Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
InVet
On-line version ISSN 1668-3498
InVet vol.20 no.1 Ciudad Autónoma de Buenos Aires 2018
COMUNICACIÃN CORTA
Hantavirus en roedores de La Ciudad de Buenos Aires circulación de hantavirus en Oligoryzomys Flavescens en Ciudad de Buenos Aires, Argentina
Hercolini, C.1; Bruno, A1; Aristegui, E.1; De Salvo, M.N.1; Vidal, J.1; Bellomo, C.2; Martinez, V.P.2; Brambati, D.F.1
1Instituto de Zoonosis Luis Pasteur, Ciudad Autónoma de Buenos Aires, Argentina.
2Instituto Nacional de Enfermedades Infecciosas ANLIS -Dr. Carlos G. Malbrán-, Ciudad Autónoma de Buenos Aires, Argentina
Recibido: 28/02/2018
Aceptado: 19/11/2018
Correspondencia e-mail: Carina Hercolini c.hercolini@gmail.com
Resumen
El síndrome pulmonar por hantavirus (SPH) es una enfermedad zoonótica. La principal causa en Argentina es el virus Andes. Oligoryzomys flavescens (Sigmodontinae) uno de los reservorios de SPH. El objetivo de esta investigación fue estimar la seroprevalencia de hantavirus en O. flavescens en la Ciudad de Buenos Aires. Se colocaron trampas de captura viva en tres parques y una reserva ecológica de la Ciudad para detectar individuos con diagnóstico positivo para virus Andes. Se capturaron un total de 286 roedores, O. flavescens fue la especie más capturada (49,65%). Se encontró serología positiva para virus Andes (genotipo Lechiguana) en ejemplares capturados en uno de los sitios en estudio (seroprevalencia = 6,62%) si bien encontramos al hospedador en otros dos parques de la Ciudad. El presente estudio confirma la presencia de roedores infectados con el virus Andes en la Ciudad de Buenos Aires, lo que implica un riesgo de transmisión en un ambiente urbano.
Palabras clave: Hantavirus; Oligoryzomys flavescens; Ciudad de Buenos Aires
Hantavirus in rodents of Buenos Aires City hantavirus circulation in Oligoryzomys Flavescens Rodents of Buenos Aires City, Argentina
Summary
Hantavirus pulmonary syndrome (HPS) is a zoonotic disease. The main cause in Argentina is Andes virus. Oligoryzomys flavescens (Sigmodontinae) was identify as one of the HPS reservoir. The objective of this research was to estimate the seroprevalence of hantavirus in O. flavescens in Buenos Aires City. We set rodents live trapping in three parklands and one ecological reserve in Buenos Aires City in order to screen hantavirus Andes infected rodents. A total of 286 rodents were captured, O. flavescens was the most frequently captured species (49.65%). Positive serology for Andes virus (Lechiguanas genotype) was found in O. flavescens in one site studied (seroprevalence = 6.62%) and we found the host in other two parks within the City. The present study confirms the presence of rodents infected with Andes virus in Buenos Aires City, which implies transmission risk in an urban environment.
Key words: Hantavirus; Oligoryzomys flavescens; Buenos Aires City.
Introduction
Hantavirus pulmonary syndrome (HPS) is a zoonotic disease caused by viruses belonging to the Hantaviridae family, and mainly harbored by some rodent species. These viruses are transmitted to humans by inhalation of aerosols generated by infected rodents27, although there is also evidence for person to person in the argentine southern region and Buenos Aires Province15, 21. The main cause of HPS in Argentina and neighboring countries is Andes virus (ANDV). Seven different ANDV genotypes have been characterized from HPS cases in Argentina 22, 24.
There are 4 geographically and ecologically distinct HPS endemic areas 16. The Central region, the second in number of cases, comprises Buenos Aires City 16, 14 where one HPS case with no history of travel outside the City was recorded and Lechiguanas genotype was characterized 11.
Several species of Sigmodontinae rodents were identified as HPS reservoirs in the central region: Akodon azarae, Necromys obscurus and Oligoryzomys flavescens 13. Particularly in Buenos Aires City few of these species were reported: A. azarae, and O. flavescens2, 4, 17, 25 . Recent studies have communicated the presence of O. flavescens in Costanera Sur Ecological Reserve 2, 4 and in Presidente Roca Park 4.
