Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
RIA. Revista de investigaciones agropecuarias
versión On-line ISSN 1669-2314
RIA. Rev. investig. agropecu. vol.44 no.1 Ciudad Autónoma de Buenos Aires abr. 2018
ARTÍCULOS
Essential oils from Argentinean native species reduce in vitro methane production
Garcia, F.1,2*; Brunetti, M.A.3; Lucini, E.I.2; Scorcione Turcato, M.C.1; Moreno, M.V.3; Frossasco, G.P.3; Colombatto, D.1,4; Martínez, M.J.3; Martínez Ferrer, J.3
1 Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina. Correo electrónico: fgarcia@agro.unc.edu.ar
2 Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba, Córdoba, Argentina.
3 Instituto Nacional de Tecnología Agropecuaria, Manfredi, Argentina.
4 Facultad de Agronomía, Universidad de Buenos Aires, Buenos Aires, Argentina.
Received: July 29th 2016
Accepted: August 15th 2017
Published online: April 24th 2018
ABSTRACT
The aim of this study was to evaluate the effect of Argentinean essential oils (EO) on methane production during in vitro fermentation compared to EO with proven effects as rumen fermentation modifiers. A complete randomized block design was used and the treatments included EO from Aloysia gratissima (50, 100, 150 and 300 mg/L), Eucalyptus globulus (5, 50, 150 and 300 mg/L), Lippia turbinata (30, 60, 120 and 240 mg/L), Mentha x piperita var. vulgaris (50, 100, 200 and 400 mg/L), Origanum vulgare ssp. hirtum cv. Compacto (0.5, 5, 50 and 250 mg/L) and cv. Mendocino (50, 150, 250 and 350 mg/L), Rosmarinus officinalis (100, 300, 500 and 700 mg/L), Schinus molle (75, 150, 300 and 600 mg/L), Tagetes minuta (5, 50, 125 and 250 mg/L), and Thymus vulgaris (5, 50, 150 and 300 mg/L). Two controls were included: control (not containing EO) and monensin (1.87 mg/L). Variables measured were: digestibility of neutral detergent fiber, gas and methane production. Compared to the control, monensin reduced methane production by 44%, but with a 15% reduction in fiber digestibility. Except for E. globulus and L. turbinata, each variable measured was significantly reduced with the higher level of inclusion compared to the control, representing that overall microbial activity was affected. L. turbinata (60.8% limonene) reduced methane by half compared to control, and by 35 to 85% when compared to monensin, without affecting digestibility of the fiber. Some essential oils from native Argentinean plants exhibited great potential to reduce enteric methane production without affecting digestibility, of which L. turbinata was the most promising alternative.
Keywords: Methane; Natural additive; Plant secondary compound; Rumen fermentation.
RESUMEN
El objetivo de este estudio fue evaluar el efecto de aceites esenciales (AE) de plantas nativas de Argentina en la producción de metano durante la fermentación in vitro, en comparación con AE con efectos comprobados como modificadores de la fermentación ruminal. Se realizó un diseño en bloque completos al azar y los AE y dosis evaluados fueron: Aloysia gratissima (0,5, 5, 50 y 250 mg/L) , Eucalyptus globulus (5, 50, 150 y 300 mg/L), Lippia turbinata (30, 60, 120 y 240 mg/L), Mentha x piperita var. vulgaris (50, 100, 200 y 400 mg/L), Origanum vulgare ssp. hirtum cv. Compacto (0,5, 5, 50 y 250 mg/L) y cv. Mendocino (50, 150, 250 y 350 mg/L), Rosmarinus officinalis (100, 300, 500 y 700 mg/L), Schinus molle (75, 150, 300 y 600 mg/L), Tagetes minuta (5, 50, 125 y 250 mg/L), y Thymus vulgaris (5, 50, 150 y 300 mg/L). Se incluyeron dos controles: control (sin AE) y monensina (1,87 mg/L). Las variables evaluadas fueron: digestibilidad in vitro de la fibra en detergente neutro, producción de gas y metano. En comparación al control, la monensina redujo la producción de metano en un 44%, pero con una reducción de un 15% en la digestibilidad de la fibra. Con excepción a E. globulus y L. turbinata, todas las variables fueron reducidas significativamente con el mayor nivel de inclusión comparado con el control, lo cual representa que la actividad microbiana se vio afectada. L. turbinata (60,8% limoneno) redujo la producción de metano a la mitad comparado al control, y entre un 35 y 85% en comparación a la monensina, sin que la digestibilidad de la fibra se vea afectada. Algunos aceites esenciales de plantas nativas de Argentina demostraron gran potencial para reducir la producción de metano entérico sin afectar la digestibilidad, de los cuales L. turbinata fue la alternativa más promisoria.
