Introduction
Trematode infections, especially fasciolosis, are some of the most economically significant helminth diseases that hinder the productivity of domestic ruminants worldwide14, 16. All species of trematodes parasites in cattle belong to Digenea subclass 19. Adult trematodes are commonly called “hepatic trematodes” and families that include parasites of great veterinary importance are Fasciolidae, Dicrocoeliidae, Paramphistomatidae and Cotylophoronia 27.
Fasciola (hepatic trematode), parafistoma (rumen/ stomach trematode) and Cotylophora (reticulum/small intestine trematode) are the most significant trematodes recorded in different parts of the world 7.
Fasciolosis is an economically significant disease of domestic cattle and sheep, and occasionally of man.
F. hepatica and F. gigantica are the two species most commonly implicated as etiological agents of fasciolosis3. Infection of adult cattle with liver flukes, unless they are serious infections, is usually clinically apparent. Therefore, under normal conditions, clinical disease is only likely in young cattle13.
However, even a moderate infection can result in a significant reduction in milk yield and quality 27, a reduction in weight gain11, 21 and reproductive performance8. The infection of calves with a large number of metacercariae (>1000), on the other hand, causes clinical fasciolosis similar to that observed in sheep characterized by weight loss, anemia and hypoproteinemia. In addition to its effect on productivity, fasciolosis is a cause of significant economic losses through the loss of the liver in slaughter 1,19.
There are several genera of paramphistomes: Paramphistomum, Cotylophoron, Calicophoron, Bothriophoron, Orthocoelium and Gigantocotyle, of which Paramphistomum is the most common and widespread in ruminants 23. Paramphistomes (Amphistomes) are traditionally considered without clinical importance11. However, a serious infection with immature trematodes, which adhere to the lining of the upper part of the small intestine, can cause serious illness and even death12.
Moderate infections of immature state can increase weight loss and reduce milk production. However, most cattle only have mild stomach infections with the adult form and those with small amounts of immature forms generally show no signs of disease12.
For arational and sustainable helminth control program, a thorough knowledge of the epidemiology of parasites and their interaction with the host in a specific climate and a management system is a prerequisite3. Therefore, this study was conducted with the objectives of establishing the prevalence and identifying the risk factors associated with trematodes infection in milk cattle in the Boyacá Highlands (Colombia).
Material and Methods
Ethics statement. Sampling for parasitism diagnosis is part of the routine of epidemiological surveillance of domestic animals on milk farms in the region and is not an invasive practice that puts the integrity of animals at risk. The research group has access to the parasitology laboratory appropriate to the techniques and purposes of the study. This study is a risk-free investigation in accordance with the provisions of resolution 8430 of 1993 Ministry of Health of Colombia. The study was approved by the institutional bioethics committee.
Study area and animals. The study was carried out in the Sotaquira Valley, located in the center of eastern Colombia, 39 km from Tunja. at a latitude and longitude of 5°45'N and 73°14'O and an average elevation of 2,860 m. The climate has an average annual rainfall of 1500 mm, humidity of 68.88% and average temperature of 16°C. The main rainy season runs from late February to mid-June and from late August to mid-December. The area has poor drainage and there is annual flooding during the rainy season, leaving bodies of water for a prolonged period even during the dry season.
The study included cattle of one year and over, of both sexes and pure breed and cross breed animals (Holstein and its crosses) managed under the traditional system of medium-sized farms, where cattle are often kept outdoors and graze all day.
Study design and sampling technique. The study was a cross-sectional study that involved 300 animals, selected using a simple random sampling method. The sample size was calculated according to Thrusfield (2005) with an estimated parasite prevalence of 20% and desired a 95% confidence interval and 5% precision.
Fecal sample and data collection. Stools was collected directly from the rectum of the study animals with gloved hands and the palpation sleeves were marked. The location, breed, age, sex and body condition of each study animal was registered at the moment of sample collection. Age was taken from the records. Cattle that are estimated to be less than 4 years old were considered as young cattle, while those of 4 years and over as adults. Body condition was classified in 3 categories 1.5 (A), 3 (B), and 4.5 (C), using Nicholson and Butterworth (1986), recommendations.
Coprological examination. It were performed in the Veterinary Microbiology Laboratory of the UPTC. Stool samples, when not examined immediately upon arrival, were stored in a refrigerator at 4°C until examination. A simple sedimentation technique was used for the detection and counting of trematode eggs 6with minor modifications. Three grams of feces were placed in a container and 40-50 ml of tap water was added and mixed thoroughly. The suspension was filtered through a strainer and allowed to stand for 5 minutes. The supernatant was carefully discarded and the sediment was resuspended in tap water.