The distribution of O. flavescens 31 showed a wide extention from the north to the center of Argentina 7, southeast Brazil 32 and Uruguay 12. This species is a good colonizer of disturbed ecosystems26, inhabits wild environments such as savannas, marshes and not very dense forests, grass and scrubland, often near water or damp zones 9, and although it is associated to wild microhabitats it has been captured in peridomiciliary urban and periurban areas 5.
Knowing the presence of O. flavescens in Buenos Aires City it becomes necessary to study if there is hantavirus circulation between them. Our aim was to estimate the seroprevalence of hantavirus in Oligoryzomys flavescens in Buenos Aires City.
Materials and Methods
Rodents were captured in four green open sites of the City during the period 2011-2014: De los Niños Park (NP: 32 hectares, 34º31'43.40''S, 58º27'33.99''O), Ribera Sur Park (RSP: 50 hectares, 34º41'57.73''S, 58º28'04.93''O), Presidente Roca Park (PRP: 154 hectares, 34º40'29.76''S, 58º26'29.79''O) and Costanera Sur Ecological Reserve (CSER: 353 hectares, 34º36'29.18''S, 58º21'03.32''O) (Figure 1). According to the characterization of environmental landscape units by Cavia et al. (11), the CSER is a natural reserve and NP and PRP are parklands. We considered RSP as parkland because it shares environmental characteristics with the other two. The CSER is occupied by riparian habitats similar to those developed along the Paraná and de la Plata rivers, like woodlands, riparian thickets, fresh water marshes and flooded grasslands. Parklands are sites of recreation, where areas of spontaneous vegetation and woodlots with planted species are included in a matrix of grass or ornamental lawn 3.
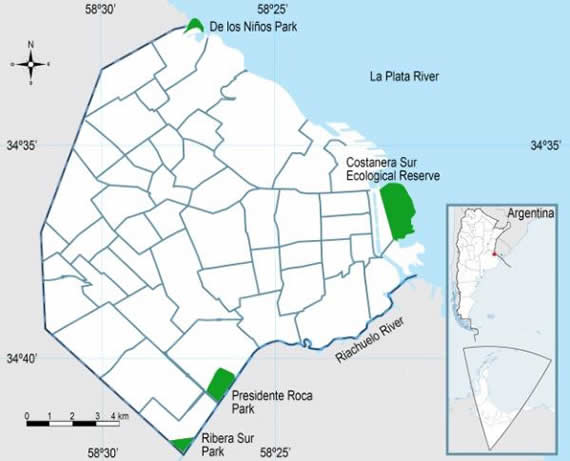
Figure 1. Studied sites in Buenos Aires City.
Rodents were captured with Sherman live traps in linear transects actively set for four consecutive nights for each sampling (Table I).
Table I: Number of traps night per season and year for each studied site

The individuals captured were identified according bibliography9 and reference material deposited at the Museo Argentino de Ciencias Naturales -Bernardino Rivadavia-. All O. flavescens specimens captured were anesthetized with isoflurane and euthanized by cervical dislocation. Body measurements, sex and reproductive status were registered. Blood and lung samples were collected for hantavirus infection analysis, following Mills et al.19. Animals were treated in accordance with the guidelines of the Sociedad Argentina para el Estudio de los Mamíferos8.
The relative abundance of each species per study area was estimated with the Trap Success Index (TS) 18. TS = (number of individuals / number of traps * nights) * 100. The correlation between the number of O. flavescens captured and number of positive individuals was calculated with Spearman Correlation Coefficient.
In order to screen ANDV antibody-positive individuals, we utilized an enzyme-linked immunosorbent assay based on the test described for human antibodies detection22. Briefly, to determine IgG antibodies against ANDV, rodent blood samples diluted 1:100 were added to 96 wells plate coated with AND recombinant nucleoprotein antigen. To determine ANDV genotype, total RNA extraction was performed on available lung tissues from seropositive rodents (5/9 individuals) using Trizol (Invitrogen) and purified by the RNAid kit (Bio 101). When it was possible (3/5 samples), viral RNA was amplified by RT-PCR and a second round of nested PCR following Ciancaglini et al.6. Briefly, RT-PCR was performed by One Step RT-PCR kit (QIAGEN) and Taq DNA Polymerase (Invitrogen) following each manufacturer instructions. For the 952 nt S-Segment fragment was used an inner primer to obtain the 530-nt fragments for S-segment (position 22-550, referred to AH1 AND strain, Gene Bank No AF324902) position. For M-segment was amplified the 461nt fragment (position 6 to 467). Amplification products were analyzed on agarose gels and sequenced. Multiple sequence alignment and comparison of nucleotide sequences were conducted using MEGA version 5 29
Results
A total of 286 rodents were captured with a total trapping effort of 13012 trap-nights. O. flavescens was the most frequently captured species (49.65%) followed by Mus musculus (36.71%), Deltamys kempi (10.14%) and Scapteromys aquaticus (3.50%). Rodents were captured in all sites but NP. In CSER we captured four species of which the trap success of O. flavescens and M. musculus were the highest. On the contrary, in RSP and PRP we only captured O. flavescens (Table II).
