Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Ecología austral
versión On-line ISSN 1667-782X
Ecol. austral vol.30 no.1 Córdoba abr. 2020
ARTÍCULO
Effects of an altitudinal gradient on benthic macroinvertebrate assemblages in two hydrological periods in a Neotropical Andean river
Raúl García-Ríos*; Dieison A. Moi & Oscar E. Peláez
Programa de Pós-Graduação em Ecologia de Ambientes Aquáticos Continentais (PEA), Universidade Estadual de Maringá (UEM), Maringá, Paraná, Brazil.
ABSTRACT
The altitudinal gradient and the hydrological dynamics affect the establishment and distribution of organisms in Andean rivers. Benthic macroinvertebrates, an essential component of lotic ecosystems, are useful to assess the influence of the altitudinal gradient and changes in environmental characteristics. We analyzed the effects of altitudinal gradient on benthic macroinvertebrates assemblages in the Chillón River (Lima, Perú), and tested two hypotheses: 1) taxa richness decreases with increasing altitude, while dominance increases, and 2) altitude is the primary driver of variation in community composition. We found 47 taxa, being the class Insecta (Arthropoda) the richest and the densest in both hydrological periods. Taxa richness had a negative linear relation with altitude, while dominance had a positive linear relation. The RDA showed that communities varied along the altitudinal gradient. Our results showed that altitude is the main driver of variation in taxa composition; however, more studies must be carried out to understand what factors are essential in organism’s distribution and how they operate in Andean lotic ecosystems.
Keywords: Species distribution; High-altitude rivers; Elevational patterns; Freshwater invertebrates.
RESUMEN
Efectos del gradiente altitudinal sobre las comunidades de macroinvertebrados bentónicos en dos períodos hidrológicos en un río altoandino neotropical.
El gradiente altitudinal y la dinámica hidrológica afectan el establecimiento y la distribución de organismos en los ríos altoandinos. Los macroinvertebrados bentónicos, un componente esencial en los ecosistemas lóticos, son útiles para evaluar la influencia del gradiente altitudinal y los cambios en las características ambientales. Analizamos los efectos del gradiente altitudinal sobre las comunidades de macroinvertebrados bentónicos del Río Chillón (Lima, Perú) y pusimos a prueba dos hipótesis: 1) la riqueza de taxones disminuye con el aumento de la altitud, mientras que la dominancia aumenta, y 2) la altitud es el factor principal de la variación en la composición de la comunidad. Registramos 47 taxones, siendo la clase Insecta (Arthropoda) el más diverso y con alta densidad en ambos períodos hidrológicos. La riqueza de taxones tuvo una relación lineal negativa con la altitud, mientras que la dominancia mostró una relación lineal positiva. El análisis de redundancia evidenció que las comunidades bentónicas variaron a lo largo del gradiente altitudinal. Nuestros resultados muestran que la altitud es el principal conductor de la variación en la composición de taxones; sin embargo, sería necesario realizar más estudios para dilucidar qué factores son esenciales en la distribución de organismos y como operan en ecosistemas lóticos andinos.
Palabras clave: Distribución de especies; Ríos de alta montaña; Patrones de elevación; Invertebrados dulceacuícolas.
Recibido: 28 de Junio de 2019
Aceptado: 5 de Noviembre de 2019
Editora asociada: Irina Izaguirre
INTRODUCTION
Andean rivers are located at more than 2000 m a. s. l. (Acosta et al. 2009), governed by a marked hydrological dynamics constituted by a fast and turbulent water flux (Jacobsen 2008), which makes it difficult for the establishment and migration of organisms (Brown et al. 2007). The altitudinal gradient also plays a crucial role in organisms’ distribution (Jacobsen 2003, 2004, 2008; Jacobsen and Brodersen 2008) and determines the physicochemical water variability (Jacobsen et al. 1997; Carrera and Gunkel 2003). Along with strong environmental gradients driven by altitude and topography, spatial isolation makes Andean rivers to exhibit a high number of endemic species (Särkinen et al. 2012). For that reason, these ecosystems are considered biodiversity hotspots (Myers et al. 2000).
Several studies show that species richness decreases at local and regional scales with increasing altitude (Jacobsen 2003, 2004; Rahbek 2005). One possible explanation for this reduction is the Andean rivers hydrological dynamics, determined by glacial melting and rains, which produces catastrophic effects on aquatic communities (Ríos-Touma et al. 2012). The fast and turbulent water flux during rainy season removes benthic organisms, and then, just the adapted ones can persist (Lytle and Poff 2004). Changes in water physicochemical variables also influence species richness. Water temperature and oxygen saturation affect the benthic macroinvertebrates communities (Jacobsen 2008). These two variables decrease with increasing altitude, influencing the survival of the organisms (Jacobsen et al. 2003). As well as affecting oxygen solubility, the temperature is a critical environmental variable determining the organisms’ metabolic rate (Culler et al. 2014). Despite oxygen solubility increases with decreasing temperature, oxygen availability decreases with altitude due to reducing atmospheric pressure and the water viscosity (Jacobsen 1998), which leads to the selection of organisms adapted to live in low oxygen levels (Jacobsen et al. 2003), being benthic macroinvertebrates the most representative group.
