INTRODUCTION
Grapevine (Vitis sp.) is a predominant crop, extensively grown worldwide, with a strong productive trend in the last decade (Seccia, Santeramo and Nardone, 2015), with more than 21 million tons in the world. In Peru, agroclimatic conditions are favorable for the crop, and it is the fifth primary product in national agriculture, representing 4.6 % of the gross value of agricultural production; areas of more intensive culture include the Ica, Piura and Lima regions, which represent 93 % of the total national production. Until 2017, the Arequipa region had a harvested area of 1336 ha, with yields of 22 139 kg ha-1 (MINAGRI, 2019).
Meloidogyne species (root-knot nematode) is one of the major causes of grapevine damage (Somavilla, Bauer Gomes and Vera, 2012). It induces the formation of root galls, which restrict the absorption of water and nutrients as well as plant growth, predisposing it to the attack of other pathogens (Perry and Moens, 2014).
Meloidogyne incognita, M. javanica, M. ethiopica, M. arenaria, and M. hapla are the most important species that affect grapevine crops worldwide (Ferris, Zheng and Walker, 2012; 2013; Aballay and Vilches, 2015). In Peru, M. arenaria (Esterase phenotype - Est A2) was identified through its esterase isoenzyme in the Arequipa, Ica, and Piura regions, M. incognita (Est. I2) in Ica and M. javanica (Est. J3) in Ica and Piura (Varas, 2018).
The use of resistant rootstocks demonstrates characteristics that are very useful for resistance or tolerance to nematodes, additionally, worldwide more than 80 % of all vineyards grow vines grafted onto rootstocks (Ollat, Bordenave, Tandonnet, Boursiquot and Marguerit, 2016). The rootstocks are resistant to phylloxera and soil fungi, they adapt to the physical and chemical properties of the soil (Somavilla et al., 2012), including tolerance to abiotic stress such as drought (Fort, Fraga, Grossi and Walker, 2017; Peccoux et al., 2018), salinity (Sohrabi, Ebadi, Jalali and Salami, 2017) and calcareous soils (Bavaresco and Lovisolo, 2015) and they provide greater vigor to the graft (Zhang, Marguerit, Rossdeutsch, Ollat and Gambetta, 2016).
In Peru, different rootstocks have been used, but there are no reports of a reaction with Meloidogyne species in the rootstocks and cultivars used under the conditions of the Arequipa region. This study aimed to evaluate the reaction of grapevine rootstocks and cultivars to M. incognita, M. arenaria and M. hapla.
MATERIALS AND METHODS
The experiment was conducted in a mesh house with a plastic cover (temperature of 25 ± 5 °C and humidity of 50 ± 5 %, conditions that are suitable for grapevine cultivation) in the Phytopathology Laboratory of the Agronomy Faculty, National University of San Agustín (16° 24' 32.79" S, 71° 31' 18.87" W; 2365 m a.s.l.), Arequipa, Peru.
The experiment followed a completely randomized design, with an 8 x 3 factorial scheme, where the factors were the rootstocks, cultivars, and nematode species. There were six replicates per treatment and each replicate consisted of a bag with a grapevine plant.
A total of six rootstocks were used: MGT 101-14 (V. ripariax V rupestris), Ritcher 110 (Vitisberlandieri x Vitis rupestris), Paulsen 1103 (V. berlandieri x V. rupestris), S04 (V berlandieri x V. riparia), Salt Creek (V candicans x V. rupestris), and K 5BB (Vt berlandieri x V. riparia). Two grapevine cultivars (Vt vinifera) were used: Quebranta and Torontel. The cuttings were disinfected with Vitavax-300 and rooted in beds with a sand and pumice substrate (2:1). Once rooted, they were transplanted into 3 kg bags with a previously sterilized substrate of fine sand and promix (3:1).
