Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
InVet
versión On-line ISSN 1668-3498
InVet vol.12 no.1 Ciudad Autónoma de Buenos Aires ene./jun. 2010
ARTÍCULO DE INVESTIGACIÓN
Non-enzymatic lipid peroxidation from liver, heart and kidney of chinchilla and rat
Gutiérrez, AM.1; Gavazza, M.2; Marmunti, M.2; Palacios, A.2*
1Cátedra de Fisiología Animal, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Calle 122 y 60 CC296, B1900 AVW, La Plata, Buenos Aires, Argentina.
2Cátedra de Bioquímica, Facultad de Ciencias Veterinarias, Universidad Nacional de La Plata, Calle 60 y 118 CC296, B1900 AVW, La Plata, Buenos Aires, Argentina. *Corresponding author. Tel: +54 221 4236663; fax: +54 221 4257980. Email:apalacios@fcv.unlp.edu.ar
Recibido: 19/05/10
Aceptado: 19/08/10
Summary
Much work has been carried out on non-enzymatic induced lipid peroxidation from different tissues of rat, but little information is available about this process in chinchilla. Studies were carried out to determine the levels of lipid peroxidation of homogenates isolated from liver, heart and kidney of chinchilla and rat. Fatty acids located in total lipids of tissues studied were determined using gas chromatography and lipid peroxidation was evaluated by chemiluminescence assay. The lipid peroxidation process was more effective in rat liver and kidney homogenates than in chinchilla. In homogenates from liver and kidney of both species a significant decrease of C20:4n6 and C22:6n3 acids were observed during lipoperoxidation. However, changes were not observed in the fatty acid profiles of heart. In the heart fatty acid profile does not appear to be responsible for the different susceptibility to the lipid peroxidation. These results indicate that a low degree of fatty acid unsaturation in liver and kidney chinchilla, may confer advantage by decreasing their sensitivity to lipid peroxidation process of this specie.
Key words: Heart; Kidney; Liver; Lipid peroxidation; Chinchilla; Rat.
Peroxidación lipídica no enzimática en hígado, corazón y riñón de chinchilla y rata.
Resumen
Peroxidación lipídica no enzimática en hígado, corazón y riñón de chinchilla y rata. Si bien se han realizado varios ensayos de peroxidación lipídica no enzimática en diferentes tejidos de rata, se tiene muy poca información acerca de este proceso en chinchillas. En el presente estudio se llevaron a cabo determinaciones comparativas acerca de los niveles de peroxidación lipídica en homogenatos aislados de hígado, corazón y riñón en chinchillas y ratas. Los ácidos grasos localizados en los lípidos totales de los tejidos estudiados fueron analizados utilizando cromatografía gaseosa y el proceso de peroxidación lipídica fue determinado mediante ensayos de quimioluminiscencia. El proceso de peroxidación lipídica fue más efectivo en homogenatos de hígado y riñón de rata que en los mismos tejidos aislados de chinchilla. Durante este proceso se observó que en homogenatos de hígado y riñón de ambas especies se produjo una disminución en el porcentaje de los ácidos grasos C20:4 n6 y C22:6 n3. Sin embargo estos cambios no fueron observados en el perfil de ácidos grasos de corazón. En este órgano, el perfil de ácidos grasos no parece ser responsable de su susceptibilidad a la peroxidación lipídica. Estos resultados indican que el bajo porcentaje de insaturación de hígado y riñón de chinchilla, podría conferir una ventaja debido a la disminución en la susceptibilidad al proceso de peroxidación lipídica en esta especie y esto podría representar una ventaja adaptativa frente a otras especies de roedores.
Palabras claves: Corazón; Riñón; Hígado; Peroxidación lipídica; Chinchilla; Rata.
Introduction
A low fatty acid unsaturation would be advantageous to longevous animals from the point of view of oxidative stress because it would decrease the sensitivity of their tissues and cellular organelles to lipid peroxidation. In addition, lipid peroxidation products are known to modify and damage other kinds of non-lipidic macromolecules including nearby proteins11,19 and DNA9.
