Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
InVet
versión On-line ISSN 1668-3498
InVet vol.16 no.1 Ciudad Autónoma de Buenos Aires jun. 2014
ARTÍCULO DE INVESTIGACIÓN
First report of Aelurostrongylus abstrusus in domestic land snail Rumina decollata, in the Autonomous city of Buenos Aires
Cardillo, N; Clemente, A; Pasqualetti, M; Borrás, P; Rosa, A; Ribicich M.
Cátedra de Parasitología y Enfermedades Parasitarias. Facultad de Ciencias Veterinarias. Universidad de Buenos Aires. Av. Chorroarin 280, 1427. Ciudad Autónoma de Buenos Aires, República Argentina.
Correspondencia e-mail: Natalia Cardillo ncardillo@fvet.uba.ar
Recibido: 07/01/2014
Aceptado: 07/11/2014
Summary
Aelurostrongylus abstrusus (Railliet, 1898) is a worldwide distributed lungworm that affects wild and domestic cats, causing bronchopneumonia of varying intensity. Cats became infected by eating slugs and snails with third infective stage larvae (L3). The aim of the study was to describe the presence of A. abstrusus in R. decollate snails. R. decollata specimens and samples of cats’ faeces were collected from the open spaces of a public institution of Buenos Aires city, inhabited by a stray cat population. Cats’ faeces were processed by Baermman´s technique and snails were digested in pool, by artificial digestion method. First stage larvae of A. abstrusus were recovered from 35.30 % (6/17) of the sampled faeces. An 80 % (20/25) snails pools were positive for the second and third larval stages. Mean value of total larvae recovered per pool was 150.64 and mean value of L3/pool was 93.89. This is the first report of the development of A. abstrusus infective larvae in R. decollate snail as intermediate host, since the relationship between high levels of infection in snails and in cats’ faeces could be demonstrated in cats’ habitat.
Key words: Aelurostrongylusabstrusus; Ruminadecollata; Intermediate host; Epidemiology.
Primer informe de Aelurostrongylus abstrusus en el caracol de tierra Rumina decollata, en la Ciudad Autónoma de Buenos Aires
Resumen
Aelurostrongylus abstrusus (Railliet, 1898) es un helminto pulmonar mundialmente distribuido que afecta a los gatos, causando bronconeumonias de variada intensidad. La infección se produce por ingestión de babosas y caracoles terrestres con larvas infectantes (L3). El objetivo del estudio fue describir la presencia de A. abstrusus en el caracol R. decollata. Se recolectaron muestras de heces felinas y caracoles presentes en una institución pública de la Ciudad Autónoma de Buenos Aires, habitada por una población de gatos sin propietario. Las heces fueron procesadas mediante la técnica de Baermman y los caracoles fueron digeridos en pool por digestión artificial enzimática. Larvas de primer estadio (L1) de A. abstrusus fueron recuperadas en el 35,30% (6/17) de las heces. El 80% (20/25) de los pooles de caracoles presentó larvas de segundo y tercer estadio. El promedio de larvas totales recuperado por pool fue de 150,64 y el valor medio de L3/pool fue de 93.89. Este es el primer hallazgo del desarrollo de larvas infectivas de A. abstrusus en el caracol doméstico R. decollata. Los altos niveles de infección encontrados en los caracoles y en las heces de los gatos demuestran el potencial de R. decollata como hospedador intermediario de A. abstrusus.
Palabras clave: Aelurostrongylus abstrusus; Rumina decollata; Hospedador intermediario; Epidemiología.
Introduction
Aelurostrongylus abstrusus (Railliet, 1898) is a worldwide distributed lungworm that affects the respiratory system of cats, and it causes bronchopneumonia of varying intensity9. In Argentina, this is an emerging disease that has been reported in urban domestic sphere of several cities in the provinces of Buenos Aires12, Corrientes14,21, and Santa Fe22. In 2005, the prevalence found in the city of Buenos Aires was 2.6%24. The prevalence increases among one-year and two-year old cats due to increased predator instinct and play kitten habits(with intermediate or paratenic hosts)9,28.
