Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agriscientia
On-line version ISSN 1668-298X
Agriscientia vol.31 no.1 Córdoba June 2014
ARTÍCULOS
Fumigant toxicity of five essential oils rich in ketones against Sitophilus zeamais (Motschulsky)
Herrera, J.M.; M.P. Zunino, Y. Massuh, R.P. Pizzollito, J.S. Dambolena, N.A. Gañan y J.A. Zygadlo
J.M. Herrera, M.P. Zunino, Y. Massuh, R.P. Pizzollito, J.S. Dambolena and J.A. Zygadlo: Instituto Multidisciplinario de Biología Vegetal (IMBIV)- CONICET. Cátedra de Química Orgánica, Facultad de Ciencias Exactas Físicas y Naturales- Universidad Nacional de Córdoba, Avenida Vélez Sarfield 1611, X5016GCA, Córdoba, Argentina. N.A. Gañan. Planta Piloto de Ingeniería Química (PLAPIQUI) - CONICET, Argentina. Correspondence to J.A. Zygadlo: juliozyg29@gmail.com
SUMMARY
Essential oils (EOs) and individual compounds act as fumigants against insects found in stored products. In fumigant assays, Sitophilus zeamais Motschulsky adults were treated with essential oils derived from Aphyllocladus decussatus Hieron, Aloysia polystachya Griseb, Minthostachys verticillata Griseb Epling and Tagetes minuta L , which are rich in ketones and their major components: a- thujone, R-carvone, S-carvone, (-) menthone, R (+) pulegone and E-Z- ocimenone. M. verticillata oil was the most toxic ( LC50: 116.6 µl /L air) characterized by a high percentage of menthone (40.1%) and pulegone (43.7%). All ketones showed insecticidal activity against S. zeamais. However, pulegone (LC50: 11.8 µl/L air), R- carvone (LC50: 17.5 µl/L air), S-carvone (LC50: 28.1 µl/L air) and E-Z-ocimenone (LC50: 42.3 µl/L air) were the most toxic. These ketones are a,b-unsaturated carbonyl. This feature could play a fundamental role in the increase of insecticidal activity against S. zeamais.
Keywords: Sitophilus zeamais; Essential oils; Aphyllocladus decussatus; Aloysia polystachya; Minthostachys verticillata; Tagetes minuta; a,b- Unsaturated carbonyl ketones
Toxicidad fumigante de cinco aceites esenciales ricos en cetonas contra Sitophilus zeamais (Motschulsky).
RESUMEN
Los aceites esenciales (AEs) y sus componentes principales actúan como fumigantes contra insectos de granos-almacenados. En ensayos fumigantes, adultos de Sitophilus zeamais Motschulsky fueron tratados con AEs, ricos en cetonas, provenientes de Aphyllocladus decussatus Hieron, Aloysia polystachya Griseb, Minthostachys verticillata Griseb Epling y Tagetes minuta L., y sus principales compuestos: a- tuyona, R-carvona, S-carvona, (-) mentona, R (+) pulegona y E-Z- ocimenona. El AE de M. verticillata fué el mas tóxico (CL50: 116,6 µl/ L aire) caracterizado por un alto contenido de mentona (40,1%) y pulegona (43,7%). Todas las cetonas mostraron actividad insecticida contra S. zeamais. Sin embargo, pulegona (CL50: 11,8 µl/ L aire), R- carvona (CL50: 17,5 µl/L aire), S-carvona (CL50: 28,1 µl/L aire) y E-Z-ocimenona (CL50: 42,3µl/L aire) fueron las más tóxicas. Estas cetonas presentan a,b-insaturaciones; dicha propiedad puede estar relacionada con el incremento de la actividad insecticida contra S. zeamais.
Palabras clave: Sitophilus zeamais; Aceites esenciales; Aphyllocladus decussatus; Aloysia polystachya; Minthostachys verticillata; Tagetes minuta; Cetonas a,b- insaturadas.
Fecha de recepción: 30/10/13;
fecha de aceptación: 27/05/14
INTRODUCTION
The central area of Argentina is the most important in the production of corn. The amount of corn exported to the United States, Europe and China during 2011 - 2012 was approximately 75,000 tons (Lezcano, 2012). Worldwide maize grain storage sites are affected by different species of beetle pests. Sitophilus zeamais (Motschulsky) is a serious primary pest of stored maize throughout the world causing quantitative and qualitative losses (Boyer et al., 2012). The control of these pests is dependent upon applications of synthetic insecticides: methyl bromide and phosphine (Benhalima, 2004; Athie & Mills, 2005; Pimentel et al., 2012) and environmental damage, which cause serious concerns about human health.
