Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agriscientia
On-line version ISSN 1668-298X
Agriscientia vol.24 no.1 Córdoba Jan./June 2007
Starch molecular fractionation of bread wheat varieties
Corcuera, V.; E. M. Salmoral, J. C. Salerno and C. R. Krisman
V. Corcuera. Instituto Fitotécnico Sta. Catalina (FCAyF, UNLP) y CIGEN (CONICET-UNLP-CIC) . E. M. Salmoral y C. R. Krisman. Grupo de Ingeniería Bioquímica, Departamento de Química, Facultad de Ingeniería, Universidad de Buenos Aires. Paseo Colón 850 (1063) Buenos Aires, Argentina. J. C. Salerno. Instituto de Genética Dr. Ewald Favret (IGEAF, CNIA-Castelar, Bs. As). Correspondencia a C. R. Krisman: gib@ fi. uba. ar
Abstract
The starch composition of bread making wheat seeds (Triticum aestivum subsp. vulgare) of the Argentine commercial varieties Buck Charrua, Buck Ombú, Buck Guaraní, Buck Catriel and Buck Poncho was analyzed by two different methods. One of these depends on the differential solubility of amylose and amylopectin in a water:butanol mixture whilst the other process is based on the use of the lectin Concanavalin A. These methods were complemented by spectrophotometric determinations to enable the identification of the á-D- glucanes and also improved the comparative quantitation of the amylose and amylopectin fractions. As a result of this, no significant variations for starch content (ANOVA, F4- 8= 0.7; p ≥ 0.05) were found among these varieties, although strong differences were found for amylose (ANOVA, F4- 8= 44.4; p ≥ 0.01) and amylopectin content (ANOVA, F4- 8= 77.1; p ≥ 0.01). These results and the fact that no differences were found for amylose (ANOVA, F2- 8= 0.3) and amylopectin among years within the same variety (ANOVA, F2- 8:0.8) at p ≥0.01 led to the conclusion that the diverse properties and end-uses of the starch mainly depend on the genotype, and that starch quality is null or scarcely influenced by the environment. This knowledge must be taken into account for wheat breeding purposes.
Keywords: Wheat; Amylopectin; Amylose; Starch fractionation.
Corcuera, V.; E. M. Salmoral, J. C. Salerno y C. R. Krisman, 2007. Fraccionamiento molecular del almidón de variedades trigo pan. Agriscientia XXIV (1): 11-18
Resumen
Se analizó la composición del almidón de granos de trigo pan (Triticum aestivum subsp. vulgare) de las variedades comerciales argentinas Buck Charrúa, Buck Ombú, Buck Guaraní, Buck Catriel y Buck Poncho mediante dos métodos diferentes. Uno de ellos depende de la solubilidad diferencial de la amilosa y amilopectina en una mezcla de agua:butanol, mientras que el otro proceso está basado en el uso de la lectina Concanavalina A. Estos métodos fueron complementados mediante determinaciones espectrofotométricas que facilitaron la identificación de los á-D-glucanos y también permitieron mejorar la cuantificación comparativa de las fracciones amilosa y amilopectina. Los resultados obtenidos indican que no existen diferencias significativas entre variedades para contenido de almidón (ANOVA, F4- 8: 0.7; p: 0.05), aunque si se hallaron fuertes diferencias para el contenido de amilosa (ANOVA, F4- 8: 44.4; p: 0.01) y amilopectina (ANOVA, F4- 8: 77.1; p: 0.01). Estos resultados y el hecho de que para una misma variedad no se encontraron diferencias entre años para el contenido de amilosa (ANOVA, F2- 8: 0.3) ni amilopectina (ANOVA, F2- 8: 0.8) permiten afirmar que las diversas propiedades y uso final del almidón dependen del genotipo y que la calidad está nula o escasamente influenciada por el medio ambiente. Este conocimiento debe ser tenido en cuenta con fines de mejoramiento genético.
Palabras clave: Trigo; Amilopectina; Amilosa; Fraccionamiento de almidón.
