Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
InVet
versión On-line ISSN 1668-3498
InVet vol.15 no.1 Ciudad Autónoma de Buenos Aires jun. 2013
ARTÍCULO DE INVESTIGACIÓN
Boar semen: complementary techniques for its evaluation
González, L.O.1; Fischman, M.L.1; Boquet, M.2; Acerbo, M.C.; Miguez, M.S.; Cisale, H.O. 1; Ferrari, M.R. 1
1Cátedra de Física Biológica. Facultad de Ciencias Veterinarias. UBA. Instituto de Investigación y Tecnología en Reproducción Animal (INITRA). Chorroarín 280 (C1427CWO). CABA. República Argentina.
2Facultad de Ciencias Naturales. Universidad Nacional de la Patagonia San Juan Bosco (UNPSJB) Sede Esquel. Chubut, Argentina.
Correspondencia e-mail: Liliana O. González lgonzalez@fvet.uba.ar
Recibido: 01/11/2012
Aceptado: 02/12/2013
Summary
Nuclear parameters of the spermatozoa (morphology, maturation and condensation degree), sperm functional capacity (membrane response to hypoosmotic medium and sperm resistance to heat incubation) and semen quality were evaluated in sixteen healthy, sexually mature and fertile boars. Sperm nuclei were stained with the Feulgen reaction to observe morphology, with Aniline Blue to determine chromatin maturation, and with Toluidine Blue to determine chromatin condensation. The mean percentage and standard error of normal nuclei in each of the three tests was: 96.6±0.8, 98.1±1.1 and 99.6±0.2 respectively. The percentage of sperm with total motility before heat incubation was 62.3±3.9, whereas that of sperm with progressive motility was 35.0±4.6 and that of Hypoosmotic Swelling Test+ (HOS+) cells 53.3±2.5. After heat incubation (Thermoresistance Test), the percentage of sperm with total motility was 37.3±3.5, that of sperm with progressive motility 13.4±3.6, and that of HOS+ cells 37.7±3.5. Nuclear parameters did not correlate significantly between each other or with the other sperm parameters studied. Total motility had correlation with: progressive motility, sperm viability, HOS test and total motility and HOS test after Thermoresistance Test. Consequently, combining different complementary tests would improve estimations of semen boar quality.
Key words: Boar; Sperm nuclei morphology; Aniline Blue; Toluidine Blue; HOS test; Thermoresistance test.
Técnicas complementarias para la evaluación de semen porcino
Resumen
Los parámetros del núcleo espermático (morfología, maduración y grado de condensación), la capacidad funcional de los espermatozoides (respuesta de la membrana al medio hipoosmótico y la resistencia térmica) y la calidad del semen se evaluaron en dieciséis cerdos sanos, sexualmente maduros y fértiles. Los núcleos espermáticos se colorearon con la reacción de Feulgen para observar su morfología, con Azul de Anilina para determinar la maduración de la cromatina y con Azul de Toluidina para determinar su condensación. El porcentaje y error estándar de los núcleos normales en las tres pruebas fue: 96.6±0.8, 98.1±1.1. 99.6±0.2 respectivamente. El porcentaje de espermatozoides con movilidad total antes de la prueba de resistencia térmica fue 62.3±3.9. mientras que la movilidad progresiva 35.0±4.6 y las células positivas a la prueba Hipoosmótica (células HOS +) 53.3±2.5. Luego de la incubación térmica el porcentaje de espermatozoides con movilidad total era 37.3±3.5, el de espermatozoides con movilidad progresiva 13.4±3.6 y el de las células HOS+ 37.7±3.5. Los parámetros nucleares no se correlacionaron entre sí ni con los demás parámetros estudiados. La movilidad total presentó correlación con: la movilidad progresiva, la viabilidad espermática, la prueba hipoosmótica y luego de la prueba de resistencia térmica con la movilidad total y la prueba hipoosmótica. Por consiguiente, la combinación de técnicas complementarias podría mejorar la estimación de la calidad del semen porcino.