In view of the problems with the current fumigants, there is a global interest in generating alternative strategies. There are numerous studies describing essential oils (EOs) and their components as potential insecticides (Pérez et al., 2010; Caballero-Gallardo et al., 2011; Kurdelas et al., 2012). This insecticidal activity has been evaluated against the maize weevil. The results showed that many EOs could control Sitophilus zeamais populations in corn grain stores (Liu et al., 2011; Suthisut et al., 2011; Yang etal., 2011). However, the insecticidal activity of synthetic products is far superior to that of EOs but the pressure generated by the consuming public about the use of synthetic pesticides has raised the option of an integrated management of insects affecting food storage sites (Yigezu et al., 2010). In this framework EOs are shown as an important alternative (Correa et al., 2011). Plant essential oils are promising in that they are easily biodegradable, do not present described resistance and they are environmentally friendly (Pérez et al., 2010).
Therefore, in the present work, we report insecticidal activity of EOs rich in ketones of Aphyllocladus decussatus Hieron, Aloysia polystachya Griseb, Minthostachys verticillata Griseb Epling, Tagetes minuta L. and their major components against S. zeamais.
MATERIALA AND METHODS
Insects
Adults of the strain of S. zeamais were obtained from Metán, Salta province, Argentina. The colony was maintained in our laboratory for one year without exposure to insecticides.
S. zeamais were reared on sterilized whole maize grain in sealed containers. Insect rearing was carried under controlled temperature and humidity (28 ºC and 60-70%) and a light/dark regime of 12:12 h (FAO, 1974). Experiments were conducted under complete darkness, at 28 ºC and 60-70% relative humidity. The unsexed adult weevils used were about 2-weeks old.
Essential oils and pure compounds
Aphyllocladus decussatus Hieron, Aloysia polystachya Griseb (population 1) were collected from La Rioja province, Argentina. Plants of Aloysia polystachya Griseb (population 2), Minthostachys verticillata Griseb Epling and Tagetes minuta L. were collected from Córdoba province, Argentina. We deposited all voucher plant specimens at the Museo Botánico de Córdoba (CORD). The EOs were obtained by hydrodistillation (Demo et al., 2005) and stored at -20ºC in airtight microtubes prior to analysis by gas chromatography-mass spectrometry (GC-MS). The a- thujone, R-carvone, S-carvone, (-) menthone and R (+) pulegone used for bioassays were purchased from Sigma Aldrich (Steinheim, Germany). The E-Z-ocimenone was isolated from T. minuta EO by supercritical carbon dioxide fractionation. The highest selectivity was obtained at 80 bar and 40 ºC, with a static period of 30 min followed by dynamic extraction at a flow rate of 0.1 g/min until complete removal of monoterpenes, leaving a residue of purified ocimenone (Figure 1).

Fig. 1. Chemical structures of natural ketones compounds studied in the present work.
Analysis of essential Oils
The EOs were analyzed by Perkin Elmer Clarus 500 chromatograph equipped with a detector FID and a capillary column DB-5 (60 m x 0.25 mm i.d. and 0.25 µm coating thickness). The temperature of the column was programmed from 60 oC to 240 oC at 4 oC/min. The detector and injector temperatures were 250 oC. Helium was used as carrier gas with a flow rate of 0.9 ml/min. The quantitative composition was obtained by peak area normalization, and the response factor for each component was considered to equal 1. For the determination of the composition, EOs samples were diluted with n-hexane. The injection volume was 1 µl. The identification of the EOs' components was carried out by GC-MS. A Perkin-Elmer Clarus 600 GC-MS coupled with an ion trap mass detector which was employed for the identification. A capillary column DB-5 (60 m x 0.25 mm i.d. and 0.25 µm coating thickness) was used for the separation of the components. Helium was used as carrier gas with a flow rate of 0.9 ml/min. The temperature program for the oven and injector was the same as that for the GC-FID. Ionization was realized by electron impact at 70 eV. Mass spectral data were acquired in the scan mode in the m/z range 35-250. Retention indices (RI) of the sample components were determined on the basis of homologous n-alkane hydrocarbons under the same conditions. The compounds were identifed by comparing their retention indices and mass spectra with published data (Adams, 2007) and libraries NIST and Adams. The main components were further identified by coinjection of authentic standards (SIGMA, USA).