Available comparative studies indicate that longevous animals have low rates of mitochondrial free radical production1, 25. While this is a relevant characteristic consistent with the free radical theory of aging 2, 14, additional factors can also lead to a low oxidative damage in longevous animals. Among tissue macromolecules, polyunsaturated fatty acids are the ones more sensitive to free radical damage, with their sensitivity increasing as a power function of the number of double bonds per fatty acid21, 22.
Aerobic metabolism entails the production of reactive oxygen species, even under basal conditions20, 24. Non enzymatic lipid peroxidation and formation of lipid peroxides can be initiated by adding ascorbate in the presence of oxygen and either Fe+3 or Fe+2 ions to various tissue preparations such as homogenates, mitochondrias, microsomal suspensions and nuclei6,12.
Lipid peroxidation proceeds by a chain reaction that includes initiation, propagation and termination. Initiation occurs when an oxidant gives rise to an initiating lipid peroxyl radical (LOO•) by reaction with either a lipid (LH) or pre-existing lipid hydroperoxide (LOOH). Propagation is cycled through rounds of LOO• abstraction of the bis-methylene hydrogen atoms of a polyunsaturated fatty acyl chain to generate additional LOO• (after O2 addition) which results in the net conversion of lipids to LOOHs. Lipid peroxidation termination involves the reaction of LOO• to form non-radical products or the reaction of one LOO• with another terminating radical to generate non-propagating radical species; the first reaction is particularly interesting as it is accompanied by emission of chemiluminescence28.The requirement of iron in lipid peroxidation has been demonstrated by Minotti et al. 199218.
The damage to different organelles and homogenates increased, because since they are rich in polyunsaturated fatty acids (PUFAs), mainly arachidonic (C20:4 n6) and docosahexaenoic (22:6 n3) acids, that are highly susceptible to lipid peroxidation28, 31. Much works were carried out about of lipid peroxidation process in different tissues of relatively short lived rodents like rat. But at this moment, little information exists about this process in Chinchilla lanígera, a relatively long lived rodent. The present study aims to compare fatty acid profiles and non enzymatic lipid peroxidation of homogenates obtained from liver, heart and kidney of chinchilla and rat. Chemiluminescence (cpm x 10-3) and fatty acid composition were used as an index of the oxidative destruction of lipids. The UI a parameter based on the maximal rate of oxidation of specific fatty acid was used to evaluate the fatty acid alterations observed during the process16.
Materials and methods
Chemicals
Butylated hydroxytoluene (BHT) and phenyl-methyl-sulfonyl fluoride (PMSF) were from Sigma (St. Louis, MO, USA). Bovine serum albumin (BSA) (Fraction V) was obtained from Wako Pure Chemical Industries, Japan. L (+) ascorbic acid and boron-trifluoride- methanol complex were from Merck. Standards of fatty acids methyl esters were from Nu Check Prep Inc, Elysian, MN, USA. All other reagents and chemicals were of analytical grade from Sigma.
Animals
Wistar AH/HOK rats, weighing 180-200 g, were obtained from Laboratory Animal Facility, Facultad de Ciencias Veterinarias, Universidad Nacional de La Plata. Chinchillas weighing 370- 500g were obtained from the farm. Both species were fed commercial chow and water was provided ad libitum. Animals were sacrificed by cervical dislocation and the organs were rapidly removed.
Preparation of liver, heart and kidney homogenates from chinchilla and rat
The organs obtained from both species were cut into small pieces and washed extensively with 0.15 M NaCl. An homogenate of each tissue was prepared in solution containing 0.25 M sucrose, 10 mM Tris-HCl pH 7.4, PMSF, phenyl-methyl-sulfonyl fluoride 0.1 mM), 3ml of solution per g of tissue, using the Potter- Elvejhem homogeneizer. All operations were performed at 4ºC. Homogenates were stored at -83ºC and used within a week of its preparation, after one cycle of freezing and thawing.