Benthic macroinvertebrates inhabit the sediment and other substrates on the bottom of the freshwater ecosystems, being the main groups found in these environments flatworms, annelids, mollusks, crustaceans, and insects (APHA 2012). They are essential components in lotic ecosystems, are a nexus in the transference of matter and energy from producers (e.g., periphyton) to superior consumers (e.g., fish) (Hussain and Pandit 2012), and have life cycles synchronized with hydrologic dynamics (e.g., reproduction and growth), mainly aquatic insects (Lytle and Poff 2004). In addition, benthic macroinvertebrates are an excellent tool to assess the influence of the altitudinal gradient and changes in environmental characteristics (Muhlfeld et al. 2011; Khamis et al. 2014). For example, it has been registered a decrease in the richness of sensitive species to oxygen deficiency (Ephemeroptera, Plecoptera, and Trichoptera (EPT)), and an increase of tolerant organisms (Oligochaeta and Chironomidae) with the increase of altitude (Jacobsen 1998, 2003).
In this work, we analyze the effects of the altitudinal gradient on the diversity and composition of the benthic macroinvertebrates community at two hydrological seasons. Also, we analyze the influence of environmental variables along an altitude gradient on benthic macroinvertebrates community composition. We test the following hypothesizes: 1) taxa richness decreases with increasing altitude, while dominance increases, and 2) altitude is the main driver of variation in community composition. The first hypothesis implies that, as altitude increases, Shannon index and evenness decrease. The second hypothesis is based on the assumption that some taxa are capable of tolerating low oxygen levels and fast water flux due to the altitudinal gradient and hydrodynamic features of the area. These conditions will be the drivers of the differences in species composition of the communities at lower and higher altitudes.
MATERIALS AND METHODS
Study area
The study was carried out in the high-altitude zone of the Chillón River (11° 28’ S - 76° 38’ W; 11° 24’ S - 76° 25’ W), which is located in the high mountain region of Canta (Lima, Perú), with an area of 456.43 km2 and a range altitude between 2500 and 4850 m a. s. l. (Figure 1). This river section has an irregular and turbulent water flux, with two hydrological periods: dry (June - November) and rainy (December - May). Geographically, this area is encompassed in two ecological regions: highlands steppes (from 1000 to 3500 m a. s. l., with riparian vegetation composed of trees and shrubs) and puna grasslands (above 3500 m a. s. l., with cold temperatures, intense solar radiation, and riparian vegetation dominated by poaceous plants).

Figure 1. Geographic location of sampling points at the high-altitude zone of Chillón River (Lima, Perú).
Figura 1. Localización geográfica de los puntos de muestreo en la zona alta del Río Chillón (Lima, Perú).
Field sampling and samples analysis
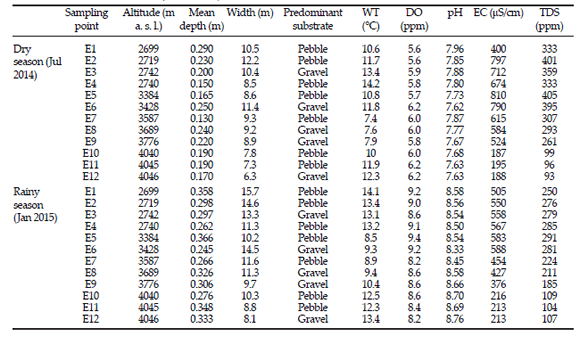
We established 12 points along the main channel, downstream of tributaries, considering the altitude range (2600 to 4100 m a. s. l.), its accessibility and performed the samplings in dry (July 2014) and rainy season (January 2015). In general, all the samplings points did not suffer the direct impact of anthropic activities, although there are fish farming activities. At each point, we measured water physicochemical variables such as water temperature (°C), dissolved oxygen (DO; ppm), pH, electrical conductivity (EC; μS/cm), and total dissolved solids (TDS; ppm), using a multiparameter probe (HANNA model HI 98129). We used data of water level and flux measured from June 2014 to May 2015 by the Servicio Nacional de Meteorología e Hidrología del Perú (SENAMHI), which has two hydrological stations in Chillón River at the villages Obrajillo and Pariacancha (2706 and 3842 m a. s. l. respectively). These data are available at snirh.ana.gob.pe/SCCRH. In addition, we registered environmental variables as river depth and width and predominant substrates to perform an environmental characterization.
