity (B, D and F) in the sampling sites (A and B), sub-basins (C and D), and hydrological order (E and F) in Inga laurina.
Figura 4. Diversidad α estimada por el Índice Shannon-Weaver (A, C y E), diversidad β (B, D y F) en los sitios de muestreo (A y B), sub-cuenca (C y D), y orden hidrológico (E y F) en Inga laurina.
DISCUSSION
Environmental and spatial influence
Regarding most beta diversity studies, our work is novel when regarding the inclusion of variables related to the detritus characteristics. The detritus of both species showed similar amounts of polyphenols and total tannic acids. However, these compounds are rapidly leached in the first hours of immersion in the water, so they probably do not exert a negative effect on the diversity of invertebrates (Hepp et al. 2009). The synergistic effects a thick leaf cuticle and high toughness, probably due to the higher structural compounds (lignin and cellulose), makes the detritus of I. laurina more stable and less chemically reactive in the water when compared to the high-quality litter of E. cloeziana (Rezende et al. 2014a; Rezende et al. 2016). Most likely, this detritus was functioning as a substrate to invertebrates.
The α diversity in E. cloeziana detritus was mainly explained by the change in stream order, followed by the sub-basin. Therefore, the invertebrate communities among different stream orders of the same sub-basin were more differentiated from each other than those of the same stream orders in different sub-basins (Ferreira et al. 2017; Tonin et al. 2017). This also explain the high β diversity levels among subbasins in this detritus. Additionally, α diversity was highest in sub-basins with detritus of I. laurina, which indicated a highest difference among sub-basins, independent of the stream order. This may explain the high β diversity levels among sampling sites of different sub-basins, because the local environmental conditions and detritus quality would reflect the regional characteristics.
Our results also indicated that environmental variables (mainly, temperature, water velocity and nutrient concentration) were important structuring forces for invertebrate communities in situ (corroborated by Ferreira et al. 2012; Hepp et al. 2012) as has been shown in other works. (Hepp and Melo 2013; Siqueira et al. 2012). Although it is not possible discard that other factors than environmental variables, not measured in our study, could explain the variation of the data, given the low explanation of the environmental matrix in the invertebrate community. Therefore, we can infer that landscapes represent a mosaic of habitats, where the composition of invertebrate communities was controlled by local environmental characteristics (Leibold et al. 2004; Ferreira et al. 2017; Tonin et al. 2017). The dispersal capacity of invertebrates in heterogeneous environments may result in local populations being affected by source-sink relationships (Leibold et al. 2004; Durães et al. 2016) and weak spatial structures (Siqueira et al. 2012; Hepp and Melo 2013). This can result in low α diversity (when viewed as networks at the landscape-scale) but high β diversity levels due to the different ability for the dispersal of invertebrates and higher-qualities habitats in headwater streams than in downstream areas (Clarke et al. 2008). This also emphasized the spatial dispersal history and explained the low values in the shared term (environment/ space). However, we cannot discard that environmental matrix could not be the sole mechanism affecting the β diversity, mainly when dispersal rates were high (Heino et al. 2015a; Durães et al. 2016).
The α and β diversities at different spatial scales
It is not new that the water velocity is important factor in determining invertebrate community. High flow velocities increase the water’s capacity to carry most fine sediment particles, leaving behind a greater percentage of coarse fractions (Rueda-Delgado et al. 2006; Santos Fonseca et al. 2012). The occurrence of coarse fractions at some particular sites increased the variety of available habitats, allowing for an increase in the invertebrate diversity in areas adjacent to colonized detritus (Ligeiro et al. 2013). Stream discharge showed a positive correlation with water velocity most likely due to the small size of sampled streams and the steep topography typical of headwater areas. In relation to orthophosphate, it is known that high concentrations support and stimulate both periphyton and microbial growth (Bae et al. 2011; Bleich et al. 2015). This provided a better detritus quality and food source for invertebrates, including shredder-detritivores, scrapers and collectors (Yoshimura et al. 2006), increasing invertebrate diversity. Therefore, our results indicated that sites that were rich in nutrients and had high water velocities had higher local invertebrate species richness (especially for Ephemeroptera, Plecoptera and Trichoptera) than other stream sites.