Palabras clave: Metano; Aditivos naturales; Compuestos secundarios de plantas; Fermentación ruminal.
Abbreviations:
ADF, acid detergent fiber;
CP, crude protein;
DM, dry matter;
D-NDFom, digestibility of organic matter neutral detergent fiber;
EO, essential oils;
GHG, greenhouse gas;
ivDMD, in vitro dry matter digestibility;
NDF, neutral detergent fiber;
NDFom, organic matter neutral detergent fiber;
NDFom d, organic matter neutral detergent fiber digested;
OM, organic matter.
INTRODUCTION
There are growing concerns over greenhouse gas (GHG) emissions due to their effects on global warming. In this regard, livestock emerges as one of the top two or three most significant contributors to climate change (Steinfeld et al., 2006), being responsible for up to 14.5% of anthropogenic GHG, where methane is the most emitted gas with about 44% of the sector’s emissions. Methane is an unavoidable by-product of anaerobic microbial fermentation of carbohydrates in the rumen carried out by the microorganisms which are in symbiosis with ruminants. In addition to the effects on the environment, it implies a loss of 2 to 12% of gross energy provided by the diet (Johnson and Johnson, 1995). Therefore, diminishing its production in the rumen is one of animal nutrition research interests. By manipulating the rumen microbial ecosystem it is feasible to reduce methane production by selective inhibition of methanogenic microorganisms (Johnson and Johnson, 1995). Antibiotics (e.g., ionophores) have long been used in the area of nutritional interventions to favorably impact on rumen fermentation in order to reduce waste input to the environment and to improve the efficiency of feed nutrients used by animals. An increasing awareness of the hazards associated with chemical feed additives, i.e., the presence of chemical residues in animal-derived foods and development of bacterial resistance to antibiotics, resulted in their prohibition for other purposes not related to specific prevention and/or treatment of animal health and well-being in many European countries (Regulation 1831/2003/EC), being under revision in several other countries as well.
In the search of natural alternatives, essential oils (EO) have been tested for their antimicrobial activity and a considerable number of studies have shown their potential to beneficially modify rumen fermentation reducing methane production (Benchaar and Greathead, 2011; Cieslak et al., 2013; Khiaosa-ard and Zebeli, 2013). Essential oils are complex mixtures of volatile lipophilic secondary metabolites (Benchaar and Greathead, 2011) having great diversity in composition depending on geographic variations, genetic factors, physiological stage at harvest, plant growing conditions, plant parts used and extraction methods (Calsamiglia et al., 2007; Figueiredo et al., 2008). The chemical composition of EO is one of the factors affecting their bioactivity in altering the rumen ecosystem, along with the dose and the base diet used (Khiaosa-ard and Zebeli, 2013; Klevenhusen et al., 2012; Lin et al., 2013).
Novel sources of EO, not yet explored in ruminant nutrition, may broad the spectrum of what has already been studied. In this sense, different EO from native Argentinean plants have been reported for their bioactivity in a variety of biological processes (Fuselli et al., 2006; García et al., 2003; Tereschuk et al., 1997), representing a promising alternative to modify rumen fermentation. The aim of this study was to evaluate the effect of four Argentinean EO on methane production during in vitro fermentation, in comparison to EO with proven effects as rumen fermentation modifiers.
MATERIALS AND METHODS
Experimental design
A completely randomized block design was used, considering incubations as blocks (repetitions), thrice-repeated in three consecutive weeks. Within each block, treatments were in duplicate, considered as replicates. Four EO from native Argentinean plants were tested together with six non-native Argentinean plants, at four doses (Table 1). Doses of EO were chosen based on the results of a preliminary study (García et al., 2014), where a broader range of doses were tested for all EO (1, 10, 100 and 100 mg/L DMi). The selection criterium to define doses was methane production inhibition without affecting overall digestion. Two controls were included: control (not containing EO) and monensin (Option 20%, Brascorp S.A., Buenos Aires, Argentina) at 1.87 mg pure monensin/L.
Table 1. Essential oils and doses evaluated.