Fecha de recepción: 04/12/06; fecha de aceptación: 22/05/07
Abreviations: WS: water soluble; BS: butanol soluble; BI: butanol insoluble; LSD: least significative difference; ConA: Concanavalin A; GBSS: granular bound starch synthase
Introduction
Wheat is one of the most widely cultivated crops in the world. All the known wheats, cultivated or not, are included within the genus Triticum that comprises 16 different species. These species may be classified into three groups according to their chromosome number: diploids (14 chromosomes), tetraploids (28 chromosomes) and hexaploids (42 chromosomes). The hexaploid bread wheat varieties grown are botanically known as Triticum aestivum subsp. v u l g a r e. Genetic improvement through decades has contributed with high quality bread wheat varieties in Argentina, worldwide famous for their excellent baking qualities.
The relative content and chemical composition of wheat starch, used in food and non-food industries (Rodriguez-Quijano et al., 2003), affect the quality of wheat derived products. Not only diversity but also composition and physical parameters give rise to diverse processing properties and therefore to many end-uses for starch (Burrell, 2003). Whereas in the old days it was thought that proteins were responsible for pasta quality, nowadays it is accepted that starch can also be a quality determining component of pasta (Burrell, 2003).
Amylose and amylopectin are the two major glucopolysaccharide components of starch. Amylose is a long linear polymer with D-glucosyl units linked by α- D - ( 1→4) glycosidic linkages, although there is now evidence that amylose is not completely linear (Curá et al., 1995).
On the other hand, amylopectin has a branched structure with some thousands of poly-glucose residues linked by α- D - ( 1→4) and α- D - ( 1→6) glycosidic linkages. The physicochemical properties of the starch depend on its amylose:amylopectin ratio.
Performing an optimum starch fractionation method usually offers difficulties that must be overcome and the accuracy of the results relies on the correct adaptation of the method to the nature of the species studied. The use of starch molecular fractionation methods gives an opportunity to obtain a better knowledge of starch components and of the amylose: amylopectin ratio. In the first instance, it is necessary to determine the starch composition through more than one fractionation method to determine which one is the most efficient for routine analysis.
For this reason, the goal of the present work was: a) to evaluate the starch composition of five wellknown and widely cultivated commercial bread wheat varieties of our country and b) to determine the efficiency of the methods used by means of the comparison of their results.
One of the methods used, is based on the differential solubility of starch components in a waterbutanol saturated solution as described by Schoch and Maywald and later modified by Curá and Krisman (1990). The other method consists on complexing the starch molecule with the protein Concanavalin A from the vegetal source ¨Concanavalina ensiformis ¨ that has a high affinity with the carbohydrates (Colonna et al., 1985) followed by chromatography (Matheson & Welsh, 1988; Yun & Matheson, 1993; Matheson, 1996).
Materials and methods
Wheat samples for starch analysis
The studies were carried out on seeds of the wellknown Argentine commercial bread wheat varieties Buck Ombú, Buck Poncho, Buck Guaraní, Buck Catriel and Buck Charrúa collected from 1997 to 1999 at the Exp. Res. Sta. INTA Castelar in the province of Buenos Aires.
Chemical reagents
Concanavalin A (Con A), methyl,α- D - m a n o p y r anoside, dimethylsulphoxide (DMSO) and analytical grade reagents were purchased from Sigma Chemical Co. Sepharose CL- 2B and Biogel P6 (Amersham Biotech) were used for gel permeation chromatography.
Buffers: A) Sodium acetate 0.2 M, pH 6.4 with NaCl 1.0 M; MnCl2 1.0 mM; MgCl2 1.0 mM and CaCl2 1.0 mM; B) Sodium acetate 0.2 M, pH 5.8 ; C) Sodium arsenite 0.1 M in 0.2 M of phosphate buffer, pH 6.
Isolation of wheat starches
To start the analysis, 5 g of seeds were defatted by the Soxhlet method, soaked in 0.005M NaHSO3 to inhibit enzyme activity, homogenized in a blender, then filtered through muslin and the granules decanted. The sediment was submitted to several washes with 0.1 N NaCl; 0.1 N NaCl: toluene (140:1), twice with 96% ethanol: water; mixtures (1:3), (2:3) and (3:1) successively. Finally, it was washed with 96% ethanol, acetone and ether, then dried and weighed.
Fractionation of starch by differential solubility in water- butanol method
A schematic overview of the method is shown in Figure 1 B.