Chemiluminescence and nonenzymatic lipid peroxidation of homogenates
Chemiluminescence and lipid peroxidation were initiated by adding ascorbate-Fe++ to homogenates preparations29. Homogenates (1 mg of protein) were incubated at 37ºC with 0,01 M phosphate buffer (pH 7.4), 0.4 mM ascorbate, final volume 1 ml. Phosphate buffer is contaminated with sufficient iron to provide the necessary ferrous or ferric iron for lipid peroxidation, (final concentration in the incubation mixture was 2.15 μM)27. Homogenates preparations which lacked ascorbate-Fe++ (control) were carried out simultaneously. Chemiluminescence was measured as counts per min (cpm) in a liquid scintillation analyzer Packard 1900 TR. Homogenate light emission was determined over 120 min period, and recorded as cpm every 10 min and the sum of the total chemiluminescence was used to calculate cpm/mg protein.
Fatty acid analysis
Homogenates lipids from samples peroxidized in presence or in the absence of ascorbic acid were extracted with chloroform/ methanol (2:1 v/v containing 0.01 % butylated hydroxytoluene, BHT as antioxidant) (Folch et al. 1957)10. Fatty acids were transmethylated with 10% F3B in methanol at 60ºC for 3 h. Fatty acid methyl esters were analyzed with a GC-14A gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a packed column (1.80 m x 4 mm i.d.) GP 10% DEGS-PS on 80/100 Supelcoport. Nitrogen was used as a carrier gas. The injector and detector temperatures were maintained at 250ºC, the column temperature was held at 200ºC. Fatty acid methyl ester peaks were identified by comparison of the retention times with those of standards. All compositions were expressed as % by area of total fatty acid.
Unsaturation index
UI was calculated according to the formula, UI = sum (fatty acid percent) x (number of double bonds)16.
Protein determination
Proteins were determined by the method of Lowry et al. 195117 using BSA as standard.
Statistical analysis
Data were expressed as means ± S.D. Statistical analysis utilized was Student's t-test. Statistical criterion for significance was selected at different p values and indicated in each case.
Ethics
Animal treatment protocols were previously approved by the local ethics committee, and are in accordance with care and treatment of laboratory animals recommended guidelines (U.S. Public Health Service, 1985).
Results
Light emission of chinchilla and rat liver, heart and kidney homogenates during non enzymatic lipid peroxidation
The values of total light emission during the non enzymatic lipid peroxidation were higher in rat than in chinchilla. The incubation of rat and chinchilla liver and kidney homogenates in the presence of ascorbate-Fe++ resulted in the peroxidation of homogenates as evidenced by the emission of light (chemiluminescence) when the control and ascorbate-Fe++ groups were compared. Table 1 shows the total light emission produced by the liver, heart and kidney homogenates of both species obtained from control and ascorbate-Fe++ treated experiments. The value of the lipid peroxidation process was 18 and 3 fold higher in rat liver and kidney homogenates than in chinchilla, respectively. Under similar conditions, changes in light emission were not observed when rat and chinchilla homogenates from heart were assayed (Fig. 1).
Table I: Total chemiluminescence of chinchilla and rat liver, heart and kidney homogenates induced by ascorbate-Fe++
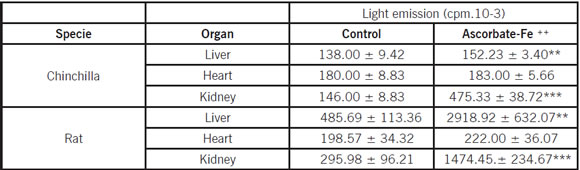
Chemiluminescence during lipid peroxidation induced by ascorbate-Fe++ of chinchilla and rat liver, heart and kidney homogenates (1 mg of protein). Control without ascorbic acid. Chemiluminescence was recorded as counts per minute was used to calculated light emission (cpm .10-3). Data are given as the mean ± S.D of three experiments. Statistically significant differences between control vs. ascorbate-Fe++ group are indicated by ***p < 0.0005 and **p < 0.005 using Student's t-test.