Palabras clave: Cerdos; Morfología del núcleo espermático; Azul de Anilina; Azul de Toluidina; Prueba Hipoosmótica; Prueba de Resistencia Térmica.
Introduction
The use of artificial insemination in the pig industry has grown during the last years. In porcine artificial insemination, single ejaculates are divided into doses and used to breed multiple females. Thus, it is economically significant to evaluate semen quality.
Routine tests usually determine sperm concentration, percentage of sperm motility, viability and normal morphology, but may not reveal subtle sperm defects19.
Some other assays try to explore the functional capacity of sperm. One of them is the hypoosmotic swelling (HOS) test. This test has been successfully related to in vivo and in vitro fertility and has been widely used in both humans and domestic species, but to now its use in boar spermatozoa has been limited12. The Thermoresistance test is carried out incubating semen at different times and temperatures and has been used to measure the sperm ability to survive in the sow's reproductive tract and retain their fertility22. Torres Bianchini et al.24 determined that a good protocol to detect differences between fresh semen samples is a combination of the slow Thermoresistance test and the HOS test. The sperm DNA quality has high importance in the prognosis of fertility, and several methods have been developed to evaluate sperm chromatin maturity and DNA integrity8. In andrological practice, mostly indirect methods are used. These methods are based on the ability of some stains to test the conformation of sperm chromatin, which depends on DNA strand breaks and DNA interaction with proteins10. Aniline Blue and Toluidine Blue are two of these stains2, 15.
Aniline Blue staining provides a specific positive reaction for lysine. The histone-rich nuclei of immature spermatozoa contain abundant lysine, which reacts positively by taking up the Aniline Blue stain, whereas the protamine-rich nuclei of mature spermatozoa with abundant arginine and cysteine react negatively and remain unstained15.
Toluidine Blue is a basic nuclear dye used for chromatin metachromatic and orthochromatic staining2, 10. The test measures the ability of the sperm chromatin DNA phosphate residues to be stained with Toluidine Blue, which is dependent on the protein state and DNA integrity25. While sperm with chromatin abnormalities are usually stained from dark blue to violet, normal ones are pale blue or not colored28.
Sperm with chromatin abnormalities frequently display abnormal head shapes and could have potential diminished fertility or could be associated with abortions6. Several studies have shown relationships between chromatin structure, DNA damage and abnormal sperm morphology11, 31. Feulgen reaction, which is a staining technique specific and stoichiometric for nuclear DNA, allows proper observations of the nuclear sperm morphology11, 28, and some authors obtained significant correlations between the observation of Feulgen-stained nuclei and results of the Sperm Chromatin Structure Assay test (SCSA test)9.
The aims of the present study, carried out on chilled semen samples obtained from healthy fertile boar, which were collected from May to October, were: i) to determine the percentage of morphologically normal and abnormal sperm nuclei and estimate the degree of maturation and compaction of the sperm nuclei according to their responses to Aniline and Toluidine Blue stains; and ii) to estimate the correlation between nuclear parameters, the HOS test, the Thermoresistance test and conventional parameters of semen evaluation (sperm viability, sperm morphology, acrosomal integrity, total motility and progressive motility).
Materials and methods
Semen collection and processing.
Commercial semen doses processed for artificial insemination were provided by Degesa Argentina S.A. and received at the Laboratory of Semen Quality and Cryopreservation of the Facultad de Ciencias Veterinarias UBA, CABA Argentina, within 24h after collection. The animals belonged to the Austral Line; this line was obtained by crossing three pure breeds (Large White, Pietrain and Hampshire). The animals were housed in the same raising and feeding conditions and submitted to a same rhythm of semen collection. Doses from ejaculate from sixteen fertile, mature sexually and healthy boars (one ejaculate for each boar) were collected from May to October (late autumn-winter-early spring). The sperm-rich fraction was collected into a pre-warmed insulated flask, using the gloved-hand technique. Immediately after collection, the semen was diluted 1:1 (v/v) in Beltsville Thawing Solution (BTS , IMV, USA), and stored for a maximum of 24 hours at 17- 18ºC.