Figure 1: Starch fractionation
According to Curá and Krisman (1990), 5 g of milled and dried seeds were defatted by the Soxhlet method and suspended in four volumes of 3 % HgCl2 pH 7 at 28 °C and stirred for an hour. The suspension was centrifuged at 5,000 x g for 15 min and the sediment was resuspended in 1-butanol: water (1: 7), autoclaved for 3 hours at 1 atmosphere, 110° C. After centrifugation at 3,000 x g for 20 min. two fractions were obtained: a) the supernatant named butanol soluble fraction (BS) contained the amylopectin and b) the sediment called butanol insoluble fraction (BI) included the amylose.
Polysaccharides from both fractions could be recovered after the addition of three volumes of 96 % ethanol, then washed with acetone and ether successively, dried and weighed for their later characterization. All the fractions were additionally purified by gel filtration chromatography on Biogel P6 Amersham Biotech (100- 200 mesh) in a of 0.8 x 10 cm diameter column in 0.1M buffer pyridine acetate pH 5. For these studies, 10 mg of each sample were solubilized with 0.1 N NaOH and neutralized to pH 5 with 0.1N HCl and applied to the column. Fractions of 1 ml were recovered, the amylose and amylopectin content were controlled in the presence of iodine reagent (Krisman, 1962) and the λmax of the determined colored complex.
The total glucose content of each polysaccharide was determined by the phenol sulphuric acid method (Dubois et al., 1956) and later calculations were done considering:
Starch %= (A x F x 1000 x 1/1000 x 100/ w x 162/ 180)
where A: absorbance against the blank absorbance; F: 100 ug of glucose/ absorbance for 100 ug of glucose; 1000: volume correction factor; 1/ 1000: conversion to milligrams; 100/w: percentage sample weight; 162/ 180: conversion free glucose to anhydre glucose.
Fractionation of starch with Concanavalin A
A schematic overview of the method (Matheson & Welsh, 1988) is shown in Figure 1 (A).
The starch was fractionated using the concanavalin A method proposed by Matheson and Welsh (1988), Yun and Matheson (1993) and Matheson (1996). The starch sample (30 mg) was solubilized in 1.0 mL DMSO to pH 6, neutralized and incubated in buffer A according to the methodology. The starch solution was mixed with Con A in a concentration of 6.0 mg of protein/ mL. Two fractions were obtained: a) the Con A soluble fraction ( s u p e rnatant) which was treated with sodium EDTA. The protein was denatured and later discolored using buffer C and NaCl 0.1 M. The mixture was chromatographed on a Sepharose CL- 2B column (1.30 x 55 cm), eluted with KOH 0.25 M (10mL/ 40 min.) and fractions of 3 ml each were recovered. The α - D glucanes collected in each fraction were controlled with the iodine reagent (Krisman, 1962) and the λmax of the colored complex was determined and analyzed.
b) The Con A insoluble fraction (sediment) was resuspended in methyl α-D-mannopyranoside (80 mg/ mL) in buffer B. After adding EDTA solution, an alkaline extraction was realized with KOH 0.1 M (neutralized with acetic acid) followed by treatment with I2: KI (2%: 20 %)
The final sediment was suspended in the buffer C and 0.1M NaCl and was chromatographed on Sepharose CL-2B with 0.25 M KOH under the same conditions described above.
Polysaccharides characterization by spectrophotometry
The methodology used is based on the fact that in the presence of the I2: KI in CaCl2 saturated solution reagent (Krisman, 1962), the α1,4 α1,6 glucopolysaccharide show absorbance values (λmax) around 380 to 700 nm. A λmax higher than 575 nm indicating the presence of a linear glucopolysaccharide (amylose) which gives a blue-colored complex. On the other hand, λmax values below 575 nm point out the existence of amylopectin that stains purple-brown. Both starch components have a linear response within the concentration range of 100- 400 ug mL-?. A Shimatzu UV spectrophotometer was used in these studies.
The estimation of the length of the external chains of the α-D glucanes confirmed the state of the polymer through a fast and practical methodology, so the ratio (parameter "A") between the maximum absorbance value found for the polysaccharide and the maximum absorbance value corresponding to the shoulder of the spectrum (Tolmasky & Krisman, 1987) were computed. Long external chains of glucopolysaccharides (amylose) usually yield a high "A" parameter (around 2-4). Smaller values of "A" parameter (around 1-1.8) are typical of the short and branched chains of amylopectin.