The sedimentation process was repeated several times until fecal debris and coloring material were removed and the supernatant was clear. After the last sedimentation process, the supernatant was removed very carefully and the sediment was recovered in a test tube and re-suspended in approximately 5 ml of tap water, a drop of blue methylene colorant was added and allowed to stand for 5 minutes. All the material was transferred to a Petri dish and examined with a low powered lens. Trematode egg counts were performed by moving the Petri dish in such a way that all fields were examined. The F. hepatica eggs took a yellow color.
The Paramphistomum sp eggs were identified using images 27. Cotylophorum sp were washed with distilled water and suspended in 70% alcohol. They were hydrated in consecutive concentrations of 50% and 25% alcohol and distilled water. Later they were colored with Carmine of Meyer, following the Jones protocol. The taxonomic determination was made using the Eduardo and Jones method 27.
Data management and statistical analysis. The data collected during sample collection and the results of coprological exams were entered and stored for analysis in the Microsoft Excel spreadsheet. The effect of age, sex, breed and body condition on trematodes infections was analyzed using a multiple logistic regression model / analysis. A univariable logistic analysis was used to assess the relationship between mixed infections and infections by individual trematodes according to body condition.
The gamma statistics of Goodman and Kruskal were used as a correlation measure for the occurrence of the three trematodes. An animal was considered positive with 1 egg for the respective trematode infection. All statistical analyzes were performed with Stata 13.1 for Windows (Stata Corporation, College Station, TX). A p value of less than 0.05 was considered significant.
Results
The specific prevalence of trematodes observed (95% CI) was of 11.6%, 9.3%, and 3.7% for Fasciola hepatica, Paramphistomum cervi and Cotylophoron cotylophorum, respectively. Mixed infections with at least two parasites were recorded in 82 27.3% cattle (95% CI) (Table 1).
Table 2 shows the results of the correlation between mixed infestations and body condition (n=300). Prevalence of F. hepatica, Paramphistomum sp and Cotylophoron sp were significantly associated with body condition and breed of the study animals (p<0.01). The prevalence of all the three trematodes was higher in animals with lean body condition than with fat body condition.
Body condition was significantly associated with concurrent infection with F. hepatica and Paramphistomum sp (p<0.01) and Paramphistomum sp and Cotylophoron sp (p<0.001). The highest prevalence of coinfections was observed in animals with lean body condition than in animals with optimum or fat body conditions. However, although co-infections related to Paramphistomum sp were significantly linked with body condition, while the single infection with Paramphistomum sp was not (p>0.05) (Table 3).
Cattle younger than four years were associated with higher prevalence of F. hepatica and Paramphistomum sp (p<0.05). F. hepatica prevalence showed a tendency to be greater in females than in males. Table 4 shows that animals with body condition 3 (53%) are less likely to be infected than cows with body condition 1.5 or 4.5. Cross animals are more resistant to Paramphistomum sp than pure Holstein cows.
Table 1 Prevalence of trematodes in milk cattle in the Boyacá Highlands (n=300).
| parasite species | 65.positive | 66.95%CI |
|---|---|---|
| FH | 69.35 | 70.11.6 |
| PC | 73.28 | 74.9.3 |
| CC | 77.11 | 78.3.7 |
| FH + PC | 81.45 | 82.15.0 |
| FH + CC | 85.24 | 86.2.4 |
| PC + CC | 89.13 | 90.8.0 |
| FH + PC + CC | 93.0 | 94.0 |
| mixed | 97.82.0 | 98.25.4 |
FH: Fasciola hepatica; PC: Paramphistomum cervi; CC: Cotylophoron cotylophorum; PV: prevalence.
Table 2 Correlation between mixed infestations and body condition BC (n=300).
| infection | 104.BC | 105.% | 106.(+) | 107.% |
|---|---|---|---|---|
| FH + PC | 110.1.5 | 111.80 | 112.14 | 113.17.5 |
| 116. | 3.0 | 117.184 | 118.28 | 119.15.2 |
| 122. | 4.5 | 123.36 | 124.3 | 125.8.3 |
| FH + CC | 128.1.5 | 129.80 | 130.10 | 131.12.5 |
| 134. | 3.0 | 135.184 | 136.12 | 137.6.52 |
| 140. | 4.5 | 141.86 | 142.2 | 143.5.55 |
| PC + CC | 146.1.5 | 147.80 | 148.5 | 149.6.25 |
| 152. | 3.0 | 153.184 | 154.7 | 155.3.80 |
| 158. | 4.5 | 159.86 | 160.1 | 161.2.77 |
BC: body condition; %: number examined; (+): positives.