Cats acquire the parasite by eating slugs and snails with third infective stage larvae (L3) of A. abstrusus15, 28. Different species of gastropods have been reported as intermediate hosts for this nematode, including Agriolimax agrestis y A. columbianus, Helminthoglypta californiensis y H. nickliniana, Helicella spp.11, Helix aspersa10,6, Mesodonthyroidus, Triodopsisalbolabris, Biomphalaria glabrata2, Cernuella virgata15, Achatinafullica19, 28. Some birds, rodents, amphibians and reptiles can act as paratenic hosts 26,27. In cats, L3 migrate to the lungs where they become adults and reproduction occurs.
The first stage larvae (L1) are coughed up and swallowed. They are eliminated in host’s faeces to the environment where they can stay alive between 45 and 60 days10. When first stage larvae reach the molluscs, they actively penetrate foot integument, and they develop through two moults to reach the third stage larvae25.
Rumina decollata (Linnaeus, 1758) is a pulmonate land snail that belongs to the Subulinidae family20.This snail is native to southern Europe, northern Africa and western Asia, and it borders the Mediterranean Sea17. It is also widely distributed in large populations in other parts of the world. In America, it was accidentally introduced into the United States4, Mexico, Bermuda, Cuba23 and Uruguay through horticultural plants4. In 1970s, it was introduced into North America as a means of biological control of garden snails Helix aspersa. In Argentina, it has only been recorded in urban areas; first reported in 1988 in Buenos Aires province16, and recently, in La Pampa and Mendoza provinces6.
R. decollata is recognized as omnivorous, and they mainly feed from decaying fresh vegetable and organic matter like animal faeces. They also prey upon other land snails, eggs, worms and insects7. Francesco and Lagiglia (2007), reported that although they do not represent a significant threat to plants or agriculture, it has high potential to be a plague in the future. This could be important to suggest as a reservoir of pathogens for animals and even men.
This study was aimed at reporting the presence of A. abstrusus in a stray cat population living in a public institution of Buenos Aires city and the evidence of infection in R. decollata snail, as potential intermediate host of the parasite in the same environment.
Materials y methods
Studied area and samples collection
The study site was the open spaces of a human public Hospital located in the Autonomous city of Buenos Aires. Most of these general hospitals are found in fenced off spaces composed of both buildings and open spaces. Animals are not permitted access but while the general characteristics of the surrounding fences do not allow the entrance of dogs this is not the case for cats. A feline stray population usually inhabits the open spaces of these hospitals, without health care but being spontaneously fed by people from the area, or belonging to the institution.
There were collected individually 17 samples of cats’ faeces from the environment of the study area. They were stored at 4 ° C before being processed. R. decollata snails were identified according to the descriptions of Dundee, D.S., (1986), with height in average of 2.18 ± 0.27 cm, and exhibit a shell with 4–7 whorls, with the upper 3 - 3.5 whorls broken and filled in the top with a septum. Seventy five adult specimens were collected from the same environment and were kept in plastic bags6. The wild population was not affected.
Samples process
Cats’ faeces: Fifteen grams of each cat sample faeces was weighted and processed by Baermann´s technique13 Fecal samples were wrapped in a triple layer of gauze and placed in a conical cup. Then were covered with no more than half full of warm water and left suspended in water over night. Sediment obtained from the bottom of the conical cup was centrifugated, and total A. abstrusus L1 was counted under optic microscope LEICA DFC 420(10X). The identification was made according to Ohlweiler et al., (2010) and Traversa et al., (2010) descriptions.