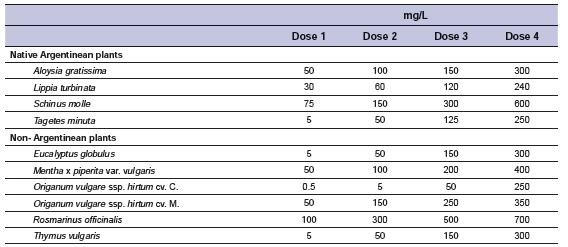
Essential oils
Native plants from Argentina: Aloysia gratissima, Lippia turbinata and Tagetes minuta were collected in Characato, Córdoba (31°28’77’’ S, 64°12’32’’ W) and Schinus molle was provided by the Faculty of Agronomy of Córdoba University (31°44’06’’ S, 64°18’48’’ W). Exotic plants: Eucalyptus globulus, Mentha x piperita var. vulgaris, Origanum vulgare ssp. hirtum cv. Compacto and cv. Mendocino, Rosmarinus officinalis and Thymus vulgaris were obtained from commercial farms in Villa Dolores, Córdoba (31°56’67’’ S, 65°11’53’’ W) and from the Faculty of Agronomy of Córdoba University. Aerial parts of plants were harvested at full flowering, air dried, and oil was obtained by steam distillation using an all-glass Clevenger-type apparatus. Oils were dried over anhydrous sodium sulphate and stored at -20°C until further use and analyses. The EO composition was analyzed by gas chromatography coupled mass spectrometry (GC-MS). The analysis was carried out on a Perkin Elmer Clarus 600 GC-MS instrument equipped with two columns: Supelcowax 10 (30 m x 0.25 μm) and DB-5 fused silica column (30 m x 0.25 μm). The carrier gas was helium at an initial temperature of 60°C for three minutes and increased 4°C/min to 240°C.
In vitro incubation
Rumen fluid was collected on the day of the experiment in a pre-warmed thermo flask from three rumen-fistulated Hereford steers (516 ± 53 kg) before morning feeding, kept on a daily ration of alfalfa hay:corn grain diet (80:20 DM basis). Once in the laboratory, all manipulations were done under a constant stream of CO2 gas. Rumen fluid was pooled and homogenized in a blender for one minute, strained through two layers of cheesecloth twice, and collected into a flask. It was constantly homogenized using a magnetic stirrer, held at 39°C in a water-bath under CO2 stream until inoculation. The substrate used for incubation was a mixture of 0.4 g of alfalfa plus 0.1 g of corn grain, which were previously freeze-dried and milled through a 2 mm sieve. The chemical composition of the substrates is shown in Table 2.
Table 2. Chemical composition (g/kg DM) of alfalfa hay and corn grain used as substrates for in vitro ruminal fermentation.
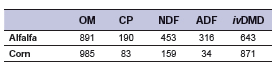
Notes: OM: Organic matter; CP: Crude protein; NDF: Neutral detergent fiber; ADF: Acid detergent fiber; ivDMD: In vitro dry matter digestibility.
Incubations were conducted in 100 mL serum bottles containing 40 mL of carbonate-phosphate buffer (Tilley and Terry, 1963) and 10 mL of strained rumen fluid. A stock solution of monensin and of each EO and dose was prepared in ethanol at the necessary concentration to achieve final doses in the bottle, and 200 μL of this solution were dispensed into the bottles immediately before inoculation with rumen fluid. An equivalent amount of ethanol was added to the control. Immediately after the rumen fluid was dispensed, the bottles were flushed with CO2 gas, sealed with butyl rubber stoppers and aluminum crimp seals, and placed in a water-bath (39°C). A blank (rumen fluid plus buffer, without substrate) was included and data was used for correction. Head-space gas pressure was measured at predetermined points during incubation using a pressure transducer (Sper Scientific Ltd., Scottsdale, Arizona, USA). Bottles were manually shaken after each reading. During the rapid gas production phase, the bottles were sampled more frequently to prevent headspace pressure from reaching 7.0 psi, as suggested by Theodorou et al. (1994), and all accumulated gas was collected in 250 mL vials for methane determination. At each sampling point, headspace gas was collected from the bottles with a gastight syring until readings reached equilibrium with the atmospheric pressure (0.00 psi). At the end of incubation (72 h) bottles were placed in ice to stop fermentation and stored at -20°C until further analyses.