Table II: Rodents captured and trap success per studied site

Positive serology for ANDV was found in O. flavescens captured in CSER where the seroprevalence was 6.62% (95% c.i.: 2.07% - 11.16%). The nine positive rodents corresponded to male individuals, seven of them were sexually active (with testicles in the scrotal position). These positive individuals were caught in different seasons: 3 in winter, 3 in spring, 2 in summer and 1 in autumn (Table III). They were found in a variety of floristic habitats, six of them were captured on the river side and the rest in different habitats of CSER. The Spearman Correlation Coefficient between number of O. flavescens captured and number of positive individuals resulted 0.81 (p < 0.001, N = 13), showing a positive relationship between both variables throughout the seasons.
Table III: Number of captured, positives and seroprevalence of Oligoryzomys flavescens in CSER 2011-2014

O. flavescens did not show any seasonal pattern in CSER and was captured in all seasons but summer 2012 and summer 2013. Although, we observed an increase of trap success since winter 2013 with a peak in spring at the same year. On the other hand, after the peak of O. flavescens, the trap success of M. musculus decreased and we also observed a slight increase of D. kempi trap success and S. aquaticus was found for the first time (Figure 2).

Figure 2. Trap success of Oligoryzomys flavescens ( ), Mus musculus ( ), Deltamys kempi ( ) and Scapteromys aquaticus ( ) per period in CSER 2011-2014.
ANDV, Lechiguanas genotype, was identified in 3 IgG positive-rodent, showing a 99.8% nucleotide identity between them in the amplified fragment. The 3 individuals were captured in winter 2012, spring 2013 and summer 2014.
Discussion
The present study confirms the presence of rodents infected with Andes virus in Buenos Aires City. This finding is not unexpected because this City is geographically placed in the Central region HPS endemic area.
Positive rodents were captured only in a natural reserve, CSER, which was the most intensely studied site. Since 2014, an exploratory study has been started in parklands in the City. In two of them, RSP and PRP, we found the host. The presence of this hantavirus reservoir species in these two places should be considered as a potential risk per se.
The seroprevalence found in CSER was 6.62%. In similar studies the seroprevalence found in O. flavescens was 7.51% in Pre Delta National Park30 and 13.51% in Exaltación de la Cruz 28. All the positive individuals were males, according to other authors that found that males are more likely to be infected 23, 28, 30. The presence of O. flavescens with positive serology for ANDV in all seasons and in different habitats located in CSER, could implies transmission risk for visitants and workers along all year.
Altought it was not part of the objective of this work, we studied the first 15 D. kempi captured for hantavirus infection analysis. All of them were negative for ANDV (data not shown). These results supports, as mention in Vadell et al. 30 that D. kempi is an uncommon host species for hantavirus and constitutes a spillover host.
We found rodent community changes along the study period in CSER. These changes can be associated with the dredging works and filling in the lagoons with water since 20132. Interestingly, we found the highest peak of O. flavescens in 2013/2014, period in which an outbreak of HPS cases was registered in Buenos Aires Province11. On the other hand, Cavia et al. 4 found in CSER a richness of 6 species, and O. flavescens and D. kempi were the dominant species; in our study richness was lower (R = 4) and O. flavescens and M. musculus were the dominant species, which could indicate that rodent community in CSER is not stable.
O. flavescens is an r strategist species, consequently its captured and reproductive activity are strongly conditioned by the density of the rest of the rodent species 26. If we take this into account and that we found a positive correlation between number of O. flavescens positive and individuals captured, it becomes necessary to continue evaluating the O. flavescens dynamics in the assemblages of rodent species. There are several variables in an infection system 10. We found that an increasing population density of O. flavescens resulted in an increasing horizontal virus transmission, according to Mills et al. 20, Hussein et al. 10 and Adler et al. 1.
Conclusions
Results presented in this work, can be used to support the adoption of preventive measures and optimize the allocation of resources to avoid disease propagation. Hantavirus´s epidemiological monitoring in rodents must be continued and new sites should be studied in the City.