Streams are influenced by canopy openness and, consequently, high temperatures (increased light availability). The nutrient cycling is affected by canopy cover (by affecting litter input and decomposition), increasing the detritivores and decomposers density and/or richness in riparian zones with higher canopy cover (Rezende et al. 2014a; Tonin et al. 2017), although the scrapers diversity can be underestimated in litterbags and can benefit with open canopy. This effect of the canopy cover may also vary differently in different hierarchical scales (e.g., reaches to watershed) by confounding factors that could co-vary (e.g., temperature, luminosity, litter biomass and litter quality) with canopy cover (for a better discussion of this topic, see Tonin et al. 2017). The degradation of the canopy cover can allow for increased sediment entrainment (Gardiner et al. 2009) which decreases habitat variety, sediment diversity and shelter availability for aquatic communities (Lancaster and Hildrew 1993; Bücker et al. 2010; Luiza-Andrade et al. 2017). This can reduce the organism pool in areas adjacent to colonized detritus, decreasing the diversity. However, our results should be interpreted with caution, since our findings are limited to invertebrate community associated with leaf breakdown. Canopy cover will also increase the exposed surface of the streams, increasing the water temperature and likely leading to a decrease in dissolved oxygen concentrations (Md Rawi et al. 2013; Uieda and Carvalho 2015). Changes in the dissolved oxygen concentrations of streams may increase the levels of suspended sediments and soil erosion (Negishi et al. 2006), deteriorating the physico-chemical parameters of the water and decreasing the overall invertebrate diversity (Dominguez-Granda et al. 2011; Luiza- Andrade et al. 2017).
CONCLUSION
The influence of the litter quality in invertebrate diversity was mostly related to the spatial scale. This study indicated that low quality riparian vegetation could locally impair but regionally support high invertebrate diversity. Environmental variables were the main structuring forces of invertebrate communities (positively correlated to discharge and orthophosphate levels; and negatively correlated to canopy openness and temperature), and the spatial influence on invertebrate communities was low. We found that the variation of α and β diversities changed with the observed spatial scales and litter traits, confirming our hypothesis. The α diversity in E. cloeziana detritus (high-quality litter) was mostly influenced by stream order, while the β diversity mostly related to subbasins. However, in the detritus of I. laurina (low-quality litter), α diversity was most influenced by sub-basins, while β diversity levels were highest among sampling sites. This may indicate that in riparian vegetation comprised of plant species with leaves of low quality, the invertebrate diversity is less resistant to ecological disturbances.
ACKNOWLEDGEMENTS
We are grateful to PROCAD-NF/CAPES (No. 173/2010), PNADB/ Edital 17/2009/CAPES (No. 1098/2010), PELD/ CNPq (No. 558233/2009-0), FAPEMIG (No. APQ-00274-12) and founds of University of Brasília (UnB - DPP; No. 121366/2011) for the financial support. We are also grateful to Cássia Alves Lima for valuable logistical support in field and laboratory works. LUH received financial support from FAPERGS (12/1354-0) and CNPq (305203/2017-7).
REFERENCES
1. Anderson, M. J. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26:32-46. https://doi.org/10.1046/j.1442-9993.2001.01070.x. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x. [ Links ]
2. Anderson, M. J., T. O. Crist, J. M. Chase, M. Vellend, B. D. Inouye, A. L. Freestone, N. J. Sanders, H. V. Cornell, L. S. Comita, K. F. Davies, S. P. Harrison, N. J. Kraft, J. C. Stegen, and N. G. Swenson 2011. Navigating the multiple meanings of beta diversity: a roadmap for the practicing ecologist. Ecology Letters 14:19-28. https://doi.org/10.1111/j.1461-0248.2010.01552.x. [ Links ]