Determination and chemical analysis
The substrate was analyzed for the following parameters: organic matter (OM), crude protein (CP), neutral detergent fiber (NDF), acid detergent fiber (ADF) and in vitro dry matter digestibility (ivDMD). Organic matter was determined at 550°C for 6 h; CP was obtained by the Kjeldahl method (AOCS: 954.01, 1998); NDF and ADF analyses were performed according to Van Soest et al. (1991) using an ANKOM200 Fiber Analyzer (Ankom®, Tech. Co., Fairport, NY, USA); and ivDMD as proposed by Tilley and Terry (1963) using a DaisyII incubator (Ankom®, Tech. Co., Fairport, NY, USA). A linear regression pressure-volume was used for regression- corrected gas-volume. Cumulative gas production was calculated by summing the regression-corrected gas volume for each measurement time and corrected by blank. Methane concentration was determined by gas chromatography using a Hewlett Packard 4890 instrument equipped with a Porapak N 80/100 analytical column (2 m) with nitrogen as the carrier gas. Injector temperature was 110°C, the column was held constantly at 90°C during analysis and the detector temperature (FID) was 250°C.
In vitro digestibility of ash free neutral detergent fiber (D-NDFom) was obtained according to Van Soest et al. (1991); briefly, the bottle content was transferred into 200 mL tubes and 100 mL neutral detergent solution plus heat stable α-amylase were added. After 1 h boiling, residues were filtered in preweighed glass crucibles (DURAN® 25- 851-32 filter crucibles) and oven-dried at 105°C for 24 h. Dry residues were weighed and ashes determined at 550°C for 4 h. The same procedure was carried out with the substrate to determine ash free neutral detergent fiber (NDFom) incubated, and by the difference with the residues, to calculate the NDFom digested (NDFom d) after blank correction. The formula used for calculation of D-NDFom was: NDFom d/ NDFom incubated * 100.
Statistical analysis
All data were analyzed using InfoStat software (Di Rienzo et al., 2011). Data were analyzed by performing analysis of variance and treatment and blocks as a fixed effect: Yij = μ + τi + βj + εij, where Yij was the observation, μ was the overall mean for each parameter, τi was the effect of treatment, βj was the effect of block and εij was the residual error. Differences between treatments were compared with the Fisher LSD test and significant differences were declared at p<0.05.
RESULTS
The EO composition was largely variable, as shown in Table 3. The number of chemical components ranged from five (e.g. E. globulus) to 40 (e.g. O. vulgare), showing great difference in the proportion represented by the first three main components: A. gratissima around 30%; S. molle 50%; O. vulgare ssp. hirtum cv. Mendocino, R. officinalis, S. molle and T. vulgaris about 60%; L. turbinata, M. x piperita var. vulgaris, O. vulgare ssp. hirtum cv. Compacto and T. minuta more than 70%; and over 90% for E. globulus. The effects of EO on gas production, D-NDFom and methane are presented in Table 4. Gas production for control was 292.0 mL/g OMi, D-NDFom of 0.477 g/g and 24.6 mg/g OMi of methane production. Compared to the control, monensin reduced gas production by around 25% and 44% mg of methane per g OMi, but at the expense of a 15% reduction in D-NDFom.
Table 3. Number of compounds detected (> 99%) and proportion of the main three compounds of the essential oils evaluated.
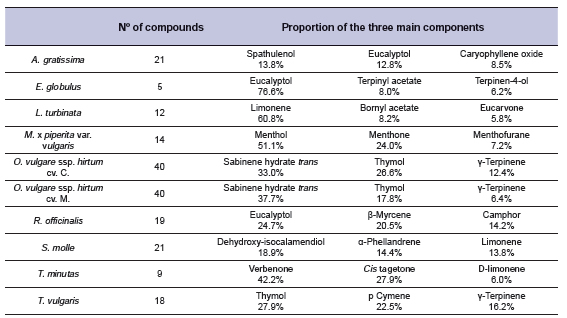
Table 4. Effect of essential oils on total gas (mL/g OMi), in vitro fiber digestibilty (D-NDFom; g/g), and methane production (%, mg/g OMi and mg/g NDFom digested).