M. incógnita (Est I2), M. arenaria(Est A2), and M. hapla (Est H1) were used to infect grapevines of the Arequipa región. The identification of Meloidogyne species was made through the morphological characterization of the female perineal pattern (Hartmann and Sasser, 1985) and the biochemical characterization of the esterase isoenzyme through electrophoresis (Carneiro and Almeida, 2001). Meloidogyne species were kept in tomato plants where they multiplied (Solanum lycopersicum cv. ‘Rio Grande') for a period of three months.
One month after transplantation, cuttings were inoculated with the nematode species. The eggs were extracted from the roots according to the method described by Hussey and Barker (1973). They were then suspended in water and inoculated, using a pipette, at a dose of 5000 eggs + J2 juveniles per bag, in four holes made in the soil around the plant. To control the viability of the inocula, susceptible tomato plants (Solanum lycopersicum cv. ‘Rio Grande') were inoculated with a suspension of 5000 eggs + J2 juveniles of each Meloidogyne species and they were installed and conducted under the same conditions as the grapevine rootstocks and cultivars.
After six months, plants were collected to evaluate their reaction to Meloidogyne species. The aerial part was separated from the roots, carefully washed to determine the number of galls (NG). Subsequently, root systems were processed according to the method of Hussey and Barker (1973) to quantify the final population of nematodes (FP). From the final nematode population in the root system, calculations of number of nematodes per gram of root (NNGR) and the reproduction factor (RF = final population / initial population) of Meloidogyne species were performed for each repetition. The grapevine rootstocks and cultivars were considered immune (RF = 0), resistant (RF <1) and susceptible (RF> 1) to the Meloidogyne species (Oostenbrink, 1966). The number of nematodes per gram of root was estimated by the ratio between the total number of nematodes and the total root mass (in grams) for each repetition.
The data for each nematode species were analyzed in the different rootstocks and cultivars (NG, NNGR, and RF variables were transformed into ). The respective analysis of variance (ANOVA) was performed, and means were compared with Duncan's multiple test (p< 0.05), the SAS® University Edition software was used for data analysis.
RESULTS AND DISCUSSION
Results of ANOVA revealed an interaction in terms of NG, NNGR and RF between M. incognita, M. arenaria, M. hapla and the different rootstocks and cultivars evaluated. This interaction was corroborated with Duncan's Test (p< 0.05). Meloidogyne species induce gall formation and can reproduce in all the rootstocks and cultivars studied (Tables 1,2 and 3).
None of the evaluated rootstocks were immune, since the three nematode species could reproduce in a limited way. Rootstocks infected with Meloidogyne species had an RF = 0.01 to 0.73 (Tables 1, 2 and 3). Most were resistant; however, the ‘Salt creek' rootstock, although resistant to M. incognita and M. arenaria, was susceptible to the attack by M. hapla, with an RF = 1.39 (Table 3).
The Quebranta and Torontel cultivars were susceptible to the three species of Meloidogyne, with the highest RF of 1.6 to 3.49. Somavilla et al., (2012), Aballay and Vilchez (2015) reported a similar susceptibility of cultivars to M. incognita, M. ethiopica, M. hapla and M. javanica.

Table 1: Number of galls (NG), number of nematodes per gram of root (NNGR), reproduction factor (RF), and reaction of rootstocks and cultivars to Meloidogyne incognita.
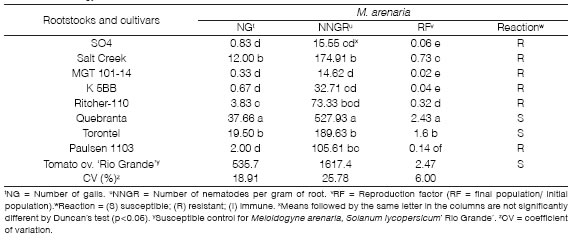
Table 2: Number of galls (NG), number of nematodes per gram of root (NNGR), reproduction factor (RF), and reaction of rootstocks and cultivars to Meloidogyne arenaria.