Fumigant Toxicity assay
The insecticidal activity against S. zeamais was evaluated using fumigant toxicity assay described by Huang et al. (2000), with some modifications. Briefy, glass vials (30 ml) were used as fumigation chambers. Different amounts of EOs and pure compounds were applied to Whatman filter paper disks (2 cm diameter) placed on the underside of the screwcap of a glass vials at the doses corresponding to 15- 600 µl/L air. A series of concentrations of each EOs and pure compound were prepared in n-hexane. Solvent was allowed to evaporate for 2 min prior to introduction of insects. Ten adults S. zeamais were placed into each vial (5 replicas / dose). Control insects were kept under same conditions without EOs and pure compounds. Insect mortality was checked after 24 h. The mortality percentages and LC50 values were calculated according to Finney (1971).
Statistical analysis
The concentration-mortality data were subjected to Probit analysis to obtain the LC50 values. The lethal concentrations LC50 and LC95 were calculated using SPSS Statistics program version 17.0 (SPPSS Inc). The values of LC50 were considered to be significantly different, if 95% confdence limits did not overlap. Treatment means were compared and separated by Duncan's test at p = 0.05 using the InfoStat software Professional 2010 (Di Rienzo et al., 2010).
RESULTS AND DISCUSSION
Essential oils
The main compounds in the EOs extracted from A. decussates, A. polystachya (population 1 and 2), M. verticillata and Tagetes minuta are presented in Table 1. We identified 32, 9, 15, 22 and 7 compounds from A. decussates, A. polystachya (population 1), A. polystachya (population 2), M. verticillata and T. minuta EOs, respectively. The principal compounds of EOs obtained from A. decussates were sabinene (12.4%), 1,8-cineole (14.6%), a-thujone (39.4%) and b-thujone (17.9%). The major component of A. polystachya (population 1) was a-thujone (98.7%), while A. polystachya (population 2) had as main components a-thujone (27.6%) and carvone (62.9%). M. verticillata EO was characterized by a high percentage of menthone (40.1%) and pulegone (43.7%). Ocimenone (43.5%) and cis-b-ocimene (42.4%) were the main components of T. minuta EO. All EOs were characterized by a high concentration of ketone type compounds, in the cases studied these compounds represented over 40% of the total EO. The exception were sabinene and 1,8-cineol in A. decussates with a concentration above 10% and the hydrocarbon cis-b-sabinene with a concentration of 42.4% in T. minuta. The following compounds showed a concentration less than 0.05% and they are components of only one or two species, from A. decussates: a-thujene, camphene, a-terpinene, cis-pinocarveol acetate, carvacrol, trans-carvil acetate, myrtenol, (neo-3)-thujanol acetate, a-terpinil acetate, neoiso-3-thujanol, sabina ketone, eugenol, cis-carvil acetate, a-humulene, calamenene, oxide caryophyllene, from A. polystachya (1), a-myrcene, myrtenol, a-curcumene, from A. polystachya (2), cis-carveol, thymol, a-humulene, spathulenol, from M. verticillata, d-2-carene, p-cymene, cis sabinene hydrate acetate, eugenol, b-bourbonene, 1S,cis-calamenene.
Table 1 Chemical constituents of essential oils from A. decussates, A. polystachya (1), A. polystachya (2), M. verticillata and T. minuta plants collected from Córdoba and La Rioja provinces, Argentina.

1 Retention index on a DB-5 column relative to homologous series of n-alkanes. GCMS: peak identifcations are based on MS comparison with fle spectra (The similarity is over 97%). Co: peak identification is based on standard comparison with relative retention time.2 tr = concentration less than 0.05%
Toxicity of essential oils
Comparison of LC50 values for the fve EOs against S. zeamais showed that M. verticillata oil was the most toxic ( LC50 =116.6 µl/L air), toxicity being twice lower at 24 h after exposure than in the rest of the EOs studied. LC50 of EO of T. minuta could not be calculated because it did not show dose dependent linear behavior (Table 2).
Table 2. Fumigant toxicity of essential oils from A. decussates. A. polystachya (1). A. polystachya (2). M. verticillata and T. minuta plants against adults of S. zeamais at 24 h after exposure.