Motility.
Subjective total motility (TM ) and progressive sperm motility (PM) was estimated in chilled semen samples using a phase-contrast microscope (400X) with a thermal stage at 37ºC29.
Viability.
To analyze viability, semen smears were stained with eosin-nigrosin and 200 spermatozoa were evaluated with a phasecontrast light microscope (1000X)17.
Hypoosmotic Swelling Test.
HOS test was conducted as previously described by Campi et al4. The test was performed by mixing 25 µl of sperm sample with 1ml of 100 mosml/l hypo-osmotic medium (490 µg sodium citrate: 900 µg fructose: 100 ml distilled water), and incubating for 10 min at 37°C. After incubation, the reaction was stopped by adding 10 µl of formaldehyde hypoosmotic solution (3 µl formaldehyde in 1000 µl hypoosmotic solution). A minimum of 200 spermatozoa were evaluated using a phase contrast microscope (400). The number of sperm with curled tails (viable) and non-curled tails (non-viable) present was recorded.
Acrosome integrity.
Was analyzed with modified Pursel and Johnson18 method. A 5µl aliquot of chilled semen was fixed in 1ml of 2,5% glutaraldehyde media to inhibit motility.
A minimum of 200 spermatozoa were evaluated using a phase contrast microscope (1000x)
Morphology.
Bengal Rose staining was performed on semen smears according to Windt et al30. In each sample, two hundred spermatozoa were observed using a light microscope (1000X).
Thermoresistance test.
The capability of spermatozoa to resist incubation at 37ºC for 1h was evaluated using the technique described by Torres Bianchini et al.24.
Nuclear tests.
The results of the following tests were estimated using a light microscope (1000X), and 250 randomly selected sperms per sample were examined by the same operator.
Feulgen reaction.
Air-dried smears, fixed in ethanol-acetic acid (3:1, v/v), were processed according to Ferrari et al.11.
Aniline Blue staining (AB).
Air-dried smears were processed according to Vieytes et al.28
Toluidine Blue Staining (TB).
Air-dried smears were stained according to Vieytes et al.28.
Statistical analysis.
Pearson's correlation was used to calculate relationships between the sperm variables measured. The results were also analyzed with descriptive statistics.
Results
The mean percentages and standard errors of the following parameters were obtained from single chilled samples of the sixteen boars: Total Motility (TM) - 62.3 ± 3.9 -, Progressive motility (PM) - 35.0 ± 4.6 -, Sperm Viability (SV) -72.6 ± 3.0 -, cells positively reacted to the Hypoosmotic Swelling Test (HOS+) - 53.3 ± 2.5 -, Acrosome Integrity (AI) - 83.3 ± 1.9 -, total motility after Thermoresistance Test- (TMTR) - 37.3 ± 3.5 -, progressive motility after Thermoresistance Test (PMTR) - 13.4 ± 3.6 -, sperm positive to the HOS Test after Thermoresistance Test (HOS+TR) - 37.7 ± 3.5 -, and Normal Morphology (NM) - 91.87 ± 1.42 -. Spermatozoa with attached cytoplasmic proximal droplets, bent tail and/or abnormal heads were considered as abnormal morphologies.
The coefficient of variation (CV) was determined for all the parameters. The lowest value was obtained for the NM (CV=6%), and the values for AI, SV, HOS, and TM were lower than 27%. The remaining parameters (PM, TMTR, PMTR, HOS+TR) had a CV higher than 35%, thus indicating an important variability between the observed ejaculates.
Figure 1 displays porcine nuclei stained with Feulgen Reaction showing normal and some abnormal nuclear morphologies. Morphologically normal nuclei were considered those that belonged to the largest population. The nuclei with normal morphology presented some variability between them. Optical observations allowed discriminating three subpopulations: quasi rectangular nuclei, sharply tapering nuclei and slightly tapering nuclei. Several types of abnormal nuclei were recognized: pyriform, large, narrow, vacuolated, putative diploid nuclei, and with abnormal condensation.