R. decollata snail:. R. decollata snails were processed in 25 pools of three adult snails each one, since its low weight made it difficult for individual processing. They were cleaned individually by brushing the foot and shell in order to remove free living nematodes attached or their eggs. Snails were killed by immersion in tepid water for 24 h15; shells were removed and bodies were weighed, minced with scissors and processed by artificial digestion technique8. Each snail macerated pool was placed in an Erlenmeyer with the digestive fluid (Per one gr. of snail tissue it was used 0.15 gr. Pepsin 1:1000, 0.15 ml HCl and 15 ml top water). It was digested for one hour in a magnetic shaker under 37 ºC and 1000 rpm. Afterwards, the fluid of the digestion was filtered in a 500 um mesh and centrifugated at 1500 rpm for three minutes. The supernatant was discharged and total larvae were counted under the light microscope. Larvae were identified according to morphological characteristics, such as the presence of stiletto in the anterior end, a lateral line throughout the body, location of genital and excretory pore and the presence of a knobed tip tail in the posterior end19, 25.
Statistical analysis. Mean value of A. abstrusus larvae recovered from cats’faeces and mean value of R. decollata positive pools were calculated. In both cases, the estimation of the confidence interval (CI) was created at the 95 % confidence level. Total larvae mean value of each pool was expressed per gram (gr.) of snail analyzed and per pool. Total L3 larvae per pool and 95 % confidence level was reported.
Results
Infection of A. abstrusus in cats
The prevalence of A. abstrusus L1 in cats’faeces was 35.30 %(6/17)and the main larvae account per gram of positive faeces was 499.40 (95 % CI: -265.25 - 1264). Mean length of L1 was348μm (95% CI: 309.38 - 386.62) and presented the posterior end with a notched S-shaped tail and a dorsal spine (Fig. 1).

Fig. 1. A. abstrusus larva 1. S-shaped caudal end with the dorsal spine (45x).
Infection of A. abstrusus in snails
Most of R. decollata snails were found on buried faeces (Fig. 2). Snails were positive for A. abstrusus larvae in 20 of 25 pools (80 %). The mean value of A. abstrusus larvae per gram of snail recovered in all positive pools was 84.58 (95 % IC: 42.70 - 126.46) and the maximum value was 323.50 larvae/gram. The mean value of A. abstrusus total larvae per pool of snail recovered in total positive pools was150.64 (95 % IC: 69.42 - 231.86) and the maximum value was 550 larvae/gram. Mean value of total L3/ pool was 93.89 (95 % IC: 33.292 - 154.51); the minimum value was 2 L3 and the maximum 455.80L3/pool (Table 1). All larvae identified as A. abstrusus presented the stiletto in the anterior end, the knobbed tip tail in the posterior end, the lateral line throughout the body and the cuticle of molting larvae (Fig. 3). Second stage larvae measured 470.33 μm (95 % IC: 436.75- 503.92)in length, and weighted between 30 – 34 μm; third stage larvae measured 576.67μm (95 % IC: 554.96 - 598.37) in length and between 22 – 24 μm in weight.

Fig. 2. Adult R. decollata snail feeding on faeces.
Table 1. Recovery of A. abstrusus larvae from R. decollata snails.
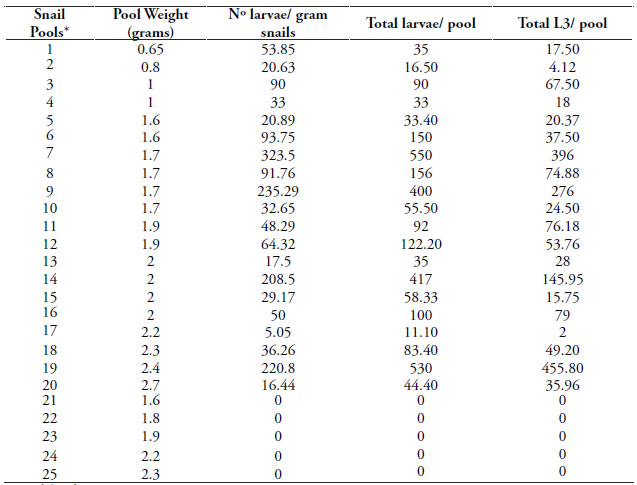
*3 snails/pool
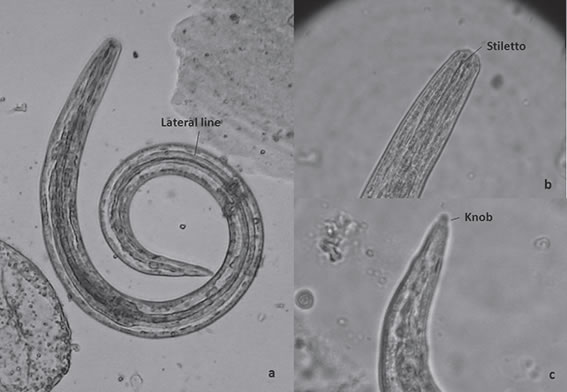
Fig. 3. a. A. abstrusus larva 3 (45x). b. Anterior region. c. Tail of infective larvae.