1 Values (means ± SE) with different letters in the same column are significantly different from each other according to Duncan's multiple range test at P ≤ 0.05. Each datum represents the mean of five replicates, each set up with 10 adults.2 ND: Not determined
Toxicity of individual compounds
All ketones showed insecticidal activity against S. zeamais. The toxicity of pure compounds can be divided into two groups from the most to the least toxic: Group 1: pulegone (LC50: 11.,8 µl/L air), R-carvone (LC50: 17.5 µl/ L air), S-carvone (LC50: 28.1µl/L air) and ocimenone (LC50: 42.3 µl/L air); Group 2: a-thujone (LC50: 65.5 µl/ L air) and menthone (LC50: 85.4 µl/ L air) (Table 3).
Table 3. Fumigant toxicity of pure compounds against S. zeamais adults at 24 h after exposure.
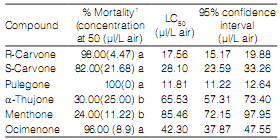
1 Values (means ± SE) with different letters in the same column are significantly different from each other according to Duncan's multiple range test at P ≤ 0.05. Each datum represents the mean of five replicates, each set up with 10 adults.
The EOs and the individual compounds act as fumigants against insects found in stored products. In the study, EO of M. verticillata was the most bioactive, which can be attributed to its content in pulegone (43.6 %) (Table 1). However, S. zeamais showed greater sensitivity to pure compounds than EOs.
Pulegone was more toxic than carvone and E-Z-ocimenone (Table 3). In addition, carvone isomers presented differences in toxicity. Carvone R was more active than S isomer. Controversial results of insecticidal activity of isomers of carvone were shown by Lee et al. (2003) and Tripathi et al. (2003).
Numerous studies have shown ketones with highest toxicity against Sitophilus in fumigant and contact assays (Lee et al., 2003; Tripathi et al., 2003; Liu et al., 2011; Germinara et al., 2012). These results suggest that the presence of carbonyl groups augments toxicity. On the other hand, ketones belonging to Group 1 were a,b-unsaturated. This feature could be playing a fundamental role in the increase of insecticidal activity. Thus, ketones of Group 1 were more toxic than those of Group 2 (Table 3). Xavier & Rauter (2008) revealed that compounds containing the a,b-unsaturated act as Michael acceptors for the addition of protein nucleophilic groups. Also, a,b-unsaturated carbonyl compounds were described as potent inhibitors of the enzymes Glutathione-S-transferases that contribute to the phase II metabolism of xenobiotics (Yu & Abo-Elghar, 2000).
In conclusion, the present results indicate thata,b-unsaturated carbonyl ketones act as potential fumigants against S. zeamais. Based on these findings, they could serve as viable alternative to synthetic insecticides.
ACKNOWLEDGEMENTS
This research was supported by grants from Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba and the Agencia Nacional de Promoción Científica y Técnica, FONCYT, PICT 2012-2146. The authors thanks Dra. Gloria E. Barboza for her valuable help. We thank Iliana A. Martinez, MA for revising the English language.
REFERENCES
1. Adams, P.R, 2007. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream, Illinois, USA. [ Links ]
2. Athie, I and K. Mills, 2005. Resistance to phosphine in stored-grain insect pests in Brazil. Brazilian. Journal. Food Technology, 8:143-147. [ Links ]
3. Benhalima, H.; M.Q. Chaudhry, K.A. Mills and N.R. Price, 2004. Phosphine resistance in stored-product insects collected from various grain storage facilities in Morocco. Journal of Stored Products Research, 40:241-249. [ Links ]
4. Boyer, S.; H. Zhang and G. Lemperiere, 2012 A review of control methods and resistance mechanisms in stored-product insects. Bulletin of Entomological Research, 102: 213-229. [ Links ]
5. Caballero-Gallardo, K.; O. Olivero-Verbel and E. Stashenko, 2011. Repellent activity of essential oils and some of their individual constituents against Tribolium castaneum Herbst. Journal of Agricultural Food Chemistry, 59: 1690-1696. [ Links ]