Table 3. Analysis of association between the prevalence of infestation by Fasciola hepatica and risk factors in cattle.
| V | 167.level | 168.FH | 169.170. | PR | 171.95%CI | 172.p | 173. 174. 175. 176.
|---|---|---|---|---|---|---|
| + - | 177. 178. 179. 180. 181. 182.||||||
| sex | 183.female | 184.33 | 185.262 | 186.0.28 | 187.0.09-0.85 | 188.0.058 | 189. 190. 191.
| male | 192.2 | 193.3 | 194. 195. 196. 197. 198.||||
| BC | 199.3.0 | 200.20 | 201.184 | 202.0.84 | 203.0.44-1.57 | 204.0.29 | 205. 206. 207.
| 1.5-4.5 | 208.15 | 209.101 | 210. 211. 212. 213. 214.||||
| age | 215.>4 years | 216.25 | 217.155 | 218.1.66 | 219.0.83-3.44 | 220.0.07 | 221. 222. 223.
| <4 years | 224.10 | 225.110 | 226. 227. 228. 229. 230.||||
| breed | 231.cross | 232.7 | 233.186 | 234.0.13 | 235.0.06-0.30 | 236.<0.0001 | 237. 238.
| 239. | Hp | 240.28 | 241.79 | 242.243. | 244. |
V: variable; FH: Fasciola hepatica; PR: prevalence ratio; + : positives, -: negatives, BC, body condition; Hp: Holstein pure; p: P value.
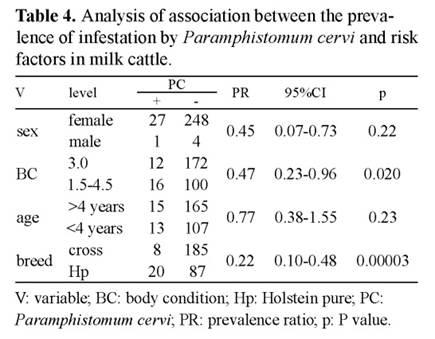
Table 4 Analysis of association between the prevalence of infestation by Paramphistomum cervi and risk factors in milk cattle. V: variable; BC: body condition; Hp: Holstein pure; PC: Paramphistomum cervi; PR: prevalence ratio; p: P value.

Table 5 Analysis of association between the prevalence of infestation by Cotylophoron cotylophorum and risk factors in milk cattle. V: variable; BC: body condition; Hp: Holstein pure; CC: Cotylophoron cotylophorum; PR: prevalence ratio; p: P value.

Table 6 Results of the association between the prevalence of mixed infestation by trematodes and body condition in cattle. BC: body condition; PR: prevalence ratio, FH: Fasciola hepatica; PC: Paramphistomum cervi; CC: Cotylophoron cotylophorum; PR: prevalence ratio; p: P value.
Table 5 shows that body condition 3.0 is more resistant to infection by Cotylophoron sp and other body conditions are more sensitive to infection. Holstein pure cows are more susceptible to Cotylophoron sp than cross animals.
There was a substantial overlap in the infection of individual animals with Fasciola sp, Paramphistomum sp and Cotylophoron sp (Table 6). The overlap was significant (gamma = 0.457) between F. hepatica and P. cervi, while it was inconclusive for Cotylophoron sp vs Fasciola sp (0.104) and Cotylophoron sp vs. Paramphistomum sp (-0.023). Table 6 shows the results of the association between the prevalence of mixed infestation by trematodes as potential predictors.
The prevalence of Fasciola sp, Paramphistomum sp and Cotylophoron sp, were significantly associated with the body condition and breed of the study animals (p<0.01). The prevalence of the three trematodes was higher in animals with a lean body condition than with a fat body condition and in Holstein pure cattle compared to the crosses. Cattle younger than four years were associated with higher prevalence of Fasciola sp and Paramphistomum sp (p<0.05).
Interestingly, no animals with fat body condition score (4.5) were found to have F. hepatica infections. But the observation number (n=3) was not enough to make a statistical comparison. The number of animals found co-infected with Fasciola sp and Cotylophoron sp (n=24) and with Cotylophoron sp, only was not sufficient (n=11) to make statistical comparisons and, therefore, were omitted from the analysis.