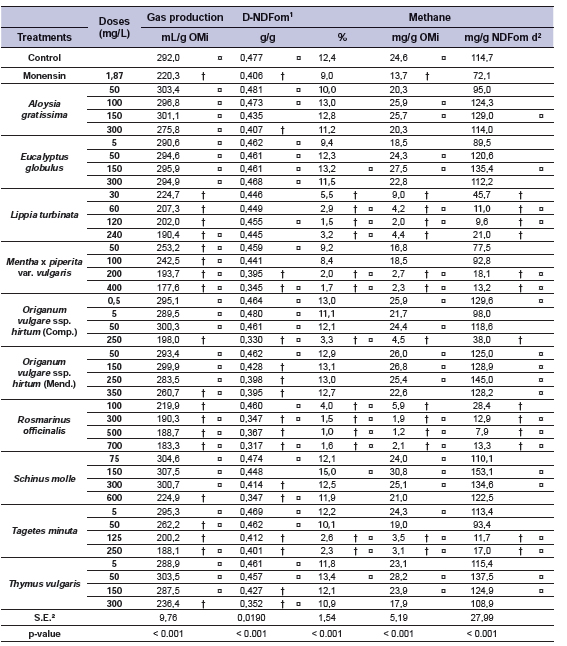
Notes: † Means within a column differ from control (p < 0.05); ¤ Means within a column differ from monensin (p < 0.05); 1 D-NDFom: in vitro digestibility of ash free neutral neutral detergent fiber; 2 NDFom d: organic matter neutral detergent fiber digested; 3 S.E.: standard error.
All levels of L. turbinata and R. officinalis at the lower level (100 mg/L) reduced gas production (23 to 35%) with greater methane inhibition (65 to 92%) when compared to the control, interestingly with no effects on fiber digestibility. Intermediate to high doses of R. officinalis (300, 500 and 700 mg/L) also reduced gas and methane production (~35% and ~90% respectively); however, fiber digestibility was severely affected (more than 20%). Compared to the control A. gratissima and E. globulus had no effects on the variables studied, except for A. gratissima at 300 mg/L that reduced fiber digestion by 14%. S. molle and T. vulgaris reduced gas production and DNDFom at higher doses, though a decrease in methane was not attained.
Although evaluated at different dose ranges, both cultivars of O. vulgare ssp. hirtum at the highest level reduced gas production and D-NDFom. When compared at similar doses, increasing the dose from 50 to 250 mg/L had a greater effect in Compacto than in Mendocino in reducing gas and methane production and fiber digestion. A reduction in gas (30 to 35%) and methane production (80 to 90%) was observed for the intermediate dose of M. x piperita var. vulgaris (200 mg/L) and intermediate to high doses of T. minuta (125 and 250 mg/L) compared to the control, with a reduction of around 15% in D-NDFom. Reductions relative to control in methane production (mg/g NDFom d) and fiber digestibility are shown in Fig. 1. Almost one third of the additives tested reduced methane production by over 50%, most of which (8 out of 13) were associated with a concomitant reduction in fiber digestion (over 10%).
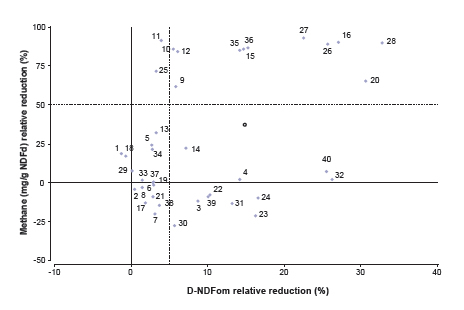
Figure 1. Reduction in methane production and fiber digestibility by EO (•) and monensin (ο) relative to control.
Notes: 1-4. Aloysia gratissima (50, 100, 150 and 300 mg/L); 2-8. Eucalyptus globulus (5, 50, 150 and 300 mg/L); 9-12. Lippia turbinata (30, 60, 120 and 240 mg/L); 13-16. Mentha x piperita var. vulgaris (50, 100, 200 and 400 mg/L); 17-20. Origanum vulgare ssp. hirtum cv. Compacto (0.5, 5, 50 and 250 mg/L); 21-24. O. vulgare ssp. hirtum cv. Mendocino (50, 150, 250 and 350 mg/L); 25-28. Rosmarinus officinalis (100, 300, 500 and 700 mg/L); 29-32. Schinus molle (75, 150, 300 and 600 mg/L); 33-36. Tagetes minuta (5, 50, 125 and 250 mg/L); 37-40. Thymus vulgaris (5, 50, 150 and 300 mg/L).