Figure 1. Examples of sperm nuclei illustrating various shapes of normal and abnormal morphologies: a, b) pyriform; c) small; d) large; e) round; f ) narrow; g) vacuolated; h) "diploid"; i) abnormal condensation; j, k, l) normal nuclei. +rectangular nuclei, ++sharply nuclei, +++slightly tapering nuclei (Magnification ca. 2000 x)
The mean percentages and standard error of the presence of different sperm nuclear morphologies were obtained from single chilled samples of the sixteen boars: normal (96.6 ± 0.8), pyriform (0.6 ± 0.3), small (0.6 ± 0.1), large (0.4 ± 0.1), round (0.4 ± 0.1), narrow (0.4 ± 0.2), vacuolated (0.6 ± 0.4), diploid (0.1 ± 0.0) and with abnormal condensation (0.2 ± 0.1). Fourteen of the samples had more than 96 % of morphologically normal nuclei. The CV of the percentage of the morphologically normal sperm nuclei was very low (3.2%), indicating that this parameter was similar in the ejaculates used in the present work. All the abnormalities were found in at least one of the animals. Vacuolated nuclei were those with the highest percentage (5.6%). Putative diploid nuclei were observed in only two of the sixteen animals, while small nuclei were found in the highest number of individuals. Aniline Blue stain allowed distinguishing three classes of nuclei with different staining intensities: unstained, partially stained and completely stained, whereas the Toluidine Blue stain allowed distinguishing two classes of nuclei: unstained and stained. The mean percentages and standard error for sperm Aniline Blue dyed are the following: unstained (98.1 ± 1.1), partially stained (0.4 ± 0.2) and completely stained (1.2 ± 1.0), and for sperm Toluidine Blue dyed: unstained (99.6 ± 0.2) and stained (0.7 ± 0.3). The percentage of nuclei unstained with AB was very high (98.1±1.1) and the CV was 3.9%. The percentage of nuclei unstained with TB was also very high (99.6±0.2%) and the CV very low (0.7%).
Table 1 shows the correlations between the twelve parameters studied in the present work. These values were obtained using the Pearson coefficient. Total motility was the parameter which showed more significant correlations with other parameters (PM, SV, HOS+, TMTR, HOS+TR). No correlation was found among nuclear parameters or between nuclear parameters and other parameters.
Table 1. Correlation coefficients between different seminal parameters in the boar semen.
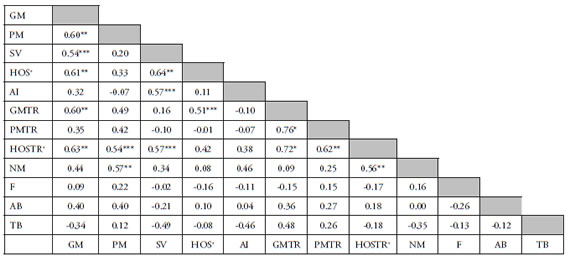
* p < 0.01 ** p < 0.03 and > 0.01 *** p < 0.05 and > 0.03 Without subscript P>0·05 GM Global Motility, PM Progressive Motility, SV Sperm Viability, HOS+ cells positively reacted to the HOS test, AI Acrosome Integrity, GMTR Global Motility after Thermoresistance, PMTR Progressive Motility after Thermoresistance, HOSTR+ cells positively reacted to the HOS test after Thermoresistance, NM Normal Morphology, F Morphological normal nuclei stained with Feulgen, AB Nuclei unstained with Aniline Blue, TB Nuclei unstained with Toluidine Blue.
Discussion
The ability to differentiate seminal quality among boars has a substantial economic impact on the swine industry12. Therefore, it is important to know the value of the semen parameters in populations of healthy and fertile animals. In this context, some non-standard methods such as the analysis of membrane function, determination of resistance to heat incubation and assessment of the chromatin characteristics are important to evaluate seminal quality8, 12.