Discussion
In this study, the highest prevalence was recorded for Fasciola sp (11.6%) followed by Paramphistomum sp (9.3%) and Cotylophoron sp (3.7%). A similar pattern of occurrence has been reported, where Fasciola hepatica exceed the prevalence followed by Paramphistomum sp and Cotylophoron sp respectively, for the three trematodes from different parts of Ethiopia22, 29 and elsewhere in Africa16, 19,20.
As opposed, investigators reported a consistently higher prevalence of paramfistomas than Fasciola gigantica, in cattle managed under different conditions in Tanzania. In the department of Quindío (Colombia), for the years 2012 and 2013, investigators reported prevalence of F. hepatica of 3.74% in cattle, much lower than that of the present study (11.6%), it can be explained that the authors had taken samples of recently wormed animals21.
In the Department of Cesar in Colombia 18, found a prevalence for F. hepatica of 3.4% and Paramphis tomum sp of 0.7%, this was also much lower than the present study (9.3). Although the humidity and temperature conditions were different, the study was not of dairy cattle or involve pure Holstein cattle.
In the municipality of Une (Cundinamarca, Colombia) in 2016, the presence of F. hepatica was 15.5% due to the presence of eggs in fecal material10 , although this study was carried out in the slaughter house, the percentage was very similar to that of the present study, which shows that the technique used in the present study was quite comparable.
While in Cuba, in 2016, investigators found a prevalence of F. hepatica (70%) in Siboney crosses with animals between 5 and 7 years, a prevalence that is very different to the data obtained in the present study, being a tropical country 22 .
The prevalence of F. hepatica observed in this study is comparable to the 24% prevalence reported by others30 (2012). However, it was lower compared to other recent reports from areas adjacent to Lake Tana 9 and reports from other parts of Africa 24, 25.
The difference in prevalence may be due to the difference in the amount of rainfall and other climatic conditions over the years in the area, and differences in ecological climatic conditions between the study areas29. The relative low prevalence of F. hepatica may also be associated with the expansion of veterinary services in the area.
The high prevalence of F. hepatica, reported in parts of the country without large permanent bodies of water compared to our study area, may show the relative importance of F. hepatica in cattle with fasciolosis in Colombia. The intermediate snail hosts of F.hepática are amphibious 3and -therefore- do not necessarily need an aquatic environment for their survival and proliferation. Several slaughterhouse surveys conducted in different parts of the country demonstrated the co-infection of cattle with Fasciola sp and Param phistomum sp 13, 29.
The prevalence of Paramphistomum sp can be explained in part by the fact that the adult parasite is considered non-pathogenic and, subsequently, is not the objective of anthelmintic treatment. It could also be related to the biology of the parasite and intermediate hosts. Adult parammphistomes can survive in the host for years, and are very prolific in producing many eggs, while the parasite multiplication in infected snails is extremely high11 .
Paramphistomum intermediate host are also ex tremely adaptable and massive12. The lack of availability of effective drugs against paramphistomas could also have contributed to the relative high prevalence of the parasite11. Common anthelmintics used for routine deworming to treat major liver nematodes and trematodes in Colombia such as albendazole, ivermectin and triclabendazole, have little or no effect on paramphistomas 25.
An increasing prevalence of paramphistomas has been documented in comparison to liver flukein France, in part due to the lack of an effective treatment against bovine paramphistomosis 14. The prevalence of Paramphistomum sp recorded in the present study (9.3%) was comparable to a previous report (22.6%) of an area similar to our study area18 characterized by a humid tropical climate with heavy rainfall.
Studies conducted in Africa3, near a large swampy area crossed by a river in northeastern Ethiopia (75%) and around a small lake in the Northern Ethiopia (65.3%), demonstrated a higher prevalence of Paramphistomum sp. However, many other studies in Ethiopia reported a lower prevalence of the parasite compared to our finding 25, 29.
The higher prevalence of Paramphistomum sp whose intermediate hosts are aquatic snails, observed in the present study could be explained by the fact that our study was conducted near permanent bodies of water compared to some of the other studies that were conducted in drier areas2.
The prevalence of Cotylophoron sp was 3.7% in this study, it is comparable to the findings which was 9.0% in the Andean region in Colombia dairy herds18. In Africa, at Lake Tana3 and western Ethiopia9, the prevalence was low compared to previous reports (28%) from others investigator30.