DISCUSSION
The effects of monensin on fermentation parameters in vitro are consistent with those reported elsewhere (Castillejos et al., 2006; Russell and Strobel, 1989; Wallace et al., 1981). Russel and Strobel (1989) reported an association with methane reduction to a decrease in acetate production caused by a reduction in the digestion of neutral detergent fiber.
Intermediate to high level of inclusion for some EO (e.g. A. gratissima at 300 mg/L and O. vulgare ssp. hirtum var. Mendocino at 150 and 250 mg/L) reduced fiber digestibility with no effect on gas production. Thus, when evaluating additives to modify rumen fermentation activity, care should be taken if using gas production as the only indicator of overall fermentation. Except for E. globulus and L. turbinata, every variable measured was significantly reduced with the higher level of inclusion compared to the control (methane, D-NDFom and gas production), representing that overall microbial activity was affected. This is in agreement with the dose-response effects previously reported for EO (Khiaosa-ard and Zebeli, 2013; Klevenhusen et al., 2012). Essential oils from native Argentinean plants have proven biological activity in several processes (Fuselli et al., 2006; García et al., 2003; Tereschuk et al., 1997); however, to our knowledge, they have not been previously evaluated to modify rumen microbial function. Therefore, the discussion will be approached by the compounds present in those EO.
Limonene is the main compound in L. turbinata, and it is also present in S. molle and T. minuta. Castillejos et al. (2006) reported toxic effects of limonene at levels of 50 and 500 mg/L, and concluded that there appears to be no benefit in using limonene as an additive to modify rumen microbial fermentation. On the contrary, in our study, among all the oils evaluated, L. turbinata (60.8% limonene) was the most effective at all inclusion levels, reducing methane by half compared to control, and showing 35 to 85% reduction when compared to monensin, without affecting fiber digestibility. With the other two EO that contain limonene, S. molle and T. minuta, fiber digestibility was severely affected at higher doses; however, in these EO limonene, represents the third main compound (13.8 and 6.0% respectively). Low levels of A. gratissima had no effects on any of the variables measured, and at the highest level (300 mg/L), this EO affected the fiber digestibility with no effects on methane. At the doses tested this EO did not represent a promising alternative to inhibit methane production in vitro. At the dose range tested for E. globulus, which is comparable with the range of doses evaluated for the rest of EO in this study, we did not find any effects on the variables studied. The absence of effect suggests low antimicrobial activity of this EO, being necessary higher levels than those evaluated here. In this regard, Patra and Yu (2012) observed a linear reduction in gas and methane production at levels ranging from 250 mg/L to 1000 mg/L. Doses in the present study may have been low to detect the effects of this EO on rumen fermentation in vitro. Mentha x piperita had no impact on fermentation at low levels. An inhibition of methane was achieved by increasing the dose; however, it was associated with a reduction in fiber digestion. The adverse effect caused by M. x piperita on the microbial population, and on cellulolytic bacteria in particular (Patra and Yu, 2014, 2012), can explain the reduction in fiber digestibility observed for levels of 200 and 400 mg/L.
The reduction in fiber digestibility found for both cultivars of O. vulgare at high levels of inclusions are in accordance with previous reports (Busquet et al., 2006; Cardozo et al., 2005; Castillejos et al., 2008). Busquet et al. (2006) evaluated different EO and their main components and concluded that carvacrol is the main active compound in oregano oil. In the present study, carvacrol was found in small proportion (0.001% for Compacto and 4% for Mendocino); and trans sabinene hydrate, thymol and γ-terpinene were the main components. It is important to highlight that even though the composition of EO of both cultivars was similar, the effects found for the same level of inclusion (250 mg/L) differed markedly between them, being the ecotype Compacto more effective than Mendocino.
Based on our results, we can suggest that R. officinalis has a strong antimicrobial activity and that the optimal inclusion level may be difficult to define because there is a narrow range of doses between those showing desirable effects (methane production inhibition) and those having negative effects (reduction of fiber degradation). Severe reduction of all the variables measured were registered at doses over 300 mg/L, which is not in agreement with the findings of Castillejos et al. (2008) who reported no reduction on rumen fermentation products (ammonia and VFA) at levels of 500 mg/L. Only high levels of T. vulgaris affected gas production and fiber digestion; however, this effect was not accompanied by a reduction in methane production. When evaluating thyme oil at 5, 50 and 500 mg/L, Castillejos et al. (2008) found an increase in total VFA at all doses of the oil, while an inclusion of its main component, thymol at 500 mg/L reduced total VFA concentration by around 30% (Castillejos et al., 2006).