Statistical analysis of data
During three years trials, five replicates of each variety were evaluated each year for total starch, amylose and amylopectin. Average values, standard deviation and coefficient of variation are presented for starch, amylose and amylopectin content. The data obtained were subjected to a two-way analysis of variance (ANOVA) and a least significant differences test (LSD) was also performed using the software STATISTICA version 2.0.
Results and discussion
Starch fractionation using lectin Concanavalin A
Starch fractionation was facilitated by the lectin complexed with the branched α D-glucan recovered in the supernatant of the insoluble Con A fraction treated with methyl α D-mannopyranoside, while the linear α-D-glucan was directly recovered in the supernatant of the soluble Con A treatment.
Starch components were analyzed in the presence of the I2 - KI in CaCl2 saturated solution reagent (Krisman, 1962) after being chromatographed on a Sepharose CL-2B column. The original methodology was supplemented by spectrophotometric determinations that allowed the characterization of the glucopolyssacharides and, later, the comparison of these results with those obtained by the method based on starch components differential solubility in a water:butanol mixture (Curá & Krisman, 1990; Curá et al., 1995).
The α D-glucanes report λmax values around 380 to 700 nm. Usually, the highest λmax values belong to the blue-colored lineal α D-glucanes and the lowest λmax values to the purple brown colored branched α D-glucanes. This fact is well observed in Table 1, where the wavelengths of maximum absorption (λmax ) for the bread wheat varieties studied are shown. We found that amylose λmax values varied from 608 nm (Buck Catriel) to 632 nm for Buck Charrúa. On the other hand, amylopectin λmax values ranked from 460nm (Buck Guaraní) to 482 nm (Buck Charrúa).
Table 1:Spectrophotometer characteristics of amylose and amylopectin

In Figure 2 (A) the starch spectral profile analyzed after Sepharose CL-2B for the variety Buck Ombú is presented. The α D-glucan from amylopectin fraction has a λmax value of 464 nm whilst the amylose fraction belonging to the unbranched á D-glucan has a λmax= 618 nm. Analogous spectral profiles were also obtained for the varieties Buck Poncho and Guaraní. Similarly, Figure 2 (B) shows that Buck Charrúa has a λmax value of 482 nm for amylopectin and 632 nm for the amylose fraction. Buck Catriel behaves similarly and the λmax for each starch fraction may be observed in Table 1.
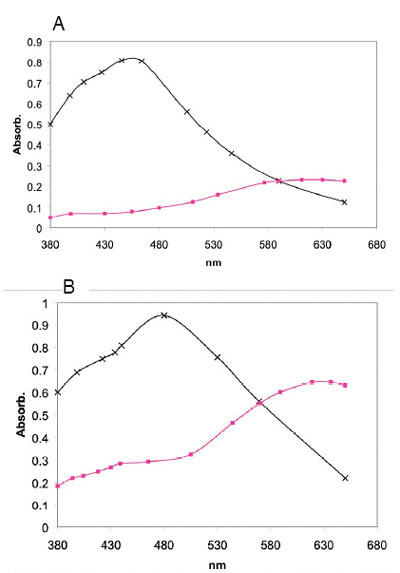
(A) Buck Ombú: amylose fraction (─♦─), amylopectin fraction ( ─x─) ; (B) Buck Charrúa: amylose (─♦─), amylopectin (─x─)
Figure 2: Starch absorption spectrum of components of Buck Charrúa and Buck Ombú wheat using concanavalin A method
Starch fractionation based on different starch solubility in butanol saturated water
Amylopectin was recovered in the buthanol soluble fraction (BS) whilst those polysaccharides extracted in the butanol insoluble fraction (BI) correspond to the amylose fraction (Curá & Krisman, 1990). None of the wheat varieties studied yielded glucopolysaccharides in the water soluble fraction (WS) which means a complete absence of the characteristic phytoglucogen like structures of this fraction. The λmax values for amylose and amylopectin obtained by this method are shown in Table 1. From this Table arises that the amylose λmax values estimated by the method based on complexing the starch with lectin ConA or the method based on differential solubility of the starch in butanol-water only differ among them up to a 0.48% depending on the wheat variety. In average, the λmax value for amylose obtained by the Concanavalin A method was 619.8 nm and 619.0 nm by the method which uses buthanol-water, so that only a difference of 0.13% was found between averages achieved by both methodologies. Similarly, amylopectin λmax values computed for the varieties analyzed varied from 0 to 0.62% depending on the method used. Only a difference of 0.04% was found for the average amylopectin λmax value obtained by either method. As similar results are achieved using both methods and considering that the methodology proposed by Curá & Krisman (1990) and Curá et al. (1995) results easier and faster to perform, it can be ruled out that it constitutes the best and most efficient way to carry through wheat starch fractionation analysis.