We collected benthic macroinvertebrates using a Surber bottom sampler (0.09 m2 and 500 μm net size), which is designed for shallow watercourses less than 50 cm deep (Merritt et al. 2008; APHA 2012). Considering two hydrological seasons, we obtained 24 samples after sampling 0.27 m2 of the rocky substrate, mainly pebble and gravel, which are the highly dominant and primary focus of benthic macroinvertebrates colonization (Domínguez and Fernández 2009). We placed the samples in 500 mL plastic pots, fixed with 70% alcohol. Benthic macroinvertebrates identification was performed using a stereomicroscope to the lowest possible taxonomic level, following of specialized taxonomic keys (Manzo 2005; Borkent and Spinelli 2007; Manzo and Archangelsky 2008; Merritt et al. 2008; Domínguez and Fernández 2009; Huamantinco and Ortiz 2010). All the classified organisms were labeled and deposited in the Departamento de Limnología, Museo de Historia Natural (MHN), Universidad Nacional Mayor de San Marcos (UNMSM), Lima, Perú.
Data analysis
We used a principal component analysis (PCA) to characterize the variation of environmental variables considering the two hydrological seasons (dry and rainy). All variables were transformed by logarithmic function (ln[x+1]), except pH. We used the function prcomp of the package stats (R Core Team 2017). To assess the effect of altitudinal gradient on environmental variables, we performed simple linear regressions, being the altitude the predictor variable, and depth, water temperature, pH, dissolved oxygen, electrical conductivity, and total dissolved solids the variables response.
To characterize the benthic macroinvertebrates community, we made a list of all the identified taxa. As identifying benthic macroinvertebrate to species level is difficult due to the lack of taxonomic keys to some groups, we obtained the values of taxa richness (not species richness) and benthic macroinvertebrates density (individuals/m2). Also, we analyzed the structure of benthic macroinvertebrates community by Shannon’s diversity (H’), evenness (J’), and Simpson’s dominance (D) indexes and their variation along the altitude gradient (2600 - 4100 m a. s. l.). We performed a Mann-Whitney U test using Statistica 7.0 (StatSoft Inc, USA) to test if there were statistically significant differences (P<0.05) between dry and rainy seasons. We used the function diversity from the vegan package (Oksanen et al. 2017) to calculate all diversity indexes.
To assess the effect of altitudinal gradient on benthic macroinvertebrates community, we performed simple linear regressions, being the altitude the predictor variable, and taxa richness, density, Shannon index, evenness, and dominance the variables response. The density of organisms was transformed by a logarithmic function (ln[x+1]) to achieve the assumptions of linear regression. We used the Shapiro-Wilk test to assess the residual normality. In addition, we analyzed the residuals homogeneity graphically.
Finally, we conducted a Redundancy Analysis (RDA) to investigate whether the variation in community composition was related to environmental variables. As most of the taxa showed a linear relationship with altitude, RDA is the most appropriate analysis for our data (Legendre and Gallagher 2001). Thus, the first two PCA axes were used as descriptors of environmental variability and the density of organisms (log-transformed) as the response variables. The RDA analysis was carried out using the function ‘rda’ of vegan package (Oksanen et al. 2017). The significance of the RDA axes describing the relationship between environment and communities was tested through 499 Monte Carlo permutations, considering P<0.05. This permutation test was performed with the function ‘permutest’ of package vegan (Oksanen et al. 2017).
RESULTS
Environmental characterization
In general, water temperature, electrical conductivity, and total dissolved solids did not differ markedly between rainy and dry seasons (Table 1). However, mean values of pH in rainy season were slightly higher than in dry season (Table 1). Dissolved oxygen values were higher in the rainy season (mean 8.8±0.4 SD) than in the dry one (mean 5.9±0.2 SD) (Table 1). Water level and flux were lower in July 2014 than in January 2015, showing a remarkable difference between dry and rainy seasons (Figure S1). Regarding the effect of altitudinal gradient on environmental variables, all measured variables had a negative linear relation with altitude (Figure S2).
Table 1. Environmental variables and physicochemical water parameters by sampling points and hydrological seasons in the high-altitude zone of the Chillón River (Lima, Perú).
Tabla 1. Variables ambientales y parámetros fisicoquímicos del agua para cada punto de muestreo y período hidrológico en la zona alta del Río Chillón (Lima, Perú).
The PCA ordination showed the environmental variability among seasons and along the elevational gradient (Figure 2). The first axes of PCA (PC1 and PC2) explained 45.20% and 32.23% of the environmental variation, respectively. The first axis (PC1) was correlated positively with altitude (0.41), depth (0.74), pH (0.82), and DO (0.79), and negatively with EC (-0.73) and TDS (-0.76). On the other hand, the second axis was correlated positively with depth (0.49), pH (0.49), EC (0.59), TDS (0.59), and DO (0.49), and negatively with altitude (-0.83). In addition, the sampling points ordinated closer together in the PCA plot in rainy season than in dry season, indicating a homogenization of the environmental variables in the rainy period.

Figure 2. Principal component analysis (PCA) of sampling points in the highaltitude zone of Chillón River (Lima, Perú) using environmental variables. Alt=altitude (m a. s. l.); Temp=water temperature (°C); DO: dissolved oxygen (ppm); EC=electrical conductivity (μS/cm); TDS=total dissolved solids (ppm).