Fig 1: Total chemiluminescence produced by liver, heart and kidney homogenates obtained from Chinchilla and Rat peroxidized (presence of ascorbate-Fe++) groups. Results are expressed as mean ±S.D. of three independent experiments. Statistically significant differences between rat vs. chinchilla are indicated by ***p<0.0005 and **p<0.005.
Fatty acid profiles of homogenates obtained from chinchilla and rat liver during non enzymatic lipid peroxidation
Tables II and III show the fatty acid composition of total lipids from control and ascorbate-Fe++ groups in liver homogenates. The saturated long chain fatty acid present in liver homogenate obtained from rat and chinchilla were mainly C16:0 and C18:0 in a percentage of approximately 40-50%. The concentration of total unsaturated fatty acids of liver homogenate presented in both species was approximately 50%. The content of monounsaturated fatty acid in chinchilla liver homogenate was higher than that present in rat. The concentration of PUFAs was different in both species: in chinchilla was approximately 9%, whereas in rat was approximately 44%. The UI was lower in chinchilla liver homogenate rather than in rat. When ascorbate-Fe++ liver homogenate was compared with control, it was observed that arachidonic (C20:4 n6), docosahexaenoic (C22:6 n3) acids and UI decreased in both species.
Table II: Fatty acid composition (area %) of chinchilla liver, heart and kidney homogenates during non-enzymatic lipid peroxidation
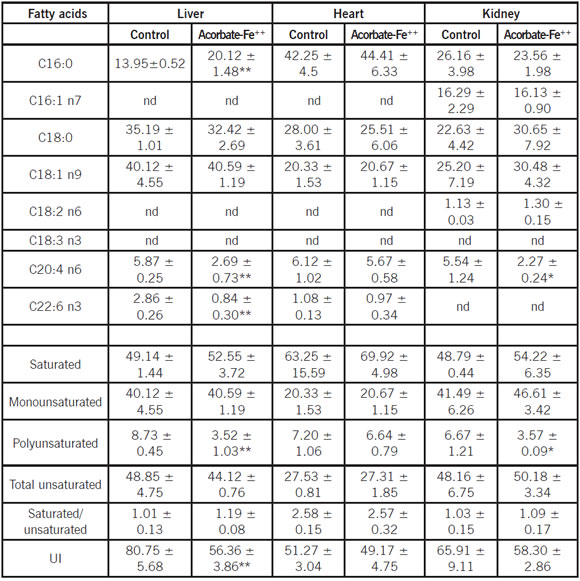
Data are given as the mean ± S.D. of three experiments. Statistically significant differences between control vs. ascorbate- Fe++ group are indicated by **p < 0.005 and *p < 0.05 using Student's t-test
Table III: Fatty acid composition (area %) of rat liver, heart and kidney homogenates during non-enzymatic lipid peroxidation

Data are given as the mean ± S.D. of three experiments. Statistically significant differences between control vs. ascorbate- Fe++ group are indicated by ***p < 0.0005, **p < 0.005 and *p < 0.05 using Student's t-test.
Fatty acid profiles of homogenates obtained from chinchilla and rat heart during non enzymatic lipid peroxidation
Tables II and III show the fatty acid composition of total lipids from control and ascorbate-Fe++ groups in heart homogenates. The saturated long chain fatty acids present in heart homogenate obtained from rat and chinchilla were mainly C16:0 and C18:0 in a percentage of approximately 30 and 60%, respectively. The concentration of total unsaturated fatty acids of heart homogenate presented in rat and chinchilla was approximately 50 and 27%, respectively. The content of monounsaturated fatty acids in chinchilla heart homogenate was higher rather than in rat. The concentration of PUFAs was different in both species: in chinchilla was approximately 7 %, whereas in rat was approximately 35%. However, when ascorbate-Fe++ heart homogenate were compared with control, no significant differences in the content of C20:4 n6, C22:6 n3 and UI in both species were observed.