The hypoosmotic swelling values obtained in the present study were below those of the eosin-unstained spermatozoa and the motile spermatozoa, thus indicating that the HOS test evaluates aspects of sperm membrane different from those evaluated by the two other sperm tests. According to different authors, viability tests measure whether the membrane is intact or not, whereas the HOS test determines functional integrity of sperm membranes3. However, good correlation coefficients have been obtained between HOS+ cells and sperm viability, total motility and total motility after Thermoresistance test, these results agree with those obtained by Gadea12. The use of the Thermoresistance test was recommended by Kozumplik and Roubal16 to evaluate the quality of fresh boar ejaculates. Both the CV of total motility and of membrane response evaluated with the HOS test increased considerably their values after incubation at 37ºC for 1 h.
The structure and functionality of the sperm nucleus are parameters of great importance in semen quality, and associations between reduced fertility or abortions and disturbances in nuclear morphology, maturation and condensation have been described in many reports6, 15. In the present study, indirect methods have been used to estimate nuclear characteristics.
As the mammalian sperm head contains little more than chromatin, nuclear and head shapes are related to DNA content and chromatin organization11, 31.
A very high percentage of nuclei with normal morphology were observed in all the sixteen boars and the very low CV (3.14%) obtained for this parameter, indicating that all animals were very similar regarding this characteristic. Although only one ejaculate from each boar was evaluate this is a reliable result because the relation between the number of normal and abnormal sperm nuclei according to González et al.14 did not differ significantly between the ejaculates of a same boar.
Morphologically distinct subpopulations of spermatozoa have been described within fresh boar ejaculates using morphometric assessments23. In the present study, these subpopulations were observed. However, the optical evaluation of these subpopulations did not allow us to establish a clear differentiation of the border between them, so we could not obtain percentage values for each one of them.
Types of morphological abnormalities of the sperm nuclei observed in the present study were similar to those described in Sus scrofa13. All the abnormalities were present in a very low percentage (between 0% and 5.63%). Other authors obtained similar values when analyzing boar sperm head morphology21. Pyriform, small, vacuolated and putative diploid nuclei ("diploid") were present in the different boar samples analyzed in this study. Spermatozoa classified as "diploid" are putative diploid because we did not determine the DNA content and the chromosome composition. However, our experience in different species and the information published by other authors indicate that diploid spermatozoa have a size optically similar to normal ones, have a slightly broader post-acrosomal zone than normal but when they are stained with Feulgen reaction and have a significant and unmistakable deeper coloration than haploid nuclei11, 20.
The sperm chromatin organizational status is characterized by a remarkable process of protein substitution and condensation5. Somatic histones, rich in lysines, are replaced by protamines. The numerous cysteine residues present in the protamines generate disulphide cross-links during the condensation process5 causing a highly compact chromatin in the mature sperm nuclei.
Aniline Blue stains the lysine residues of sperm nuclear histones specifically. Results obtained in the present study showed a very low number of nuclei stained with Aniline Blue and a mean of 98.11% of unstained nuclei, indicating a great replacement of histones by protamines. This result is in agreement with previous studies that have reported that protamination in boars is almost complete27. The very low Coefficient of Variation obtained (CV=3.9%) points out that all the animals were very similar for this characteristic.
Toluidine Blue is a classic nuclear dye used for metachromatic (strong blue) and orthochromatic (slightly blue) chromatin staining. The methachromatic TB reaction represents a simple cytochemical approach to detect sperm chromatin abnormalities based on differences in -SS- crosslinking1. In the present study, most of the nuclei were unstained (99.56±0.22) and all the animals had similar values according to the very low value of CV obtained (CV=0.7%). These results indicate that the chromatin compaction is high in all the animals.
According to our results, boar sperm nuclei with putative abnormalities were infrequent. Other authors, using the Acridine Orange test, also observed low chromatin instability7, 26.
In most artificial insemination centers, total motility is estimated routinely as a valuable technique for identification and elimination of subfertile ejaculates. The good correlation observed in the present study between TM and other parameters such as PM, SV, HOS+, TMTR and, HOS+TR might be explained by the fact that these parameters are related to mean cellular structures that contribute to the maintenance of motility.