Discussion and Conclusions
In the city of Buenos Aires, there are free populations of cats living in semi-wild conditions in public institutions which offer them shelter and protection5, 24.
Feral cats have structured defecations habits. They usually bury their faeces in sectored and community places and in wet surface areas around trees1, 29. In this study, we agree with the aforementioned authors regarding these cats’ defecation habits since we found most of semiburied faeces in these kinds of places. The high proportion of feces positives for A. abstrusus (35.30%), confirms that the life cycle of the parasite takes place in the area. Most of snails were found feeding on buried faeces so coprophagus habits of R. decollata were confirm. Their slow movement may be relevant when they feed on faeces because A. abstrusus larvae can actively penetrate R. decollata foot at this moment.
The susceptibility of molluscs to a protostrongylid parasite is defined by the ability of L1 to penetrate the foot, by the possibility of developing into L3 and by the time required to complete this process30. Cabaret et al. (1990) reported that the snail infection rate is a good estimation of L1 penetration capability3. In this study, the infection rate per pool was 93.89L3 (95 % IC: 33.292 - 154.51); and the maximum value was 455.80L3/pool. That showed a high parasite development in R. decollata snail. Most of them were found to be in the L3 infective stage or in the transition period between L2 and L3 stage because it was possible to observe the knobbed tip tail (according to Ohlweiler et al., 2010 description). We do not know which the infective dose was. Thiengo et al. (2008) in experimental infection in Cornuella virgata with 500 A. abstrusus L1 obtained 49.8 larvae/mollusc infection rate and Hamilton (1969), in Helix aspersa experimental infection with 100,000 A. abstrusus L1 recovered 316 larvae/ snail. It may be necessary to improve the experimental infection of A. abstrusus in R. decollata to obtain the optimum infection dose, the time required to complete the development to L3 and the infection rate for cats.
It seems that faeces provide food for snails and protective substrate for A. abstrusus larvae by perpetuating the snail and parasite cycle in contaminated environments. These conditions would represent a risk for rodents and birds as paratenic hosts and also for cats. Studies in England and Wisconsin have documented that well-fed domestic cats continue hunting natural preys as well as wild cats do18.
R. decollatais a highly invasive snail, but they do not reach long distances. However, human activities and lack of natural predators could lead to rapid dispersal in the country, as mentioned by Francesco and Lagiglia(2007), for R. decollata, and by Thiengo et al. (2008), and Ohlweiler et al. (2010),for Achatina fulica snail which presents similar behaviour.
This is the first record of A. abstrusus in R. decollata snail. The high prevalence of larvae in snails (80 %), their coprophagous habits, their slowness of movement (compared to Helix aspersa snail), and the predation habits on this species recorded in this study postulate R. decollate as an efficient intermediate host of the parasite.
The increase of cats’ populations which live in semi-wild conditions in urban areas without health care needs special attention from sanitary authorities. They may consider the role of these cats in the circulation of emerging pathogens (like A. abstrusus) and as reservoirs of other diseases, which include some zoonotic infections. Moreover, it is necessary to improve epidemiological surveillance of ecological species such as R. decollata, which could become intermediate hosts of animals’ parasites and contribute to maintain them in the environment.