The "A" value ranked from 1.17 to 1.30, when the λmax ranged from 460 to 485 nm (Tolmasky & Krisman, 1987). On the other hand, when λmax ranged from 608 to 632 nm, "A" values varied from 2.93 to 3.33, suggesting the presence of long and scarcely branched chains of amylose.
The spectrophotometer profiles of BS and BI for Buck Charrúa can be seen in Figure 3. A similar behaviour was observed during the fractionation process of the varieties Buck Catriel, Buck Ombú, Buck Poncho and Buck Guaraní.

Figure 3: Starch absorption spectrum of components of Buck Charrúa wheat using method based on different solubility in butanol saturated water. Buck Charrúa: BS (butanol soluble fracion) (—x—), BI (butanol insoluble fraction) ( ——-)
Starch components
Total starch, amylose and amylopectin content for the five bread wheat varieties studied during three years running are presented in Tables 2a, 2b and 2c. These hard red winter bread wheat varieties can be classified according to the number of days from plant emergence to flowering as: 1) precocious (Buck Guaraní, Buck Ombú, Buck Charrúa) and 2) intermediate (Buck Poncho, Buck Catriel).
Table 2 . a- Starch content bread wheat Buck varieties during three years running

b- Amylose content of wheat Buck varieties during three years

c- Amylopectin content wheat Buck varieties during three years

As it may be seen in Table 2a, the average starch content of the varieties analyzed ranged from 58.0% to 59.9%. Through a two-way analysis of variance and least significant difference analysis (LSD) we did not find significant differences for starch content among the five bread wheat varieties studied (p≥ 0.05) neither among years (environment) within each variety (p≥0.05). In average, the intermediate cycle length varieties starch content was 58.6% ± 0.6 (c.v.= 1.0%) and 59.1% ± 1.0 (c.v.= 1.7%) for the precocious ones (also see Table 2a). Amylose and amylopectin average content for the same groups of varieties can be observed in Tables 2b and 2c respectively. As expected, the results confirm that there are no statistical significant differences at p ?0.01 between precocious and intermediate varieties for starch (±Student´s= 1.9), amylose (±Student´s t= 0.5) nor amylopectin (±Student´s t= 0.7) as the number of days to flowering does not play any role in the determination of starch quantity and quality. Nevertheless, amylose and amylopectin content strongly differed among varieties as it is shown in Tables 2b and 2c respectively. Significant differences between varieties were found both for amylose (p≥0.05) and amylopectin (p≥0.05) (see Figure 4). The LSD test allowed detecting two homogeneity groups for amylose and three for amylopectin (see Table 3). These results and the fact that no differences among years within the same variety were found for amylose (p≥0.01) and amylopectin ( p≥0.01) let us keep like other authors (Zhao et al., 2005) that starch quality mainly depends on the variety genotype with null or scarce environment influence during grain filling. This knowledge must be well considered for wheat breeding purposes. Whether historically classic wheat breeding focused on increasing grain yields, new approaches for further genetic improvement of its functional quality through conventional breeding or biotechnology may be of great importance. The chemical and physical properties of the storage components could be modified to increase their usefulness in conventional applications and for novel uses.

Figure 4: Starch content and quality in different bread wheat Buck varieties.

In the present study we found that the varieties Buck Catriel and Buck Charrúa have 41.2% and 41% amylose respectively. Therefore, according to our results both varieties beat in a 5% the maximum value of the amylose content range accepted for wheat (15 to 35%) and 64% over the average (25%).