3. Anderson, M. J., K. E. Ellingsen, and B. H. McArdle 2006. Multivariate dispersion as a measure of beta diversity. Ecology Letters 9:683-693. [ Links ]
4. APHA (American Public Health Association). 1995. Standard methods for the examination of water and wastewater, 20th ed. APHA, Washington, D.C. [ Links ]
5. Bae, M.-J., Y. Kwon, S.-J. Hwang, T.-S. Chon, H.-J. Yang, I.-S. Kwak, J.-H. Park, S.-A. Ham, and Y.-S. Park. 2011. Relationships between three major stream assemblages and their environmental factors in multiple spatial scales. Annales de Limnologie - International Journal of Limnology 47:S91-S105. https://doi.org/10.1051/limn/2011022. [ Links ]
6. Bailey, R. C. 1992. Hierarchical analysis of community and habitat structure. Coenoses 7:127-135. [ Links ]
7. Bambi, P., R. S. Rezende, T. M. S. Cruz, J. E. A. Batista, F. G. G. Miranda, L. V. Santos, and J. F. Gonçalves Jr. 2016. Diversidade da Flora Fanerogâmica de três matas de galeria no bioma Cerrado. Heringeriana 10:147-167. [ Links ]
8. Baselga, A. 2012. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Global Ecology and Biogeography 21:1223-1232. https://doi.org/10.1111/j.1466-8238.2011.00756.x. [ Links ]
9. Bleich, M. E., M. T. F. Piedade, A. F. Mortati, and T. André. 2015. Autochthonous primary production in southern Amazon headwater streams: Novel indicators of altered environmental integrity. Ecological Indicators 53:154-161. https://doi.org/10.1016/j.ecolind.2015.01.040. [ Links ]
10. Borcard, D., and P. Legendre. 2002. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecological Modelling 153:51-68. https://doi.org/10.1016/S0304-3800(01)00501-4. [ Links ]
11. Bücker, A., M. Sondermann, H.-G. Frede, and L. Breuer 2010. The influence of land-use on macroinvertebrate communities in montane tropical streams - a case study from Ecuador. Fundamental and Applied Limnology / Archiv für Hydrobiologie 177:267-282. https://doi.org/10.1127/1863-9135/2010/0177-0267. [ Links ]
12. Clarke, A., R. Mac Nally, N. Bond, and P. S. Lake 2008. Macroinvertebrate diversity in headwater streams: a review. Freshwater Biology 53:1707-1721. https://doi.org/10.1111/j.1365-2427.2008.02041.x. [ Links ]
13. Costa, S. S., and A. S. Melo 2008. Beta diversity in stream macroinvertebrate assemblages: among-site and amongmicrohabitat components. Hydrobiologia 598:131-138. https://doi.org/10.1007/s10750-007-9145-7. [ Links ]
14. Cottenie, K. 2005. Integrating environmental and spatial processes in ecological community dynamics. Ecology Letters 8:1175-82. https://doi.org/10.1111/j.1461-0248.2005.00820.x. [ Links ]
15. De Nadaï-Monoury, E., F. Gilbert, and A. Lecerf. 2014. Forest canopy cover determines invertebrate diversity and ecosystem process rates in depositional zones of headwater streams. Freshwater Biology 59:1532-1545. https://doi.org/10.1111/fwb.12364. [ Links ]
16. Dominguez-Granda, L., K. Lock, and P. L. M. Goethals. 2011. Using multi-target clustering trees as a tool to predict biological water quality indices based on benthic macroinvertebrates and environmental parameters in the Chaguana watershed Ecuador. Ecological Informatics 6:303-308. https://doi.org/10.1016/j.ecoinf.2011.05.004. [ Links ]
17. Durães, L., F. O. Roque, T. Siqueira, A. M. Santos, M. A. Borges, and R. S. Rezende. 2016. Simulating the role of connectivity in shaping stream insect metacommunities under colonization cycle dynamics. Ecological Modelling 334:19-26. https://doi.org/10.1016/j.ecolmodel.2016.04.020. [ Links ]
18. Ferreira, V., A. C. Encalada, and M. A. S. Graça. 2012. Effects of litter diversity on decomposition and biological colonization of submerged litter in temperate and tropical streams. Freshwater Science 31:945-962. https://doi.org/10.1016/j.ecolind.2016.08.042. [ Links ]