Table 3: Number of galls (NG), Number of nematodes per gram of root (NNGR), reproduction factor (RF), and reaction of rootstocks and cultivars to Meloidogyne hapla.
The SO4, Salt Creek, MGT-101-14, K5BB, Ritcher 110, and Paulsen 1103 rootstocks were resistant to M. incognita. According to Boubals (1992), the Paulsen 1103 rootstock is moderately resistant to M. incognita. Similarly, SO4 is resistant to M. incognita and M. arenaria (McKenry and Anwar, 2006). Somavilla et al. (2012) also reported that SO4, Salt Creek, and K 5BB are resistant to M. incognita. Moura et al. (2014) reported that MGT 101-14 and K 5BB are resistant to M. incognita. As described by Gutierrez- Gutierrez, Palomares-Rius, Jiménez-Díaz and Castillo (2011), the Ritcher 110 rootstock has a nematode-resistant reaction, which agrees with the results obtained in this experiment. The K5BB and Ritcher 110 rootstocks showed the lowest reproduction rates of M. incognita, with a RF= 0.29 and 0.28, respectively. Quebranta and Torontel cultivars show a high susceptibility, with an RF = 2.97 and 2.28, respectively (Table 1). However, no other studies have indicated the susceptibility reaction of these cultivars, although similar behavior was evident to the cultivars evaluated by Somavilla et al. (2012), Aballay and Vilches (2015).
M. arenaria exhibited the lowest population levels in the experiment, with an RF= 0.02 to 0.73. All rootstocks were considered resistant, as the RF was low compared to the previous Meloidogyne species evaluated. According to Somavilla et al. (2012), SO4, Salt Creek, and K 5BB were equally resistant to M. arenaria. Nevertheless, Paulsen 1103 was susceptible to the nematode, although this result may have been affected by crop conditions, such as soil characteristics, irrigation, and carrier clonal differences. Gutierrez-Gutierrez et al. (2011) indicated that Ritcher 110 and SO4 rootstocks are resistant to M. arenaria. Furthermore, Ferris et al. (2012) found that the MGT 101-14 rootstock was resistant and Ritcher 110 moderately resistant to M. arenaria. The authors also found Paulsen 1103 was susceptible, which is corroborated in this research. Moreover, Quebranta and Torontel cultivars, with an RF = 2.43 and 1.6, respectively, are considered susceptible to M. arenaria (Table 2).
Regarding the reaction of the rootstocks to M. hapla, SO4, MGT 101-14, K 5BB, Ritcher 110, and Paulsen 1103 were resistant, except Salt Creek, which was susceptible, with an RF = 1.39. According to Télis and Landa (2007), Salt Creek, SO4, and K5BB are resistant to M. hapla. Moreover, according to Aballay and Vilches (2015), SO4, K5BB, Paulsen 1103, and MGT 101-14 are not immune to M. hapla, but rather are more resistant than a grapevine cultivar. Ritcher 110 and SO4 rootstocks are resistant to M. hapla (Gutierrez- Gutierrez et al., 2011). These reports differed from the results obtained in the experiment. On the contrary, Dalmasso and Cuani (1976) reported that SO4 is susceptible to M. hapla. Finally, Quebranta and Torontel cultivars presented the highest population levels, with a RF = 3.49 and 3.37, indicating susceptibility (Table 3).
CONCLUSIONS
Results indicate that SO4, K5BB, Ritcher 110, MGT 101-14, Paulsen 1103, and Salt Creek rootstocks are resistant to M. incógnita and M. arenaria. Only the Salt Creek rootstock is susceptible to M. hapla. The cultivars Quebranta and Torontel are susceptible to M. incógnita, M. arenaria and M. hapla. As a conclusion, it is suggested to continue the studies of reaction to Meloidogyne with other rootstocks and root-knot nematodes, which is essential for a right choice of the rootstock to use.