Fatty acid profiles of homogenates obtained from chinchilla and rat kidney during non enzymatic lipid peroxidation
Tables II and III show the fatty acid composition of total lipids from control and ascorbate-Fe++ groups in kidney homogenates. The saturated long chain fatty acids present in kidney homogenate obtained from rat and chinchilla were mainly C16:0 and C18:0 in a percentage of approximately 42-50%. The concentration of total unsaturated fatty acid of kidney homogenate presented in both species was approximately 55%. The content of monounsaturated fatty acids in chinchilla kidney homogenate was higher than that present of rats. The concentration of PUFAs was different in both species: in chinchilla was approximately 7%, whereas in rat was approximately 42%.
When ascorbate-Fe++ kidney homogenate were compared with control, it was observed in chinchilla kidney homogenate only arachidonic acid (C20:4 n6) diminished, whereas in rat kidney homogenate C20:4 n6 and C22:6 n3 decreased significantly. The UI was lower in chinchilla kidney homogenate rather than in rat.
Discussion
Lipid peroxidation can alter the cellular structure of membrane-bound enzymes by changing the membrane phospholipids fatty acid composition. Lipid peroxidation has gained renewed attention with increasing evidence showing its biological role in aging animals. The assessment of lipid peroxidation levels in vivo is difficult partly because lipids are oxidized by different oxidants by different mechanisms to give versatile types of products, which may undergo metabolism and secondary reactions30.
Chemiluminescence is the light emission derived from a chemical reaction such as the last reaction of the lipid peroxidation in which chemically excited molecules decay to the electronic ground state and emit photons.
Measurement of light emission from a chemical reaction is very useful from an analytical point of view because, under appropriate experimental conditions, light output is directly related to the analytic concentration, thus allowing precise and sensitive quantitative analysis. In addition, the light emission is usually represented by steady-state kinetics, which simplifies sample handling and measurement procedures. Chemiluminescence has widely been used as an indicator of reactive oxigen species formation in cells and whole organs, thus allowing the study of a number of pathophysiological conditions related to oxidative stress23.
A complementary evolutionary reason for the low number of double bonds of mammals with large body size could be a relationship with longevity. Individual acyl chains differ greatly in their chemical propensity for oxidative damage. The n-3 PUFA are more peroxidation-prone than n-6 PUFA and within each PUFA class there is a 4-fold increase in peroxidizability between the short- and long-chain fats. C22:6 n3 is 320-fold more susceptible to peroxidation than 18:1 n9 15.
In addition, lipid peroxidation products are known to damage nearby macromolecules including DNA9,5, with expected long-term consequence for aging7. It is logical to think that the low degree of fatty acid unsaturation of longevous mammals will protect their tissues against oxidative damage, while at the same time it may also contribute to lowering their basal metabolic rates8.
Previous investigations of our laboratory have shown that the degree of unsaturation of fatty acids and the sensitivity to lipid peroxidation of liver mitochondria is lower in longevous pigeon than in the short-lived rat13. Therefore, from the point of view of unsaturated C20:4 n6 was lower in pigeon than in the rat; as a consequence, the UI was significantly lower in pigeon than in rat liver mitochondria13.
On the other hand, in a previous report12, we have demonstrated that the PUFAs content was lower in liver mitochondria from bovine than in rat, but similar in kidney mitochondria of both species.
In agreement with previous studies of liver mitochondria of rats, pigeons, and humans19 and between different mammalian species20, in the present comparative study describes for the first time the fatty acid composition and sensitivity to lipid peroxidation of homogenates obtained from liver, heart and kidney of chinchilla (Chinchilla lanigera).