19. Ferreira, W. R., L. U. Hepp, R. Ligeiro, D. R. Macedo, R. M. Hughes, P. R. Kaufmann, and M. Callisto. 2017. Partitioning taxonomic diversity of aquatic insect assemblages and functional feeding groups in neotropical savanna headwater streams. Ecological Indicators 72:365-373. https://doi.org/10.1016/j.ecolind.2016.08.042. [ Links ]
20. Gardiner, E. P., A. B. Sutherland, R. J. Bixby, M. C. Scott, J. L. Meyer, G. S. Helfman, E. F. Benfield, C. M. Pringle, P. V. Bolstad, and D. N. Wear. 2009. Linking stream and landscape trajectories in the southern Appalachians. Environmental monitoring and assessment 156:17-36. https://doi.org/10.1007/s10661-008-0460-x. [ Links ]
21. Goering, H. K., and P. J. Van Soest. 1970. Forage fiber analysis (apparatus, reagents, procedures and some applications). Pp 1-2 in Agricultural handbook. US Department of Agriculture, Washington, D.C. [ Links ]
22. Golterman, H. L., R. S. Clymo, and D. M. A. M. Ohnsta. 1978. Methods for chemical analysis of freshwater. Blackwell Scientific Publications, Oxford. [ Links ]
23. Graça, M. A. S., C. Cressa, M. O. Gessner, M. J. Feio, K. A. Callies, and C. Barrios. 2001. Food quality, feeding preferences, survival and growth of shredders from temperate and tropical streams. Freshwater Biol 46:1-11. https://doi.org/10.1046/j.1365-2427.2001.00729.x. [ Links ]
24. Graça, M. A. S., F. Barlocher, and M. O. Gessner. 2005. Methods to Study Litter Decomposition: A Practical Guide. Springer, The Netherlands, Dordrecht. https://doi.org/10.1007/1-4020-3466-0. [ Links ]
25. Graça, M. A. S., V. Ferreira, C. Canhoto, A. C. Encalada, F. Guerrero-Bolaño, K. M. Wanen, and L. Boyero. 2015. A conceptual model of litter breakdown in low order streams. International Review of Hydrobiology 100:1-12. https://doi.org/10.1002/iroh.201401757. [ Links ]
26. Hamada, N., J. L. Nessimian, and R. B. Querino 2014. Insetos aquáticos na Amazônia brasileira: taxonomia, biologia e ecologia. INPA, Manaus. [ Links ]
27. Heino, J., M. Grönroos, J. Ilmonen, T. Karhu, M. Niva, and L. Paasivirta. 2013. Environmental heterogeneity and β diversity of stream macroinvertebrate communities at intermediate spatial scales. Freshwater Science 32:142-154. https://doi.org/10.1899/12-083.1.
28. Heino, J., A. S. Melo, and L. M. Bini. 2015a. Reconceptualising the beta diversity-environmental heterogeneity relationship in running water systems. Freshwater Biology 60:223-235. https://doi.org/10.1111/fwb.12502. [ Links ]
29. Heino, J., A. S. Melo, L. M. Bini, F. Altermatt, S. A. Al-Shami, D. G. Angeler, N. Bonada, C. Brand, M. Callisto, K. Cottenie, O. Dangles, D. Dudgeon, A. Encalada, E. Gothe, M. Gronroos, N. Hamada, D. Jacobsen, V. L. Landeiro, R. Ligeiro, R. T. Martins, M. L. Miserendino, C. S. Md Rawi, M. E. Rodrigues, F. de O. Roque, L. Sandin, D. Schmera, L. F. Sgarbi, J. P. Simaika, T. Siqueira, R. M. Thompson, and C. R. Townsend. 2015b. A comparative analysis reveals weak relationships between ecological factors and beta diversity of stream insect metacommunities at two spatial levels. Ecology and evolution 5:1235-48. https://doi.org/10.1002/ece3.1439. [ Links ]
30. Hepp, L. U., R. Delanora, and A. Trevisan. 2009. Compostos secundários durante a decomposição foliar de espécies arbóreas em um riacho do sul do Brasil. Acta Botanica Brasilica 23:407-413. https://doi.org/10.1590/S0102-33062009000200012. [ Links ]
31. Hepp, L. U., V. L. Landeiro, and A. S. Melo. 2012. Experimental Assessment of the Effects of Environmental Factors and Longitudinal Position on Alpha and Beta Diversities of Aquatic Insects in a Neotropical Stream. International Review of Hydrobiology 97:157-167. https://doi.org/10.1002/iroh.201111405. [ Links ]
32. Hepp, L. U., and A. S. Melo. 2013. Dissimilarity of stream insect assemblages: effects of multiple scales and spatial distances. Hydrobiologia 703:239-246. https://doi.org/10.1007/s10750-012-1367-7. [ Links ]