1. Afonso,E.; Lemoine M., et. al. Spatial distribution of soil contamination by Toxoplasma gondii in relation to cat defecation behaviour in an urban area. Int. J. Parasitol.2008; 38(8-9): 1017-1023. [ Links ]
2. Ash, L.R. Diagnostic morphology of the thirdstage larvae of Angiostrong ylus cantonensis, Angiostrongylusvasorum, Aelurostrongylus abstrusus and Anafilaroides rostratus (Nematoda: Metastrongyloides). J. Parasitol. 1970; 56:249–253.
3. Cabaret, J., Weber, H., Girard, R. Dual or single infection of the terrestrial snail Solatopupa similes (Bruguiére, 1792) with two protostrongylid nematodes, Muelleriuscapillaris (Mueller, 1889) and Neostrongyluslinearis (Marotel, 1913). Ann. Vet. Res. 1990; 21:131–136.
4. Cowie, R.H. Can snails ever be effective and safe biocontrol agents? Int. J. Pest. Manag. Sci. 2001; 47(1):23–40.
5. Daprato, B., Cardillo, N., Kunic, M., Berra, Y., Sommerfelt I. Persistencia de la contaminación ambiental por huevos de Toxocara cati en un espacio público. Argentina. Una Salud. Revista Sapuvet de Salud Pública. 2011; 2(1): 24- 35. [ Links ]
6. De Francesco, C.G., Lagiglia, H. A predatory land snail invades central-western Argentina. Biol. Inv. 2007; 9:795–798.
7. Dundee, D.S. Notes on The habits and anatomy of the introduced land snails, Rumina and Lamellaxis (Subulinidae). Nautilus. 1986; 100(1):32-37. [ Links ]
8. Gamble, H.R., Bessonov, A.S., et. al.International Commission on Trichinellosis: Recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet. Parasitol. 2000, 93: 393–408.
9. Grandi, G., Calvi, L.E., et. al. Aelurostrongylus abstrusus (cat lungworm) infection in five cats from Italy. Vet. Parasitol. 2005; 134: 177-182. [ Links ]
10. Hamilton, J.M., 1969. On the migration, distribution, longevity and pathogenicity of larvae of Aelurostrongylus abstrusus in the snail, Helix aspersa. J. Helminthol. 3/4:319–325
11. Hobmaier, M., Hobmaier, A., 1935. Intermediate host of Aelurostrongylus abstrusus of the cat. Proc. Soc. Exp. Biol. Med 32:1641–1647.
12. Idiart, J.R., Martín, A.A., Venturini, L., Ruager, J., 1986. Neumonía por Aelurostrongylus abstrusus en gatos. Primeros hallazgos en el Gran Buenos Aires y La Plata. Vet. Arg. 3(23): 229-237. [ Links ]
13. Lacorcia, L., Gasser, R.B., Anderson, G.A., Beveridge, I., 2009. Comparison of bronchoalveolar lavage fluid examination and other diagnostic techniques with the Baermann technique for detection of naturally occurring Aelurostrongylus abstrusus infection in cats. J. Am. Vet. Med. 235 (1): 43-49. [ Links ]
14. Lombardero, O.J., Días B.E., 1967. Primeros casos de Aelurostrongylus abstrusus (Raillet, 1898) en la Argentina (Nematoda: Metastrongylidae). Rev. Med. Vet. 48(3): 279- 83. [ Links ]
15. López, C., Panadero, R., Paz, A., Sánchez-Andrade, R., Díaz, P., Díez-Baños, P., Morrondo, P., 2005. Larval development of Aelurostrongylus abstrusus (Nematoda, Angiostrongylidae) in experimentally infected Cernuella (Cernuella) virgata (Mollusca, Helicidae) Parasitol. Res. 95: 13–16.