Our results indicated that light emission was 18 and 3 fold higher in rat liver and kidney homogenates rather than in chinchilla, respectively. These results are consistent with the low UI and low sensitivity to lipid peroxidation observed in Chinchilla.
Thus, a low degree of fatty acid unsaturation may have been selected in longevous animals in order to protect their tissue lipids and proteins against oxidative damage, while maintaining an appropriate environment for membrane function in terms of transition temperature, and hence in the membrane fluidity 3,26. These last parameters are strongly affected only by the first and second double bond introduced in the fatty acid chain4. The main differences in unsaturated fatty acids observed in this work are a substitution of 22:6 n3 and C20:4 n6 in short-lived animals for C18:2 n6 and C18:1 n9 in long-lived animals. It is reasonable to think that the lower degree of fatty acid unsaturation of longevous animals will protect their tissues against oxidative damage. The low UI of longevous animals can thus be responsible in part for their relatively higher resistance to lipid peroxidation.
Another interesting finding in this study is that heart homogenates of both species exhibited less sensitivity to in vitro lipid peroxidation and showed lower values of UI than homogenates isolated from liver and kidney. Nevertheless, the PUFAs content was lower in heart homogenate from chinchilla than in rat, but in both species the heart homogenated was not affected by the lipid peroxidation process.
The relative longevity and the presence of high levels of monounsaturated fatty acids, essentially incapable of being peroxidized allow us to think that Chinchilla lanígera could be an excellent model for laboratory studies.
1. Barja, G. Mitochondrial free radical generation: sites of production in states four and three, organ specificity and relationship with aging rate. J Bioenergetics Biomembr. 1999; 31: 347-365. [ Links ]
2. Beckman, K., Ames, B. The free radical theory of aging matures. Physiol Rev. 1998; 78: 547-581. [ Links ]
3. Brand, M.D., Couture, P., Hulbert, A.J. Liposomes from mammalian liver mitochondria are more polyunsaturated and leakier to protons than those from reptiles. Comp. Biochem. Physiol. 1994; 108 B: 181-188. [ Links ]
4. Brenner, R.R. Effect of unsaturated acids on membrane structure and enzymes kinetics. Prog Lipid Res. 1984; 23(2): 69-96. Review. [ Links ]
5. Box, H., Maccubin, A. Lipid peroxidation and DNA damage. Nutrition. 1997; 13: 920-921. [ Links ]
6. Catalá, A., Cerruti, A. Non enzymatic peroxidation of lipids isolated from rat liver microsomes, mitochondria and nuclei. Int J Biochem Cell Biol.1997; 29: 541-546. [ Links ]
7. Chen, J., Yu, B. Detoxification of reactive aldehydes in mitochondria: effects of age and dietary restriction. Aging Clin Exp Res. 1996; 8: 334-340. [ Links ]
8. Cutler, R. Peroxide-producing potential of tissues: inverse correlation with longevity of mammalian species. Proc Natl Acad Sci.1985; 82: 4798-4802. [ Links ]
9. Draper, H. Effects of peroxidative stress and age on the concentration of a deoxyguanosine-malonaldehyde adduct in rat DNA. Lipids. 1995; 30: 959-961. [ Links ]
10. Folch, J., Lees, N., Sloane Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem.1957; 226: 497-509. [ Links ]
11. Fosmark Andree, P., Dallner, G., Ernster, L. Endogenous ubiquinol prevents protein modification accompanying lipid peroxidation in beef heart submitochondrial particles. Free Rad Biol Med. 1995; 19: 749-757. [ Links ]