Acknowledgements
We thanks the authorities of Costanera Sur Ecological Reserve, Presidente Roca Park, De los Niños Park, Ribera Sur Park, personal of Instituto de Zoonosis Luis Pasteur: Yamila Bechara, Carolina Zotter, Javier Marengo and Javier Donacimiento for field work assistance and Yanina Berra for cooperation in the field work in Costanera Sur Ecological Reserve.
1. Adler FR, Pearce-Duvet JMC, Dearing MD. How host population dynamics translate into time-lagged prevalence: An investigation of Sin Nombre virus in deer mice. Bull Math Biol. 2008; 70:236-252. [ Links ]
2. Arístegui A, Hercolini C, Brambati D, Vidal J, Bruno A, Sassaroli JC, Beaudoin J. Primer registro de Scapteromys aquaticus y Cryptonanus chacoensis en la Ciudad Autónoma de Buenos Aires, Argentina. Nótulas Faunísticas: Segunda Serie. 2014; 169: 1-5. [ Links ]
3. Cavia, R. Rodents in the city of Buenos Aires: an approach to their control, PhD Thesis, University of Buenos Aires, Buenos Aires. (in Spanish). 2006. [ Links ]
4. Cavia R, Cueto G R, Suarez OV. Changes in rodent communities according to the landscape structure in an urban ecosystem. Landscape and Urban Planning. 2009 90: 11-19. [ Links ]
5. Calderón G, Pini N, Bolpe J, Levis S, Mills J, Segura E, Guthmann N, Cantoni G, Becker J, Fonollat A, Ripoll C, Bortman M, Benedetti R, Sabattini M,Enria D. Hantavirus Reservoir Hosts Associated with Peridomestic Habitats in Argentina. Emerg Infect Dis. 1999; 5 (6): 792-797. [ Links ]
6. Ciancaglini M, Bellomo CM, Torres Cabreros CL, Alonso D, Bassi SC, Iglesias AA, Martínez VP. Hantavirus pulmonary syndrome in Tucumán province associated to an unexpected viral genotype. Medicina (B Aires). 2017; 77(2):81-84. [ Links ]
7. Cirignoli S, Teta P, Pardiñas UFJ, D-Elía G. Tribu Oryzomyini. In: Barquez RM, Díaz MM, Ojeda RA (eds.) Mamíferos de Argentina, Sistemática y distribución. Sociedad Argentina para el Estudio de Mamíferos, Mendoza; 2006. p. 166-175. [ Links ]
8. Giannoni SM, Mera Sierra R, Brengio S y Jiménez Baigorria L. Guía para el uso de animales en investigaciones de campo y en cautiverio. Comisión de Ãtica de la Sociedad Argentina para el Estudio de los Mamíferos [Internet]. 2003. Available from: https://www.sarem.org.ar/legislacion/ [ Links ]
9. Gómez Villafañe IE, Miño M, Cavia R, Hodara K, Courtalón P, Suárez O, Busch M. Roedores: guía de la provincia de Buenos Aires. L.O.L.A. Buenos Aires. 2005; p 100. [ Links ]
10. Hussein K, Hornfeld B, Evander M, Magnusson M, Olsson G, Ecke F. Dynamics and Drivers of Hantavirus Prevalence in Rodent Populations. Vector-Borne and Zoonotic Diseases. 2014; 14(8): 537-551. [ Links ]
11. Iglesias AA, Bellomo CM, Martínez VP. Síndrome pulmonar por hantavirus en Buenos Aires, 2009-2014. Medicina (B Aires). 2016; 76: 1-9. [ Links ]
12. Langguth A. Las especies uruguayas del género Oryzomys (Rodentia-Cricetidae). Com. Zool. Mus. Hist. Nat. Mont. Urug. 1963; 7 (99): 1-22. [ Links ]
13. Levis S, Morzunov SP, Rowe JE, Enria DA, Pini N, Calderon G, Sabattini M, St Jeor SC. Genetic diversity and epidemiology hantavirus in Argentina. J Infect Dis. 1998; 177529-538. [ Links ]
14. Martínez VP, Colavecchia S, García Alay M, Suzuki B, Trincheri A, Busto S, Rabinovich R, Padula P. Hantavirus pulmonary syndrome in Buenos Aires Province. Medicina (B Aires). 2001; 61(2):147-56.3. [ Links ]
15. Martinez VP, Bellomo C, San Juan J, Pinna D, Forlenza R, Elder M, Padula PJ. Person-to-person transmission of Andes virus. Emerg Infect Dis Dec. 2005; 1(12):1848-53. [ Links ]