Figura 2. Análisis de componentes principales (ACP) de los puntos de muestreo en la zona alta del Río Chillón (Lima, Perú) usando las variables ambientales. Alt=altitud (m s. n. m.); Temp=temperatura del agua (°C); DO: oxígeno disuelto (ppm); EC=conductividad eléctrica (μS/cm); TDS=sólidos disueltos totales (ppm).
Benthic macroinvertebrates community structure
We found 47 benthic macroinvertebrates taxa, of which one was identified at the level of phylum, 7 to class, 1 to subclass, 10 to family, 10 to subfamily, and 18 to genus (Table 2). The class Insecta (Arthropoda) was the richest (35 taxa) and presented high values of density in both hydrological seasons. Within this class, the order Diptera was the richest (17 taxa) and the most abundant (73556 individuals/ m2). According to the Mann-Whitney test, taxa richness, the density of organisms and evenness differed between dry and rainy seasons (U=24.5, P=0.0045; U=2.0, P<0.001; U=23, P=0.004; respectively). In general, the dry season presented the highest values of taxa richness (S=46, mean 23.83±4.45 SD) and density of organisms (98381.48 individuals/ m2, mean 8198.46±4178.96 SD) (Figure S3), while evenness was the highest in rainy season (mean 0.565±0.089 SD) (Figure S3).
Table 2. Benthic macroinvertebrates community taxa composition and total density of organisms (individuals/m2) collected in dry (July 2014) and rainy seasons (January 2015) from the high-altitude zone of the Chillón River (Lima, Perú).
Tabla 2. Composición taxonómica y densidad total de macroinvertebrados bentónicos colectados en las épocas seca (julio 2014) y lluviosa (enero 2015) en la zona alta del Río Chillón (Lima, Perú).

Effect of altitude on the benthic macroinvertebrates community
The attributes of the benthic macroinvertebrates community responded in different ways along the altitudinal gradient. Taxa richness, Shannon diversity and evenness had a negative linear relation with altitude (Radj=0.311, P<0.01; Radj=0.389, P<0.01; Radj=0.201, P<0.05, respectively Figures 3a, c, d). On the other hand, dominance presented a positive linear relation with altitude (Radj=0.366, P<0.01 Figure 3e). Density of organisms did not present relation with altitude (Rad=-0.039, P=0.723), with values oscillating along to altitudinal gradient (Figure 3b). In general, values of taxa richness, Shannon diversity, and evenness were highest at lower altitudes (Figures 3a, c, d). Conversely, values of dominance were highest at higher altitudes (Figure 3e).

Figure 3. Regression analysis between altitude and taxa richness (a), organisms density (b), Shannon index (c), evenness (d), and dominance (e) of benthic macroinvertebrates community in the high-altitude zone of Chillón River (Lima, Perú). Black circles represent sampling points at dry season; grey circles represent sampling points at rainy season.
Figura 3. Análisis de regresión entre altitud y riqueza de taxa (a), densidad de organismos (b), índice de Shannon (c), equitabilidad (d) y dominancia (e) de las comunidades de macroinvertebrados bentónicos en la zona alta del Río Chillón (Lima, Perú). Los círculos negros representan a los puntos de muestreo en la época seca; los círculos grises, en la época lluviosa.
The relationship between benthic macroinvertebrates community composition and environmental variables
The RDA showed that macroinvertebrates community varied along the altitudinal gradient (RDA1 36.6% of explained variance; F=3.44, P=0.02), as well as among seasons (RDA2 8.9% of explained variance; F=14.06, P<0.001) (Figure 4). Thus, the first RDA axis showed that communities varied in response to altitude (r=0.80), dissolved oxygen (r=- 0.51) and pH (r=-0.45). On the other hand, the second RDA axis showed the variation in composition among seasons in response to pH (r=0.76), dissolved solids (r=-0.74), and dissolved oxygen (r=-0.69). This variation in composition was related to changes in abundances of few taxa. At low altitudes of the sampling gradient, communities were characterized by having a higher density of Baetodes (Ephemeroptera) and Turbellaria, while high altitude communities had a higher density of Chironomidae, mainly Orthocladiinae (Diptera).

Figure 4. a) Redundancy analysis (RDA) showing sampling points in relation to environmental variables and benthic macroinvertebrates in two hydrological periods. Taxa abbreviations in (a): Hydro=Hydrozoa; Turb=Turbellaria; Ostra=Ostracoda; Ande=Andesiops sp.; Baet=Baetodes sp.; Neo=Neoelmis sp.; Podo=Podonominae; Ortho=Orthocladiinae.
Figura 4. a) Análisis de redundancia (RDA) mostrando los puntos de muestreo en relación a las variables ambientales y los macroinvertebrados bentónicos en ambos períodos hidrológicos. Abreviaturas de los grupos taxonómicos en (a): Hydro=Hydrozoa; Turb=Turbellaria; Ostra=Ostracoda; Ande=Andesiops sp.; Baet=Baetodes sp.; Neo=Neoelmis sp.; Podo=Podonominae; Ortho=Orthocladiinae.