12. Gavazza, M., Marmunti, M., Catalá, A. Sensitivity of mitochondria isolated from liver and kidney of rat and bovine to lipid peroxidation: A comparative study of light emission and fatty acid profiles. Mol Cell Biochem. 2005; 280: 77-82. [ Links ]
13. Gutiérrez, A., Reboredo, G., Arcemis, C., Catalá, A. Non enzymatic lipid peroxidation of microsomes and mitochondria isolated from liver and heart of pigeon and rat. Int J Biochem Cell Biol. 2000; 32: 73-79. [ Links ]
14. Harman, D. Extending functional life span. Exper Gerontol. 1998; 33: 95-112. [ Links ]
15. Holman, R.T. Autooxidation of fats and related substances. In: Holman, R.T.; Lundberg, W.o., Malkin, t: (Eds), Progress in chemistry of fats and other lipids, vol 2. Pergamon Press, London, 1954, pp. 51-98. [ Links ]
16. Lanillo, M., Sanchez Yague, J., Checa, A., Martín-Valmaseda, E., Felipe, A. Phospholipid and fatty acid composition in stored sheep erythrocytes of different densities. Exp Hematol. 1995; 23: 258-264. [ Links ]
17. Lowry, O., Rosebrough, N.; Farr, A.; Randall, R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193: 265-275. [ Links ]
18. Minotti, G., Aust, S. Redox cycling of iron and lipid peroxidation. Lipids. 1992; 27: 219-226. [ Links ]
19. Pamplona, R., Prat, J., Cadenas, S., Rojas, C., Pérez-Campo, R., López Torres, M.; Barja, G. Low fatty acid unsaturation protects against lipid peroxidation in liver mitochondria from long lived species: the pigeon and human case. Mech Age Dev. 1996; 86: 53-66. [ Links ]
20. Pamplona, R., Portero-Otin, M., Riba, D., Ruiz, C., Prat, J., Bellmunt, M., Barja, G. Mitochondrial membrane peroxidizability index is inversely related to maximum life span in mammal. J Lipid Res. 1998; 39: 1989-1994. [ Links ]
21. Pamplona, R., Portero-Otin, M., Ledo, D., Gredilla, R., Barja, G. Heart fatty acid unsaturation and lipid peroxidation, and aging rate, are lower in the canary and the parakeet than in the mouse. Aging Clin Exp Res. 1999; 11: 44-49. [ Links ]
22. Pamplona, R., Portero-Otin, M., Ruiz, C., Gredilla, R., Herrero, A., Barja, G. Double bond content of phospholipids and lipid peroxidation negatively correlate with maximum longevity in the heart of mammals. Mech Ageing Dev. 2000; 112: 169-183. [ Links ]
23. Roda, A., Pasini, P., Guardigli, M., Baraldini, M., Musiani, M., Mirasoli, M. Bio and chemiluminescence in bioanalysis. J Anal Chem. 2000; 366: 752-759. [ Links ]
24. Sies, H. Strategies of antioxidant defense. Eur J Biochem. 1993; 215: 213-219. [ Links ]
25. Sohal, R., Weindruch, R. Oxidative stress, caloric restriction and aging. Science. 1996; 273: 59-63. [ Links ]
26. Stubbs, C.D., Smith, A.D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984; 779:89-137. [ Links ]
27. Tadolini, B., Cabrini, L., Menna, C., Pinna, G., Hakim, G. Iron [III] stimulation of lipid hydroperoxide-dependent lipid peroxidation. Free Rad Res. 1997; 27: 563-576. [ Links ]
28. Vladimirov, Y., Olenev, V., Suslova, T., Cheremisina, Z. Lipid peroxidation in mitochondrial membrane. Adv. Lipid Res. 1980; 17: 173-249. [ Links ]
29. Wright, J., Rumbaugh, R., Colby, H., Miles, P. The relationship between chemiluminescence and lipid peroxidation in rat hepatic microsomes. Arch Biochem Biophys. 1979, 192: 344-351. [ Links ]
30. Yoshida, Y., Saito, Y., Hayakawa, M., Habuchi, Y., Imai, Y., Sawai, Y., Niki, E. Levels of lipid peroxidation in human plasma and erythrocytes: comparison between fatty acids and cholesterol. Lipids. 2007; 42: 439-49. [ Links ]