16. Martinez VP, Bellomo CM, Cacace ML, Suarez P, Bogni L, Padula PJ. Hantavirus pulmonary syndrome in Argentina, 1995-2008. Emerg Infect Dis Dec. 2010; 16(12):1853-60. [ Links ]
17. Massoia E, Fornes A. Rodents collected in the Federal Capital City (Caviidae, Cricetidae, Muridae). IDIA. 1967; 47-53. [ Links ]
18. Mills JN, Ellis BA, McKee KT, Maiztegui JI, Childs JE. Habitat Associations and Relative Densities of Rodent Populations in Cultivated Areas of Central Argentina. Other Publications in Zoonotics and Wildlife Disease. Lincoln. 1991; p. 82. [ Links ]
19. Mills JN, Yates TL, Childs JE, Parmenter RR, Ksiazek TG. Guidelines for Working with Rodents Potentially Infected with Hantavirus. Other Publications in Zoonotics and Wildlife Disease. Lincoln; 1995. p. 84. [ Links ]
20. Mills JN, Ksiazek TG, Peters CJ, Childs JE. Long-term studies of hantavirus reservoir populations in the southwestern United States: A synthesis. Emerg Infect Dis. 1999; 5:135. [ Links ]
21. Padula, PJ, Edelstein A, Miguel SDL, López NM, Rossi CM, Rabinovich RD. Hantavirus Pulmonary Syndrome Outbreak in Argentina: Molecular Evidence for Person-to-Person Transmission of Andes Virus. Virology. 1998; 241: 323-330. [ Links ]
22. Padula PJ, Colavecchia SB, Martínez VP, Gonzalez Della Valle MO, Edelstein A, Miguel SD, Russi J, Riquelme JM, Colucci N, Almirón M, Rabinovich RD. Genetic diversity, distribution, and serological features of hantavirus infection in five countries in South America. J Clin Microbiol. 2000; 3 8(8):3029-35. [ Links ]
23. Padula P, Figueroa R, Navarrete M, Pizarro E, Cadiz R, Bellomo C, Jofre C, Zaror L, Rodriguez E, Murúa R. Infection in Wild Sigmodontine Rodents Transmission Study of Andes Hantavirus. J Virol. 2004; 78(21):11972-11979. [ Links ]
24. Padula P, Martinez VP, Bellomo C, Maidana S, San Juan J, Tagliaferri P, Bargardi S, Vazquez C, Colucci N, Estévez J, Almiron M. Pathogenic hantaviruses, northeastern Argentina and eastern Paraguay. Emerg Infect Dis. 2007; 13(8):1211-4. [ Links ]
25. Pearson OP. Age estructure and reproductive dynamic in a population of meadow mice, Akodon azarae. Physis. 1967; XXVII (74), 53-58 [ Links ]
26. Sánchez López MI. Factores que limitan la abundancia de los roedores múridos en el delta del Paraná. PhD Thesis, Universidad de Buenos Aires, Buenos Aires. 1998 p. 150. [ Links ]
27. Schmaljohn CS. Molecular biology of hantaviruses. In Elliott RM (ed). The Bunyaviridae. Plenum Press. New York, N.Y. 1996; 63-90. [ Links ]
28. Suárez OV, Cueto GR, Cavia R, Gómez Villafañe IE, Bilenca DN, Edelstein A, Martínez P, Miguel S, BellomoC, Hodara K, Padula PJ, Busch M. Prevalence of Infection with Hantavirus in Rodent Populations of Central Argentina. Mem Inst Oswaldo Cruz, Rio de Janeiro. 2003; 98(6): 727-732. [ Links ]
29. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, and Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol. 2011. [ Links ]
30. Vadell MV, Bellomo C, San Martin A, Padula P, Gomez Villafane I. Hantavirus ecology in rodent populations in three protected areas of Argentina. Trop Med Int Health. 2011; 16 (10): 1342-1352. [ Links ]
31. Waterhouse GR. Characters of new species of the genus Mus, from the collection of Mr. Darwin. Proceedings of the Zoological Society of London. 1837; 5(50-51):15-21. [ Links ]
32. Weksler M, Bonvicino CR. Taxonomy of pigmy rice rats genus Oligoryzomys Bangs, 1900 (Rodentia, Sigmodontinae) of the Brazilian cerrado, with the description of two new species. Arquivos do Museu Nacional, Rio de Janeiro. 2005; 63:113-130. [ Links ]