6. Correa, A.; E. Pereira, E. Cordeiro, L. Braga and R. Guedes, 2011. Insecticide resistance, mixture potentiation and fitness in populations of the maize weevil (Sitophilus zeamais). Crop Protection, 30:1655-1666. [ Links ]
7. Demo, M.; M. Oliva, M. López, M.P. Zunino and J. Zygadlo, 2005. Antimicrobial activity of essential oils obtained from aromatics plants of Argentina. Pharmaceutical Biology, 43: 129-134. [ Links ]
8. Di Rienzo, J.A.; F. Casanoves, M.G. Balzarini, L. Gonzalez, M. Tablada and C.W. Robledo, 2010. Infostat versión 2010. Grupo Infostat, FCA, Universidad Nacional de Córdoba, Argentina. [ Links ]
9. FAO, 1974. Boletín Fitosanitario de la FAO. Método provisional para gorgojos adultos importantes en cereales almacenados con malation o lindano. Método Nº 15 de la FAO, 22:127-137. [ Links ]
10. Finney, D.J, 1971. Probit Analysis. Cambridge University Press, London.333 pp [ Links ]
11. Germinara, G.; A. De Cristofaro and G. Rotundo, 2012. Bioactivity of short-chain aliphatic ketones against adults of the granary weevil, Sitophilus granarius (L.) Pest Management Science, 68: 371-377. [ Links ]
12. Huang, Y.; S.L. Lam and S.H. Ho, 2000. Bioactivities of essentialoil from Elletaria cardamomum (L.) Maton. to Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst). J.Stored Prod. Res. 36:107-117. [ Links ]
13. Kurdelas, R.; S. López, B. Lima, G. Feresin, J. Zygadlo, S. Zacchino, M. López, A. Tapia and M. Freile, 2012. Chemical composition, anti-insect and antimicrobial activity of Baccharis darwinii essential oil from Argentina, Patagonia. Industrial Crops and. Products, 40:261-267. [ Links ]
14. Lee, S.; C. Peterson and J. Coats, 2003. Fumigation toxicity of monoterpenoids to several Stored Product Insects. Journal of Stored Products Research, 39:77-85. [ Links ]
15. Lezcano, E., 2012. Productos de maíz. Alimentos Argentinos, 54:18 - 38. [ Links ]
16. Liu, Z.; S. Chu and G. Jiang, 2011. Toxicity of Schizonpeta multifida essential oil and its constituent compounds towards two grain storage insects. Journal of the Science of Food and Agriculture, 91: 905-909. [ Links ]
17. Pérez, S.; M. Ramos-López, M. Zavala-Sánchez and N. Cárdenas-Ortega, 2010. Activity of essential oils as a biorational alternative to control coleopteran insects in stored grains. Journal of Medicinal Plants Research, 4: 2827-2835. [ Links ]
18. Pimentel, M.; L. Faroni, R. Guedes, A. Sousa and M. Tótola, 2009. Phosphine resistance in Brazilian populations of Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Journal of Stored Products Research 45:71-74. [ Links ]
19. Pimentel, M.; L. Faroni and R. Guedes, 2012. Phosphine-induced walking response of the lesser grain borer (Rhyzopertha dominica). Pest Management Science, 68: 1368-1373. [ Links ]
20. SPSS, Inc, 2008. SPSS-vs.17. User's Guide. [ Links ]
21. Suthisut, D.; P. Fields and A. Chandrapatya, 2011. Fumigant toxicity of essential oils from three Thai plants (Zingiberaceae) and their major compounds against Sitophilus zeamais, Tribolium castaneum and two parasitoids. Journal of Stored Products Research, 47:222-230. [ Links ]
22. Tripathi, A.; V. Prajapati and S. Kumar, 2003. Bioactivities of l-Carvone, d-Carvone, and Dihydrocarvone Toward Three Stored Product Beetles. Journal of Economic Entomology, 96:1594-1601. [ Links ]
23. Xavier, N and A. Rauter, 2008. Sugars containing a,b- unsaturated carbonyl systems: synthesis and their usefulness as scaffolds in carbohydrate chemistry. Carbohydrate. Research. 343: 1523-1539. [ Links ]
24. Yang, K.; Y. Zhou, C. Wang, S. Du, Z. Deng, Q. Liu and Z. Liu, 2011. Toxicity of Rhododendron anthopogonoides essential oil and its constituent compounds towards Sitophilus zeamais. Molecules, 16: 7320-7330. [ Links ]
25. Yigezu, A.; E. Corinne, V. Paul, L. Maier, J. Woloshuk and D. Moog, 2010. Economics of Integrated Insect Management in Stored Corn. Journal of Economic Entomology, 103: 1896-1908. [ Links ]
26. Yu, S. and G. Abo-Elghar, 2000. Allelochemicals as Inhibitors of Glutathione S-Transferases in the Fall Armyworm. Pesticide Biochemestry and Physiology, 68:173-183. [ Links ]