33. Hieber, M., and M. O. Gessner. 2002. Contribution of stream detritivores, fungi, and bacteria to leaf breakdown based on biomass estimates. Ecology 83:1026-1038. https://doi.org/10.1890/0012-9658(2002)083[1026:COSDFA]2.0.CO;2. [ Links ]
34. Hubbell, S. P. 2001. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton, Princeton University Press. [ Links ]
35. Hutchinson, G. E. 1957. Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology 22:15-427. https://doi.org/10.1101/SQB.1957.022.01.039. [ Links ]
36. Koleff, P., K. J. Gaston, and J. J. Lennon. 2003. Measuring Beta Diversity for Presence-Absence Data. Journal of Animal Ecology 72:367-382. https://doi.org/10.1046/j.1365-2656.2003.00710.x. [ Links ]
37. Koroleff, F. 1976. Determination of nutrients. Pp. 117-181 in K. Grasshoff (ed.). Methods of Sea Water Analysis. Verlag Chemie Weinhein. [ Links ]
38. Lancaster, J., and A. G. Hildrew. 1993. Flow Refugia and the Microdistribution of Lotic Macroinvertebrates. Journal of the North American Benthological Society 12:385-393. https://doi.org/10.2307/1467619. [ Links ]
39. Legendre, P., D. Borcard, and P. R. Peres-Neto. 2005. Analyzing beta diversity: partitioning the spatial variation of community composition data. Ecological Monographs 75:435-450. https://doi.org/10.1890/05-0549. [ Links ]
40. Legendre, P., and L. Legendre. 1998. Numerical Ecology. English Edition. Elsevier, London. [ Links ]
41. Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau, and A. González. 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7:601-613. https://doi.org/10.1111/j.1461-0248.2004.00608.x. [ Links ]
42. Lennon, J. J., P. Koleff, J. J. D. Greenwood, and K. J. Gaston. 2001. The geographical structure of British bird distributions: diversity, spatial turnover and scale. Journal of Animal Ecology 70:966-979. https://doi.org/10.1046/j.0021-8790.2001.00563.x. [ Links ]
43. Ligeiro, R., R. M. Hughesb, P. R. Kaufmannc, D. R. Macedo, K. R. Firmiano, Ferreira, D. Oliveira, A. S. Melo, and M. Callisto. 2013. Defining quantitative stream disturbance gradients and the additive role of habitat variation to explain macroinvertebrate taxa richness. Ecological Indicators 25:45-57. https://doi.org/10.1016/j.ecolind.2012.09.004. [ Links ]
44. Luiza-Andrade, A., L. S. Brasil, N. L. Benone, Y. Shimano, A. P. J. Farias, L. F. Montag, S. Dolédec, and L. Juen. 2017. Influence of oil palm monoculture on the taxonomic and functional composition of aquatic insect communities in eastern Brazilian Amazonia. Ecological Indicators 82:478-483. https://doi.org/10.1016/j.ecolind.2017.07.006. [ Links ]
45. Magurran, A. E. 2001. Ecological diversity and its measurement. Chapman and Hall, London. [ Links ]
46. McCabe D. J., and N. J. Gotelli. 2000. Effects of disturbance frequency, intensity, and area on assemblages of stream macroinvertebrates. Oecologia 124:270-279. https://doi.org/10.1007/s004420000369. [ Links ]
47. Md Rawi, C. S., S. A. Al-Shami, M. R. Madrus, and A. H. Ahmad. 2013. Local effects of forest fragmentation on diversity of aquatic insects in tropical forest streams: implications for biological conservation. Aquatic Ecology 47:75-85. https://doi.org/10.1007/s10452-012-9426-8. [ Links ]
48. Merritt, R. W., and K. W. Cummins. 1996. An introduction to the aquatic insects of North America. Kendall/Hunt Publishing Company, Dubuque. [ Links ]
49. Negishi, J. N., R. C. Sidle, S. Noguchi, A. R. Nik, and R. Stanforth. 2006. Ecological roles of roadside fern Dicranopteris curranii on logging road recovery in Peninsular Malaysia: Preliminary results. Forest Ecology and Management 224: 176-186. https://doi.org/10.1016/j.foreco.2005.12.017. [ Links ]
50. Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. Henry, H. Stevens, and H. Wagner. 2013. Community Ecology Package: Ordination, Diversity and Dissimilarities. Version 2.0-8.
51. Oksanen, J., R. Kindt, P. Legendre, B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, and H. Wagner. 2008. Adonis function. Pp. 15-0 in Vegan: Community Ecology Package. R package version 1.13-1.