These results indicate that the action of the main compounds isolated may not directly have the same behavior of the primary complex mixture of chemical structures. An explanation of this phenomenon is the interaction that may occur between compounds, such as the synergic effect of p cymene in carvacrol/thymol-based EO (Macheboeuf et al., 2008). In addition, EO comprise a large number of we consider it is essential a large number of components and it is likely that their mode of action involves several targets in the bacterial cells (Castillejos et al., 2006). The diversity in EO composition, which depends on environmental conditions and genetic aspects, results in different bioactivity of the EO related to plant ecotype and origin. Hence, when using plants as source of oil, we considered it is essential to include a description of the EO composition as part of the study.
CONCLUSIONS
Essential oils from native Argentinean plants are capable of modifying rumen fermentation in vitro. Lippia turbinata is a promising natural alternative to in-feed antibiotics, considering that it has great potential to reduce enteric methane production without affecting digestibility. Considering the complex composition of essential oils and their resulting bioactivity, a description of the chemical composition should be included in this kind of evaluations.
ACKNOWLEDGMENTS
This study was completed at the National Institute of Agricultural Research (INTA Manfredi’s Experimental Station, Cordoba, Argentina), and was made possible by a grant from the National Program of Animal Production of INTA (PNPA-1126023).
REFERENCES
1. BENCHAAR, C.; GREATHEAD, H. 2011. Essential oils and opportunities to mitigate enteric methane emissions from ruminants. Anim. Feed Sci. Technol. 166-167, 338–355. doi:10.1016/j.anifeedsci. 2011.04.024
2. BUSQUET, M.; CALSAMIGLIA, S.; FERRET, A.; KAMEL, C. 2006. Plant extracts affect in vitro rumen microbial fermentation. J. Dairy Sci. 89, 761–771. doi:10.3168/jds.S0022-0302(06)72137-3
3. CALSAMIGLIA, S.; BUSQUET, M.; CARDOZO, P.W.; CASTILLEJOS, L.; FERRET, A. 2007. Invited Review: Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 90, 2580– 2595. doi:10.3168/jds.2006-644
4. CARDOZO, P.W.; CALSAMIGLIA, S.; FERRET, A.; KAMEL, C. 2005. Screening for the effects of natural plant extracts at different pH on in vitro rumen microbial fermentation of a high-concentrate diet for beef cattle. J. Anim. Sci. 83, 2572–2579.
5. CASTILLEJOS, L.; CALSAMIGLIA, S.; FERRET, A. 2006. Effect of essential oil active compounds on rumen microbial fermentation and nutrient flow in in vitro systems. J. Dairy Sci. 89, 2649–2658. doi:10.3168/jds.S0022-0302(06)72341-4
6. CASTILLEJOS, L.; CALSAMIGLIA, S.; MARTÍN-TERESO, J.; TER WIJLEN, H. 2008. In vitro evaluation of effects of ten essential oils at three doses on ruminal fermentation of high concentrate feedlot-type diets. Anim. Feed Sci. Technol. 145, 259–270. doi:10.1016/j.anifeedsci.2007.05.037
7. CIESLAK, A.; SZUMACHER-STRABEL, M.; STOCHMAL, A.; OLESZEK, W. 2013. Plant components with specific activities against rumen methanogens. Animal 7, 253–265. doi:10.1017/ S1751731113000852
8. DI RIENZO, J.A.; CASANOVES, F.; BALZARINI, M.G.; GONZALEZ, L.; TABLADA, M.; ROBLEDO, C.W. 2011. InfoStat. [ Links ]
9. FIGUEIREDO, A.C.; BARROSO, J.G.; PEDRO, L.G.; SCHEFFER, J.J.C. 2008. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragr. J. 23, 213–226. doi:10.1002/ffj.1875
10. FUSELLI, S.R.; GARCÍA DE LA ROSA, S.B.; GENDE, L.B.; EGUARAS, M.J.; FRITZ, R. 2006. Antimicrobial activity of some Argentinean wild plant essential oils against Paenibacillus larvae, causal agent of American foulbrood (AFB). J. Apic. Res. 45, 02–07.
11. GARCÍA, C.C.; TALARICO, L.; ALMEIDA, N.; COLOMBRES, S.; DUSCHATZKY, C.; DAMONTE, E.B. 2003. Virucidal activity of essential oils from aromatic plants of San Luis, Argentina. Phytother. Res. 17, 1073–1085. doi:10.1002/ptr.1305.