In summary, sperm nuclear abnormalities are infrequent in healthy and fertile boars. No correlation was found among nuclear parameters or between nuclear parameters and others parameters in this work. These results seem to indicate that the genetic and/or physiological origin of the structures evaluated by these parameters would be different. Consequently, combining different complementary tests would improve estimations of semen boar quality.
Acknowledgements This research was conducted in Argentina and supported by grants from the Universidad de Buenos Aires UBAC yT V028, V008, 2002010010081. We thank Degesa Argentina S.A for kindly providing the cooled boar semen used in this study.
1. Andreetta, AM.; Stockert, JC.; Barrera, CA. A simple method to detect sperm chromatin abnormalities: cytochemical mechanism and possible value in predicting semen quality in assisted reproductive procedures. Int J Androl. 1995; 18:23-8. [ Links ]
2. Beletti, ME.; Mello, MLS. Comparison between the toluidine blue stain and the Feulgen reaction for evaluation of rabbit sperm chromatin condensation and their relationship with sperm morphology. Theriogenology. 2004; 62:398-402. [ Links ]
3. Cabrita, E.; Alvarez, R.; Anel, E.; Herráez, MP. The hypoosmotic swelling test performed with coulter counter: a method to assay functional integrity of sperm membrane in rainbow trout. Anim Reprod Sci. 1999; 55:279-87. [ Links ]
4. Campi, S.; Blasi, C.; Fischman, M.; García, C.; Cisale, H. Comparación entre dos test de funcionalidad de membrana para valorar semen de verraco. Veterinaria Argentina. 2004; 21:421-6. [ Links ]
5. Caron, C.; Govin, J.; Rousseaux, S; Khochbin, S. How to pack the genome for a safe trip. Prog Mol Subcell Biol. 2005; 38:65-89 . [ Links ]
6. Chemes, EH.; Rawe, YV. Sperm pathology: a step beyond descriptive morphology. Origin, characterization and fertility potential of abnormal sperm phenotypes in infertile men. Hum Reprod Update. 2003; 9:405-28. [ Links ]
7. De Ambrogi, M.; Ballester, J.; Saravia, F.; et.al. Effect of storage in short-and long-term commercial semen extenders on the motility, plasma membrane and chromatin integrity of boar spermatozoa. Int J Androl. 2006; 29:543-52. [ Links ]
8. Didion, BA.; Kasperson, KM.; Wixon RL.; Evenson, DP. Boar fertility and sperm chromatin structure status: a retrospective report. J Androl. 2009; 30:655-60. [ Links ]
9. Dobrinski, I.; Hughes, HPA.; Barth, AD. Flow cytometric and microscopic evaluation and effect on fertility of abnormal chromatin condensation in bovine sperm nuclei. J Reprod Fertil. 1994; 101:531-8. [ Links ]
10. Erenpreisa J.; Erenpreiss, J.; Freivalds, T.; et al. Toluidine blue test for sperm DNA integrity and elaboration of image cytometry algorithm. Cytometry. 2003; 52:19-27. [ Links ]
11. Ferrari, MR.; Spirito, SE.; Giuliano, SM.; Fernández, HA. Chromatin cytophotometric analysis of abnormal bovine spermatozoa. Andrologia. 1998; 30:85-9. [ Links ]
12. Gadea, J. Sperm factors related to in vitro and in vivo porcine fertility. Theriogenology. 2005; 63:431-44. [ Links ]
13. González, LO.; Giuliano, SM.; Ferrari, MR.; Spirito, SE.; Cisale, HO. Estudios preliminares de la morfología del espermatozoide y del núcleo espermático del jabalí (Sus scrofa). Revista de Medicina Veterinaria. 2005 86:64-66. [ Links ]