52. Peres-Neto, P. R., P. Legendre, S. Dray, and D. Borcard. 2006. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614-2625. https://doi.org/10.1890/0012-9658(2006)87[2614:VPOSDM]2.0.CO;2. [ Links ]
53. Rezende, R. S., M. A. S. Graça, A. M. Santos, A. O. Medeiros, P. F. Santos, Y. R. Nunes, and J. F. G. Junior. 2016. Organic Matter Dynamics in a Tropical Gallery Forest in a Grassland Landscape. Biotropica 48:301-310. https://doi.org/10.1111/btp.12308. [ Links ]
54. Rezende, R. S., M. M. Petrucio, and J. F. Jr. Gonçalves. 2014a. The Effects of Spatial Scale on Breakdown of Leaves in a Tropical Watershed. Plos One 9:e97072. https://doi.org/10.1371/journal.pone.0097072. [ Links ]
55. Rezende, R. S., A. M. Santos, C. Henke-Oliveira, and Jr J. F. Gonçalves 2014b. Effects of spatial and environmental factors on benthic a macroinvertebrate community. Zoologia Curitiba 31:426-434. https://doi.org/10.1590/S1984-46702014005000001. [ Links ]
56. Rueda-Delgado, G., K. M. Wantzen, and M. B. Tolosa. 2006. Leaf-Litter Decomposition in an Amazonian Floodplain Stream: Effects of Seasonal Hydrological Changes. Journal of the North American Benthological Society 25:233-249. https://doi.org/10.1899/0887-3593(2006)25[233:LDIAAF]2.0.CO;2. [ Links ]
57. Santos Fonseca, A. L., I. Bianchini, C. M. M. Pimenta, C. B. P. Soares, and N. Mangiavacchi. 2012. The flow velocity as driving force for decomposition of leaves and twigs. Hydrobiologia 703:59-67. https://doi.org/10.1007/s10750-012-1342-3. [ Links ]
58. Schneider, D. C. 2001. The rise of the concept of scale in ecology. BioScience 51:545-554. https://doi.org/10.1641/0006-3568(2001)051[0545:TROTCO]2.0.CO;2. [ Links ]
59. Siqueira, T., L. M. Bini, F. O. Roque, and K. Cottenie. 2012. A metacommunity framework for enhancing the effectiveness of biological monitoring strategies. Plos One 7:e43626. https://doi.org/10.1371/journal.pone.0043626. [ Links ]
60. Strickland, J. D. H., and T. R. Parsons. 1960. A manual of seawater analysis. Fisheries Research Board of Canada, Ottawa. [ Links ]
61. Tonin, A. M., L. U. Hepp, and J. F. Jr. Gonçalves. 2017. Spatial Variability of Plant Litter Decomposition in Stream Networks: from Litter Bags to Watersheds. Ecosystems 21:567-581. https://doi.org/10.1007/s10021-017-0169-1. [ Links ]
62. Uieda, V., and E. Carvalho. 2015. Experimental manipulation of leaf litter colonization by aquatic invertebrates in a third order tropical stream. Brazilian journal of biology 75:405-13. https://doi.org/10.1590/1519-6984.15013. [ Links ]
63. Whittaker, R. H. 1956. Vegetation of the Great Smoky Mountains. Ecological Monographs 26:1-80. https://doi.org/10.2307/1943577. [ Links ]
64. Whittaker, R. H. 1960. Vegetation of the Siskiyou Mountains, Oregon and California. Ecological Monographs 30:279- 338. https://doi.org/10.2307/1948435. https://doi.org/10.2307/1943563. [ Links ]
65. Whittaker, R. H. 1972. Evolution and Measurement of Species Diversity. Taxon 21:213-251. https://doi.org/10.2307/1218190. [ Links ]
66. Wiens, J. A. 1989. Spatial Scaling in Ecology. Functional Ecology 3:385-397. https://doi.org/10.2307/2389612. [ Links ]
67. Yoshimura, C., K. Tockner, T. Omura, and O. Moog. 2006. Species diversity and functional assessment of macroinvertebrate communities in Austrian rivers. Limnology 7:63-74. https://doi.org/10.1007/s10201-006-0170-4. [ Links ]