DISCUSSION
Our results showed changes in macroinvertebrates community (richness, Shannon, evenness, and dominance), which were driven by the environmental gradient related to the altitude, confirming our first hypothesis. Thus, while richness and Shannon decreased, dominance increases with altitude, also validating our second hypothesis. This fact agrees with Jacobsen (2004), who analyzed the Andean rivers of Ecuador and showed a notable decrease in invertebrate orders (e.g., Diptera, Coleoptera, Trichoptera) with increasing altitude. Likewise, many studies have shown a monotonic reduction of benthic macroinvertebrates diversity with the altitude (e.g., Kuhn et al. 2011; Loayza-Muro et al. 2013). Another possible explanation to low macroinvertebrates diversity is food availability, which usually decreases because primary productivity has a negative relationship with an increase in altitude (Simpson 1983). Andean rivers at higher altitudes are surrounded by small vegetation, mainly gramineous plants, which do not support exogenous organic matter; while in low and middle elevations, there are shrubs and trees, providing organic matter like decaying leaves and so more food availability to macroinvertebrates (Jacobsen 2008). Previous theoretical and experimental studies had shown a positive relationship between productivity and diversity (e.g., Tilman 1999; Tilman et al. 2001), which was affected by altitude in our study. Thus, our findings reinforce that this is a general pattern in ecology.
It is known that the reduction in dissolved oxygen availability with altitude affects sensitive taxa like Trichoptera and Ephemeroptera; however, the families Baetidae and Leptohyphidae (Ephemeroptera) tolerate a wide range of temperature and, to a certain point, organic pollution (Flowers and De la Rosa 2010). That would explain the dominance of Andesiops sp. (Baetidae) in the sampling points located at low altitudes, which might be influenced by domestic and fish farming wastewater. Also, high altitude can promote the dominance of some aquatic insects in the communities. For instance, we found several organisms with adaptations to low temperatures, as the family Chironomidae (Diptera), which has a freezing tolerance (Sømme and Zachariassen 1981), and with adaptations to fast water flux like Claudioperla sp., a genus that has strong tarsal claws (Froehlich 1969). Thus, it is suggested that high altitudes act as an environmental filter, selecting well-adapted species.
Furthermore, contrary to taxa richness, Shannon index, and evenness, organisms density did not suffer the effect of the altitudinal gradient. This may be explained by some tolerant species, such as the family Chironomidae, which replace the sensitive ones at higher altitude, decreasing the species diversity. Besides of Orthocladiinae, other organisms also were abundant at higher altitude, such as the class Oligochaeta (Annelida), the family Empididae (Diptera), and the genera Hyalella (Amphipoda), Meridialaris (Ephemeroptera), and Claudioperla (Plecoptera). These organisms would have contributed to an increase in dominance. On the other hand, several organisms were present at low and middle elevations, such as the genera Physa (Mollusca), Baetodes, and Trichorythodes (Ephemeroptera), Smicridea, Atopsyche, and Metrichia (Trichoptera), and Neoelmis (Coleoptera). This species distribution along the altitudinal gradient might be produced by oxygen availability, temperature, or an effect of both, or water flux (Crespo-Pérez et al. 2016). Indeed, Dos Santos et al. (2018) showed that the genera Andesiops and Baetodes are cold- and warmadapted, respectively. Thus, the temperature is a critical factor acting on Ephemeroptera species distribution and could affect the presence of other groups.
High altitude constitutes a barrier for species dispersal and imposes extreme environmental conditions to which some organisms cannot resist (Jacobsen 2004). In our study, for example, the distribution range of Baetodes sp. (Ephemeroptera) is restricted to altitudes lower than 2800 m a. s. l., which agrees with previous results obtained by Villamarín (2008) and Acosta (2009) for Andean streams in Ecuador and Perú. The genus Claudioperla was the unique organism belonging to the order Plecoptera reported in our study. Villamarín (2008) and Acosta (2009) also reported this genus with a distribution range restricted to Western Andes. Regarding the order Trichoptera, Acosta (2009) also found the same families in the high-altitude zone of the Cañete River (Lima), in the similar altitudinal gradient (2500-4500 m a. s. l.). The genus Anomalocosmoecus (Limnephilidae) was abundant at higher altitudes, which agrees with the results of Huamantinco and Ortiz (2010), who pointed out that the distribution of this genus is strongly influenced by the altitude, inhabiting rocky substrates. The genus Austrelmis (Elmidae, Coleoptera) was found along the altitudinal gradient. Acosta (2009) reported the same distribution range for this genus. In general, the orders Diptera, Coleoptera, and Trichoptera showed the highest richness (17, 7, and 6, respectively), in agreement with observations of Ríos-Touma (2008), Villamarín (2008), and Acosta (2009) in Andean lotic environments. Also, Balian et al. (2008) highlighted these three orders as the most representative in freshwater ecosystems.