12. GARCÍA, F.; MARTÍNEZ FERRER, J.; CORA, A.; BRUNETTI, M.A.; FROSSASCO, G.; LUCINI, E.; MORENO, M.V.; MARTÍNEZ, M.J.; COLOMBATTO, D. 2014. Effect of increasing doses of essential oils on in vitro ruminal fermentation, in: Revista Argentina de Producción Animal Vol. 34 (Supl. 1). 387 p. [ Links ]
13. JOHNSON, K.A.; JOHNSON, D.E. 1995. Methane emissions from cattle. J. Anim. Sci. 73, 2483–2492.
14. KHIAOSA-ARD, R.; ZEBELI, Q. 2013. Meta-analysis of the effects of essential oils and their bioactive compounds on rumen fermentation characteristics and feed efficiency in ruminants. J. Anim. Sci. 91, 1819–1830. doi:10.2527/jas2012-5691
15. KLEVENHUSEN, F.; MURO-REYES, A.; KHIAOSA-ARD, R.; METZLER-ZEBELI, B.U.; ZEBELI, Q. 2012. A meta-analysis of effects of chemical composition of incubated diet and bioactive compounds on in vitro ruminal fermentation. Anim. Feed Sci. Technol. 176, 61–69. doi:10.1016/j.anifeedsci.2012.07.008
16. LIN, B.; WANG, J.H.; LU, Y.; LIANG, Q.; LIU, J.X. 2013. In vitro rumen fermentation and methane production are influenced by active components of essential oils combined with fumarate. J. Anim. Physiol. Anim. Nutr. (Berl). 97, 1–9. doi:10.1111/j.1439- 0396.2011.01236.x
17. MACHEBOEUF, D.; MORGAVI, D.P.; PAPON, Y.; MOUSSET, J.-L.; ARTURO-SCHAAN, M. 2008. Dose-response effects of essential oils on in vitro fermentation activity of the rumen microbial population. Anim. Feed Sci. Technol. 145, 335–350. doi:10.1016/j. anifeedsci.2007.05.044
18. PATRA, A.K.; YU, Z. 2014. Effects of vanillin, quillaja saponin, and essential oils on in vitro fermentation and protein-degrading microorganisms of the rumen. Appl. Microbiol. Biotechnol. 98, 897–905. doi:10.1007/s00253-013-4930-x
19. PATRA, A.K.; YU, Z. 2012. Effects of essential oils on methane production and fermentation by, and abundance and diversity of, rumen microbial populations. Appl. Environ. Microbiol. 78, 4271– 4280. doi:10.1128/AEM.00309-12
20. RUSSELL, J.B.; STROBEL, H.J. 1989. Effect of ionophores on ruminal fermentation. Appl. Environ. Microbiol. 55, 1–6.
21. STEINFELD, H.; GERBER, P.; WASSENAAR, T.; CASTEL, V.; ROSALES, M.; DE HAAN, C. 2006. Livestock’s long shadow: Environmental issues and options, FAO, Rome, Italy,. doi:10.1007/ s10666-008-9149-3
22. TERESCHUK, M.L.; RIERA, M.V.Q.; CASTRO, G.R.; ABDALA, L.R. 1997. Antimicrobial activity of flavonoids from leaves of Tagetes minuta. J. Ethnopharmacol. 56, 227–232. doi:10.1016/S0378- 8741(97)00038-X
23. THEODOROU, M.K.; WILLIAMS, B.A.; DHANOA, M.S.; MCALLAN, A.B.; FRANCE, J. 1994. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 48, 185–197. doi:10.1016/0377-8401(94)90171-6
24. TILLEY, J.M.A.; TERRY, R.A. 1963. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 18, 104– 111. doi:10.1111/j.1365-2494.1963.tb00335.x
25. VAN SOEST, P.J.; ROBERTSON, J.B.; LEWIS, B.A. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi:10.3168/jds.S0022-0302(91)78551-2
26. WALLACE, R.J.; CZERKAWSKI, J.W.; BRECKENRIDGE, G. 1981. Effect of monensin on the fermentation of basal rations in the Rumen Simulation Technique (Rusitec). Br. J. Nutr. 46, 131–148. doi:10.1079/BJN19810016