16. Miquel, S.E., 1988. Reciente introducción de un gasterópodo terrestre en la República Argentina. Neotrop. 33(90):88. [ Links ]
17. Neck, R.W., 1986. On a collection of land and freshwater gastropods from Cameron County, Texas. Texas Conchol. 22(3-4):107-111. [ Links ]
18. Ogan, C.V., Jurek, R.M., 1997. Biology and ecology of feral, free-roaming and stray cats. Mesocarnivores of Northern California: Biology, Management, and Survey Techniques, Workshop Manual. Eds. Harris, John E. & Chester V. Ogan. Humboldt State Univ., Arcata, CA. The Wildlife Society, California North Coast Chapter, Arcata, CA. 12-15: 87-91. [ Links ]
19. Ohlweiler, F.P., Yoshika Takahashi, F., Manas Eduardo, J. Current distribution of Achatinafulica, in the state of São Paulo including records of Aelurostrongylus abstrusus (Nematoda) larvae infestation.Inst. Med. Trop. S. Paulo. 2010; 52 (4): 211-214. [ Links ]
20. Rascop, A.M., 1960. The biology of Rumina decollata (Linnaeus), Pulmonata: Achatinidae. Unpublished Master’s Thesis, University Of Arizona. 65 Pp.
21. Santa Cruz, A.M., Lombardero, O.J. Primeros hallazgos en el Nordeste de Aelurostrongylus abstrusus (Raillet, 1911) adultos en gatos. Vet. Arg. 1987; 4(32):155-157. [ Links ]
22. Schiaffi, A.L., Bela, M.G., Bola, L.N., Peruzzo, L.E. Aelurostrongylus abstrusus: Diagnostico en la ciudad de Rosario. Vet. Arg. 1995; 12 (117): 480-3. [ Links ]
23. Selander, R.K., Kaufman, D.W. Self-fertilization and genetic population structure in a colonizing land snail. Proceedings of the National Academy of Science. 1973; 70(4):1186–1190.
24. Sommerfelt, I.E, Cardillo, N., López, C., Ribicich, M., Gallo, C., Franco, A. Prevalence of Toxocara cati and other parasites in cats’ faeces collected from the open spaces of public institutions: Buenos Aires, Argentina. Vet. Parasitol. 2006; 140: 296-301.
25. Thiengo, S.C., Fernandez, M.A., Torres, E.J.L, Coelho, P.M., Lanfredi, R.M. First record of a nematode Metastrongyloidea (Aelurostrongylusabstrusus larvae) in Achatina (Lissachatina) fulica(Mollusca, Achatinidae) in Brazil. J Invest. Pathol. 2008; 98(1): 34-39. [ Links ]
26. Traversa, D., Di Cesare, A., Conboy, G. Canine and feline cardiopulmonary parasitic nematodes in Europe: emerging and underestimated. Parasit. Vectors. 2010; 3: 1- 62. [ Links ]
27. Traversa, D., Guglielmini, C. Feline aelurostrongylosis and canine angiostrongylosis: A challenging diagnosis for two emerging verminous pneumonia infections. Vet. Parasitol. 2008; 157: 163- 174. [ Links ]
28. Traversa, D., Lia, R.P., Iorio, R., et. al. Diagnosis and risk factors of Aelurostrongylus abstrusus (Nematoda, Strongylida) infection in cats from Italy. Vet. Parasitol. 2008;153 (1-2):182-186. [ Links ]
29. Uga, S., Minami, T., Nagata, K. Defecation habits of cats and dogs and contamination by Toxocara eggs in public park sandpits. Am. J. Trop. Med. Hyg.1996; 54(2): 122-126. [ Links ]
30. Urban, E. Studies on lung nematodes (Protostrongylidae, Dictyocaulidae) in sheep on the Podhale region, Tatra Highlands. I. The incidence of the infection and diagnostic methods. ActaParasitol. Polonica. 1980; 27: 53-62. [ Links ]