In the Chillón River, environmental variables varied with the hydrological period, showing remarkable changes in water level at all sampling points in rainy season. This seasonal variation explained changes in composition between seasons, and it is typical of Andean rivers, where temporal patterns in water flux determine the structure and function of the fluvial ecosystem (Ríos-Touma 2008). It is known that glacial ice melting and rain in summer contribute to the sudden rise in water level, which is reflected in a turbulent water flux (Allan et al. 2006). This water flux at rainy season is crucial because it transports nutrients, substrate, and organisms across the stream (Death 2008). As evidence, we registered high values of electrical conductivity and total dissolved solids in the rainy season, which is the product of the erosion of the stream bed by water turbulence. On the other hand, during the dry season, the reduced water flux could isolate some stream sections, changing the environmental variables and enhancing the environmental heterogeneity (Allan et al. 2006), which was evidenced by the difference in water temperature and total dissolved solids between samplings points at low and high altitudes. For that reason, we argue that rainy season acts as a natural disturbance, affecting environmental characteristics (Lake 2003), and combined with altitude produces an environmental gradient.
Overall, our results have important implications for the understanding of the diversity patterns and organisms distribution in high altitude stream, showing that the altitude is the main driver of diversity and variation in composition for the macroinvertebrates communities. Nevertheless, seasonal variation also leads to changes in community composition over time. That is, although communities mainly differed in the altitudinal gradient, they also were dissimilar among seasons due to the influence of environmental factors. Our findings show that at spatial scale, the altitudinal gradient plays a vital role in environmental features and water physicochemical, affecting richness, abundance and distribution range of benthic macroinvertebrates. At temporal scale, the change in water level and flux had a significant influence on water physicochemical and habitat stability, being the dry season the most stable, when we found the highest values of taxa richness and abundance. However, more studies would be necessary to elucidate what factors are essential in organisms distribution and how they operate according to the scale (local or regional) in Andean lotic ecosystems.
ACKNOWLEDGMENTS
We thank I. Samanez and H. Ortega for providing sampling devices and J. Pereira for drawing up the map of the study area. We are also very grateful to D. García, J. Meléndez, A. Romaní, and L. Valdez for providing sampling support. This research was funded by the authors’ resources.
REFERENCES
1. Acosta, R. 2009. Estudio de la Cuenca altoandina del río Cañete (Perú): Distribución altitudinal de la comunidad de macroinvertebrados bentónicos y caracterización hidroquímica de sus cabeceras cársticas. Tesis Doctoral. Universitat de Barcelona. [ Links ]
2. Acosta, R., B. Ríos-Touma, M. Rieradevall, and N. Prat. 2009. Propuesta de un protocolo de evaluación de la calidad ecológica de ríos altoandinos (CERA) y su aplicación a dos cuencas en Ecuador y Perú. Limnetica 28:35-64. [ Links ]
3. Allan, J. D., A. S. Flecker, S. Segnini, D. C. Taphorn, E. Sokol, and G. W. Kling. 2006. Limnology of Andean piedmont rivers of Venezuela. Journal of the North American Benthological Society 25:66-81. https://doi.org/10.1899/0887-3593(2006)25[66:LOAPRO]2.0.CO;2. [ Links ]
4. APHA, WEF, AWWA. 2012. Standard Methods for the Examination of Water and Wastewater. 22th Edition. American Public Health Association. Washington, D.C. [ Links ]
5. Balian, E. V., H. Segers, C. Lévèque, and K. Martens. 2008. The Freshwater Animal Diversity Assessment: an overview of the results. Hydrobiologia 595:627-637. https://doi.org/10.1007/s10750-007-9235-6. https://doi.org/10.1007/s10750-007-9246-3. https://doi.org/10.1007/978-1-4020-8259-7_61. [ Links ]
6. Borkent, A., and G. R. Spinelli. 2007. Neotropical Ceratopogonidae (Diptera: Insecta). Aquatic Biodiversity in Latin America (ABLA). Vol. 4. Pensoft, Sofia-Moscow. [ Links ]
7. Brown, L. E., D. M. Hannah, and A. M. Milner. 2007. Vulnerability of alpine stream biodiversity to shrinking glaciers and snowpacks. Global Change Biology 13:958-966. https://doi.org/10.1111/j.1365-2486.2007.01341.x. [ Links ]