The variations observed between studies on the prevalence of trematodes in general can be attributed to the differences in ecological climatic conditions between the study areas, the difference in rainfall between the years of study, the differences in the study stations and the difference in animal management practices.
The prevalence of the three trematodes considered in this study, were higher in thin animals compared to animals with medium and fat body condition. Serious infection with F. hepatica in cattle, especially in young cattle, can cause a serious disease characterized by anemia, hypoalbuminemia (edema), body condition problems and weight loss 4, 14, 28. Similarly, a serious infection with immature stomach flukes can cause de creased appetite, apathy and weight loss 21.
Even moderate infections with F. hepatica 11, 21 and immature Paramphistomum sp 12 can affect weight gain. Loss of appetite, which could contribute to poor body condition, is also one of the clinical signs of chronic fasciolosis 13. However, it should be borne in mind that it is difficult to separate the effects of different genera of trematodes on the body condition, since they tend to occur together. Our finding supports previous reports that associated fasciolosis19, Paramphistomum sp 14 and Cotylophoron sp with poor body condition.
The significant association of fasciolosis (when an animal was considered F. hepatica positive regardless of its status to the other two trematodes) with the body condition observed in this analysis was not repeated when animals infected with F. hepatica alone (single infection) were considered.
It is possible that the result was influenced by those animals that were also coinfected with other trematodes (especially Paramphistomum sp) in the former, since
F. hepatica, positive animals were more likely to be positive for Paramphistomum sp than their F. hepatica negative counterparts. It is also possible that animals found with single Fasciola sp infection in the study, were wormed with effective anthelmintics against liver flukes and possibly against nematodes.
The result may even suggest an additive or synergistic pathogenic effect of coinfection with trematodes. A high mortality rate has been reported in concurrent infections involving F. gigantica and Cotylophoron sp, in dairy cows2. After observing a positive correlation (r2 = 0.12) between Fasciola sp and Paramphistomum sp, worm count in naturally infected cattle, other investigators suggested that the heterologous interaction of these two parasites may aggravate the economic effects of liver fluke in the livestock industry29. The prevalence of F. hepatica and P. cervi were higher in adult cattle, over 4 years old, compared to their young counterparts. Simultaneously with this finding, other researchers also recorded a higher prevalence of F. hepatica in younger cattle3,17. However, many studies on Paraphistomum cervi in Ethiopia and elsewhere, found no difference in prevalence between age groups 15, 19.
The variation could be attributed in part to differences in the classification of age categories between studies. The development of immunity due to exposure to Fasciola sp, which limits the lifespan of the primary infection, slows the migration of the secondary infection and ultimately reduces the number of established trematodes 28, may be responsible for a lower prevalence of Fasciola sp in older cattle. Similarly, the development of a better acquired immunity against param phistomas has been established22,24,26, 30.
It was found that pure cattle were more affected with the three trematodes and excreted a greater number of eggs in their feces than crossed animals, contrary to some previous reports from Ethiopia 9,11. This difference is likely due to the inequality in exposure rather than the difference in natural resistance, since studies involving F. hepatica suggested that cattle (Bos indicus) appear to be more resistant than Bos taurus to infection with Fasciola sp 5,7,26.
It is possible that more attention was given to valuable animals that their probability of grazing in areas infested with snails was limited and/or they were dewormed more frequently than crossed animals. According to this observation, some studies reported a higher prevalence of trematodes in cross cattle than in pure animals 24,25, 30.
In this study, few animals (n = 24) harbored both Fasciola sp and Cotylophoron sp, and neither the appearance nor the EPG (eggs per gram) of these trematodes were correlated. Investigators recorded a similar finding and explained it with the important liver pathology caused by both trematodes, which may exclude the establishment of the other when an infection is established 29.
In conclusion, the present study showed that the main trematodes of significance for the health and welfare of animals are relatively prevalent in the study area, especially in adult cattle with a high rate of mixed infections. The finding suggests that there is considerable economic loss due to trematode infections through the reduction of livestock production efficiency in the study area.
The prevalence of Fasciola sp and Cotylophoron sp, also show the risk that these parasites could pose for public health. Also, measures should be considered that help minimize the exposure of livestock to parasites, such as keeping livestock away from grazing in high- risk areas and the strategic use of effective anthelmintics against mixed infections of trematodes, especially in young cattle. Empirical diagnosis and treatment of cattle, mainly showing poor growth or weight loss, in the study area should take into account trematodes.