On the other hand, Buck Ombú showed a high amylopectin content (71.2%) and also Buck Guaraní (67%). This result is in total agreement with SDSPAGE and molecular studies carried out on the latter variety point out that its genetic background carries the waxy mutant gene Wx1- B1/ b (Pfluger L., 2006, personal communication). The variety Buck Poncho has been found to contain 66% amylopectin (only 1% over the minimum to be classified as w a x y) which is known to be mutant for the gene waxy1 ( genotype aae) (Pfluger, L., 2006, personal communication) that promotes the change of only one amino acid and may restrict or not the production of the granulebound starch synthase (GBSS) also called "waxy protein" responsible for amylose biosynthesis on wheat. According to this, these varieties lack at least one GBSS, for this reason they may be considered partial waxy mutants and could perhaps be useful to produce noodles.
Conclusions
Whether wheat starch molecular fractionation may be appropriately and successfully done by a) a method based on the starch differential solubility in butanol saturated water or by b) the carbohydrateprotein complex lectin Concanavalin A method, the first results easier, shorter and consequently more efficient. The five varieties showed similar starch content but as starch qualitative composition varied dramatically among them, this would surely promote different usefulness for each one. Although a small number of bread wheat varieties were analyzed, these results agree with those of previous authors in the sense that starch composition seems to be null or scarcely modified by the environment as no differences in starch composition were found among years within the same genotype.
Acknowledgements
The authors thank to SECYT-UBA, I-043 and ANPCyT PICT, 12137 for the grants accorded that made possible the conclusion of these studies.
Literature cited
1. Burrell, M.M., 2003. The need for improved quality or quantity: an overview. Journal of Experimental Botany 54 (382): 451- 456. ç [ Links ]
2. Colonna, P.; V.Biton and C. Mercier, 1985. Interactions of concanavalin A with α-D glucans. Carbohydrate Research 137: 151-166. [ Links ]
3. Curá, J.A and C.R. Krisman, 1990. Cereal grains: A study of their (α1,4)- (α1,6) glucopolysaccharides composition. Starch/ Stärke 42: 71-175. [ Links ]
4. Curá, J.A.; Per-Erik Jansson and C.R.Krisman, 1995. Amylose is not strictly linear. Starch / Stärke 47: 207-209. [ Links ]
5. Dubois, M.; K.Gilles; J.Hamilton.; P.Rebers and F. Smith, 1956. Colorimetric method based on phenol sulfuric acid. Analytical Chemistry 28: 356. [ Links ]
6. Krisman, C.R., 1962. A method for the colorimetric estimation of glycogen with iodine. Analytical Biochemistry 4: 17-23. [ Links ]
7. Matheson, N.K. and L.A. Welsh, 1988. Estimation and fractionation of the essentially unbranched amylose and branched amylopectin component of starches with concanavalin A. Carbohydrate Research 180: 301-313. [ Links ]
8. Matheson, N.K., 1996. The chemical structure of amylose and amylopectin fractions of starch tobacco leaves during development and diurnally nocturnally. Carbohydrate Research 282 (2): 247-262. [ Links ]
9. Rodriguez-Quijano, M.; R. Lucas and J.M. Carrillo, 2003. Waxy proteins and amylose content in tetraploid wheats Triticum dicoccum Schulb, Triticum durum L. and Triticum polonicum L. Euphytica 134: 97-101. [ Links ]
10. Schoch, T.J. and E.C.Maywald, 1956. Fractionation of starch by selective precipitation with butanol. Analytical Chemistry 28: 382-389. [ Links ]
11. Tolmasky, D.S. and C.R. Krisman, 1987. The degree of branching in (a1,4) (a1,6) linked glucopolysaccharides is dependent on intrinsic properties of the branching enzymes. European Journal of Biochemistry 168: 393- 397. [ Links ]
12. Yun, S.H. and N.K. Matheson, 1993. Structures of the amylopectins of waxy, normal amylose extender and wx: ae genotypes and of phytoglycogen of maize. Carbohydrate Research 243(2): 307-321. [ Links ]
13. Zhao, C.; T.Ning; N.Jiao; B.Han and Z. Li, 2005. Effects of genotype and environment on protein and starch quality of wheat grain. The Journal of Applied Ecology 16( 7): 1257-60. [ Links ]