8. Carrera, P., and G. Gunkel. 2003. Ecology of a high Andean stream, Rio Itambi, Otavalo, Ecuador. Limnologica 33: 29-43. https://doi.org/10.1016/S0075-9511(03)80005-1. [ Links ]
9. Crespo-Pérez, V., P. Andino, R. Espinosa, O. Dangles, and D. Jacobsen. 2016. The altitudinal limit of Leptohyphes Eaton, 1882 and Lachlania Hagen, 1868 (Ephemeroptera, Leptohyphidae, Oligoneuriidae) in Ecuadorian Andes streams: searching for mechanisms. Aquatic Insects 37:1-18. https://doi.org/10.1080/01650424.2015.1109128. [ Links ]
10. Culler, L. E., M. A. McPeek, and M. P. Ayres. 2014. Predation risk shapes thermal physiology of a predaceous damselfly. Oecologia 176:653-660. https://doi.org/10.1007/s00442-014-3058-8. [ Links ]
11. Death, R. G. 2008. The effect of floods on aquatic invertebrate communities. Pp. 103-121 in J. Lancaster and R. A. Briers (eds.). Aquatic Insects: Challenges to Populations. CAB International, Wallingford, UK. https://doi.org/10.1079/9781845933968.0103. [ Links ]
12. Domínguez, E., and H. R. Fernández. 2009. Macroinvertebrados Bentónicos Sudamericanos. Sistemática y Biología. Fundación Miguel Lillo, Tucumán, Argentina. [ Links ]
13. Dos Santos, D. A., C. Molineri, C. Nieto, M. C. Zuñiga, D. Emmerich, P. Fierro, P. Pessacq, B. Ríos-Touma, J. Márquez, D. Gomez, F. F. Salles, A. C. Encalada, R. Príncipe, G. C. Gómez, C. Valdovinos Zargues, and E. Domínguez. 2018. Cold/Warm stenothermic freshwater macroinvertebrates along altitudinal and latitudinal gradients in Western South America: A modern approach to an old hypothesis with updated data. Journal of Biogeography 45:1571-1581. https://doi.org/10.1111/jbi.13234. [ Links ]
14. Flowers, R. W., and C. De la Rosa. 2010. Ephemeroptera. Revista de Biología Tropical 58:63-93. [ Links ]
15. Froehlich, C. G. 1969. Studies on Brazilian Plecoptera 1. Some Gripopterygidae from the Biological Station at Paranapiacaba, State of São Paulo. Beitr Neotrop Fauna 6:17-39. https://doi.org/10.1080/01650526909360412. [ Links ]
16. Huamantinco, A., and W. Ortiz. 2010. Clave de géneros de larvas de Trichoptera (Insecta) de la Vertiente Occidental de Los Andes, Lima, Perú. Revista Peruana de Biología 17:075-080. https://doi.org/10.15381/rpb.v17i1.54. [ Links ]
17. Hussain, Q. A., and A. K. Pandit. 2012. Macroinvertebrates in streams: A review of some ecological factors. Int J Fish Aquac 4:114-123. https://doi.org/10.5897/IJFA11.045. [ Links ]
18. Jacobsen, D. 1998. The effect of organic pollution on the macroinvertebrate fauna of Ecuadorian highland streams. Archiv für Hydrobiologie 143:179-195. https://doi.org/10.1127/archiv-hydrobiol/143/1998/179. [ Links ]
19. Jacobsen, D. 2003. Altitudinal changes in diversity of macroinvertebrates from small streams in the Ecuadorian Andes. Archiv für Hydrobiologie 158:145-167. https://doi.org/10.1127/0003-9136/2003/0158-0145. [ Links ]
20. Jacobsen, D. 2004. Contrasting patterns in local and zonal family richness of stream invertebrates along Andean altitudinal gradient. Freshwater Biology 49:1293-1305. https://doi.org/10.1111/j.1365-2427.2004.01274.x. [ Links ]
21. Jacobsen, D. 2008. Low oxygen pressure as a driving factor for the altitudinal decline in taxon richness of stream macroinvertebrates. Oecologia 154:795-807. https://doi.org/10.1007/s00442-007-0877-x [ Links ]
22. Jacobsen, D., and K. P. Brodersen. 2008. Are altitudinal limits of equatorial stream insects reflected in their respiratory performance? Freshwater Biology 53:2295-2308. https://doi.org/10.1111/j.1365-2427.2008.02050.x. [ Links ]
23. Jacobsen, D., S. Rostgaard, and J. J. Vásconez. 2003. Are macroinvertebrates in high altitude streams affected by oxygen deficiency? Freshwater Biology 48:2025-2032. https://doi.org/10.1046/j.1365-2427.2003.01140.x. [ Links ]
24. Jacobsen, D., R. Schultz, and A. Encalada. 1997. Structure and diversity of stream invertebrate assemblages: the influence of temperature with altitude and latitude. Freshwater Biology 38:247-261. https://doi.org/10.1046/j.1365-2427.1997.00210.x. [ Links ]
25. Khamis, K., D. M. Hannah, L. E. Brown, R. Tiberti, and A. M. Milner. 2014. The use of invertebrates as indicators of environmental change in alpine rivers and lakes. Science of the Total Environment 493:1242-1254. https://doi.org/10.1016/j.scitotenv.2014.02.126. [ Links ]