14. González, L.; Campi, SH.; Ferrari, M.; Fischman, ML.; Cisale, HO. Análisis intraindividuo de la frecuencia de núcleos espermáticos con distintas morfologías en eyaculados porcinos. InVet. 2010; 12:205-11. [ Links ]
15. Hammadeh, ME.; Al-Hasani, S.; Stieber, M.; et al. The effect of chromatin condensation (Aniline Blue staining) and morphology (strict criteria) of human spermatozoa on fertilization, cleavage and pregnancy rates in an intracytoplasmic sperm injection programme. Hum Reprod. 1996; 11:2468-71. [ Links ]
16. Kozumplík, J.; Roubal, M. Relation of heat resistance tests and sperm survival to pregnancy and fertility in sows. Vet Med (Praha). 1990; 35:725-32. [ Links ]
17. Pintado, B.; De La Fuente, J.; Roldan, E. Permeability of boar and bull spermatozoa to the nucleic acid stains propidium iodide or Hoechst 33258, or to eosin: accuracy in the assessment of cell viability. J Reprod Fertil. 2000; 118:145-52. [ Links ]
18. Pursel, V.; Johnson, L. Glutaraldehyde fixation of boar spermatozoa for acrosome evaluation. Theriogenology. 1974; 1:63. [ Links ]
19. Sharma, RK; Said, T.; Agarwal, A. Sperm DNA damage and its clinical relevance in assessing reproductive outcome. Asian J Androl. 2004; 6:139-48. [ Links ]
20. Spirito, S.; Campi, S.; Boquet, M.; Fernández, H.; Ferrari, M. Equine sperm nuclei with different ploidy levels: relationship between the nuclear DNA content and the nuclear area. Andrologia. 2011; 43:248-53. [ Links ]
21. SutkeviÄienÄ-, N.; Žilinskas, H. Sperm morphology and fertility in artificial insemination boars. Veterinarija ir Zootechnika. 2004; 26:11-3. [ Links ]
22. Tardif, S.; Laforest, JP; Cormier, N.; Bailey, JL. The importance of porcine sperm parameters on fertility in vivo. Theriogenology. 1999; 52:447-59. [ Links ]
23. Thurston, LM.; Watson, PF.; Mileham, AJ.; Holt WV. Morphologically distinct sperm subpopulations defined by fourier shape descriptors in fresh ejaculates correlate with variation in boar semen quality following cryopreservation. J Androl. 2001; 22:382-94. [ Links ]
24. Torres Bianchini, L.; Blasi, C.; García, C.; Jorge, A.; Fischman, M.; Cisale, H. Thermal resistance test for porcine spermatozoa. Preliminary report. Biocell. 2004; 28:110. [ Links ]
25. Tsakmakidis, I.A.; Lymberopoulos, AG.; Khalifa, TAA. Relationship between sperm quality traits and field-fertility of porcine semen. J Vet Sci. 2010; 11:151-4. [ Links ]
26. Tsarev, I.; Bungum, M.; Giwercman, A.; et al. Evaluation of male fertility potential by Toluidine Blue test for sperm chromatin structure assessment. Hum Reprod. 2009; 24:1569-74. [ Links ]
27. van der Heijden, GW.; Ramos L.; Baart EB.; et al. Sperm-derived histones contribute to zygotic chromatin in humans. BMC Dev Biol. 2008; Mar 31;8:34. [ Links ]
28. Vieytes, AL.; Cisale, HO.; Ferrari, MR. Relationship between the nuclear morphology of the sperm of 10 bulls and their fertility. Vet Rec. 2008; 163:625-9. [ Links ]
29. WHO laboratory manual for the examination and processing of human semen. 5th ed. 2010; Geneva, WHO Press, World Health Organization. pp.32-54. [ Links ]
30. Windt, ML.; De Beer, PM.; Franken, DR.; et al. Sperm decondensation and semen parameters: utilization of a simple staining technique for the evaluation of human sperm decondensation. Andrología 1994; 26, 67-72. [ Links ]
31. Zini, A.; Boman, JM.; Belzile, E.; Ciampi, A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2008; 23:2663-8. [ Links ]