26. Kuhn, J., P. Andino, R. Calvez, R. Espinosa, L. Hamerlik, S. Vie, O. Dangles, and D. Jacobsen. 2011. Spatial variability in macroinvertebrate assemblages along and among neighbouring equatorial glacier-fed streams. Freshwater Biology 56:2226-2244. https://doi.org/10.1111/j.1365-2427.2011.02648.x. [ Links ]
27. Lake, P. S. 2003. Ecological effects of perturbation by drought in flowing waters. Freshwater Biology 28:1161-1172. https://doi.org/10.1046/j.1365-2427.2003.01086.x. [ Links ]
28. Legendre, P., and E. D. Gallagher. 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129:271-280. https://doi.org/10.1007/s004420100716. [ Links ]
29. Loayza-Muro, R. A., M. L. de Baat, E. J. Palomino, P. Ku Perus, M. H. S. Kraak, W. Admiraal, and J. A. J. Breeuwer. 2013. Metals and altitude drive genetic diversity of chironomids in Andean streams. Freshwater Biology 59:56-63. https://doi.org/10.1111/fwb.12245. [ Links ]
30. Lytle, D. A., and N. L. Poff. 2004. Adaptation to natural flow regimes. Trends in Ecology and Evolution 19:94-100. https://doi.org/10.1016/j.tree.2003.10.002. [ Links ]
31. Manzo, V. 2005. Key to the South America genera of Elmidae (Insecta: Coleoptera) with distributional data. Studies on Neotropical Fauna and Environment 40:201-208. https://doi.org/10.1080/01650520500140619. [ Links ]
32. Manzo, V., and M. Archangelsky. 2008. A key to known larvae of South American Elmidae (Coleoptera: Byrrhoidea), with a description of the mature larva of Macrelmis saltensis Manzo. Annales de Limnologie - International Journal of Limnology 44:63-74. https://doi.org/10.1051/limn:2008023. [ Links ]
33. Merritt, R. W., K. W. Cummins, and M. B. Berg. 2008. An Introduction to the Aquatic Insects of North America. Fourth Edition. Kendall/Hunt. Co. [ Links ]
34. Muhlfeld, C. C., J. J. Giersch, F. R. Hauer, G. T. Pederson, G. Luikart, D. P. Peterson, C. C. Downs, and D. B. Fagre. 2011. Climate change links fate of glaciers and an endemic alpine invertebrate. Climatic Change 106:337-345. https://doi.org/10.1007/s10584-011-0057-1. [ Links ]
35. Myers, N., R. A. Mittermeier, C. G. Mittermeier, G. A. B. da Fonseca, and J. Kent. 2000. Biodiversity hotspots for conservation priorities. Nature 403:853-858. https://doi.org/10.1038/35002501. [ Links ]
36. Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs, and H. Wagner. 2017. vegan: Community Ecology Package. R package version 2.4-4. URL: CRAN.R-project.org/package=vegan.
37. R Development Core Team. 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: www.R-project.org. [ Links ]
38. Rahbek, C. 2005. The role of spatial scale and the perception of large-scale species-richness patterns. Ecology Letters 8:224-239. https://doi.org/10.1111/j.1461-0248.2004.00701.x. [ Links ]
39. Ríos-Touma, B. 2008. Comunidades de macroinvertebrados en un río altoandino: importancia del microhábitat, dinámica de la deriva, papel de la materia orgánica y relevancia de la ovoposición. Tesis Doctoral. Universitat de Barcelona. [ Links ]
40. Ríos-Touma, B., A. C. Encalada, and N. Prat. 2012. Leaf litter dynamics and its use by invertebrates in a high-altitude Andean stream. International Review of Hydrobiology 94:357-371. https://doi.org/10.1002/iroh.200811161. [ Links ]
41. Särkinen, T., R. T. Pennington, M. Lavin, M. F. Simon, and C. E. Hughes. 2012. Evolutionary islands in the Andes: persistence and isolation explain high endemism in Andean dry tropical forests. Journal of Biogeography 39:884-900. https://doi.org/10.1111/j.1365-2699.2011.02644.x. [ Links ]
42. Simpson, B. 1983. A historical phytogeography of the Andean flora. Revista Chilena de Historia Natural 56:109-122. [ Links ]
43. Sømme, L., and K. E. Zachariassen. 1981. Adaptations to low temperature in high altitudes insects from Mount Kenya. Ecological Entomology 6:199-204. https://doi.org/10.1111/j.1365-2311.1981.tb00606.x. [ Links ]
44. Tilman, D. 1999. The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80: 1455-1474. https://doi.org/10.2307/176540. https://doi.org/10.1890/0012-9658(1999)080[1455:TECOCI]2.0.CO;2. [ Links ]
45. Tilman, D., P. B. Reich, J. Knops, D. Wedin, T. Mielke, and C. Lehman. 2001. Diversity and productivity in a long-term grassland experiment. Science 294:843-845. https://doi.org/10.1126/science.1060391. [ Links ]
46. Villamarín, C. P. 2008. Estructura y composición de las comunidades de macroinvertebrados en ríos altoandinos de Ecuador y Perú. Diseño de un sistema de medida de la calidad del agua con índices multimétricos. Tesis Doctoral. Universitat de Barcelona. [ Links ]














